Abstract
Intradermal vaccination via gene gun efficiently delivers DNA vaccines into dendritic cells (DCs) of the skin, resulting in the activation and priming of antigen-specific T cells in vivo. We have previously demonstrated that intradermal delivery of DNA vaccines encoding single-chain trimer (SCT) composed of the most immunogenic epitope of human papillomavirus type 16 (HPV-16) E6 protein (aa49-57), β2-microglobulin, and MHC class I heavy chain (SCT-E6) can bypass antigen processing and lead to stable cell-surface presentation of E6 peptides. We also showed that co-administration of DNA vaccines with DNA encoding anti-apoptotic proteins can prolong the survival of DNA-transduced DCs, resulting in significant enhancement of antigen-specific CD8+ T cell immune responses. In the current study, we hypothesized that combining the SCT strategy and antiapoptotic strategy may further enhance DNA vaccine potency by augmenting antigen-specific CD8+ T cell immune responses and antitumor effects in vaccinated mice. Here, we show that C57BL/6 mice vaccinated with SCT-E6 DNA combined with antiapoptotic protein Bcl-xL DNA generated enhanced E6-specific CD8+ T cell immune responses compared to mice vaccinated with SCT-E6 DNA and a non-functional mutant Bcl-xL (mtBcl-xL) DNA. Furthermore, we show that mice treated with SCT-E6 and Bcl-xL DNA generated enhanced anti-tumor effects against E6-expressing tumor cells (TC-1/Luciferase) compared to mice treated with SCT-E6 and mtBcl-xL DNA. We conclude that the combination of a strategy to bypass antigen processing and presentation mechanisms and a strategy to prolong the life of DCs can lead to significant enhancement of DNA vaccine potency.
Keywords: Human papillomavirus-16 (HPV-16), E6, single chain trimer (SCT), dendritic cells, cancer immunotherapy, BclxL, DNA vaccines, tumor treatment
Introduction
DNA vaccination has emerged as an approach with great potential for the therapy of cancers (for review, see [1]). Naked plasmid DNA vaccines are relatively safe and simple to prepare in large quantities of high purity, and the inherent stability of their molecular structure is an added benefit compared to biological agents such as vector-based or protein-based vaccines [2, 3]. Additionally, DNA vaccines may be administered repeatedly without concern for losing their ability to be boosted, unlike bacterial or viral vectors. While DNA has considerable advantages, one major drawback is its limited potency. DNA vaccine potency may be enhanced by targeting DNA to the antigen-presenting cells and by modifying the properties of DNA-transfected APCs (for review, see [4]). Furthermore, naked DNA has no cell type specificity, thus it is essential to determine the optimal route for delivery of DNA vaccines.
Administration of DNA vaccines can be accomplished via different routes, but perhaps one of the most efficient routes of delivery involves intradermal administration by gene gun. This procedure consists of the acceleration of gold particles coated with DNA encoding a protein of interest, i.e., a tumor antigen, into the skin of the recipient, using high-pressure inert gas as the propellant. Within the epidermal tissue, the key professional antigen-presenting cells (APCs), the Langerhans cells, receive the DNA and express the encoded antigen [5]. These antigen-expressing cells mature, and are then able to migrate to draining lymph nodes, where they interact with specific naïve T cells, resulting in their activation to effector T cells specific for the antigen encoded by the DNA vaccine [6]. Thus, the administration of DNA vaccines via gene gun serves as an efficient method to directly deliver DNA into the professional APCs and represents a system to test the various strategies that modify the properties of APCs to enhance DNA vaccine potency.
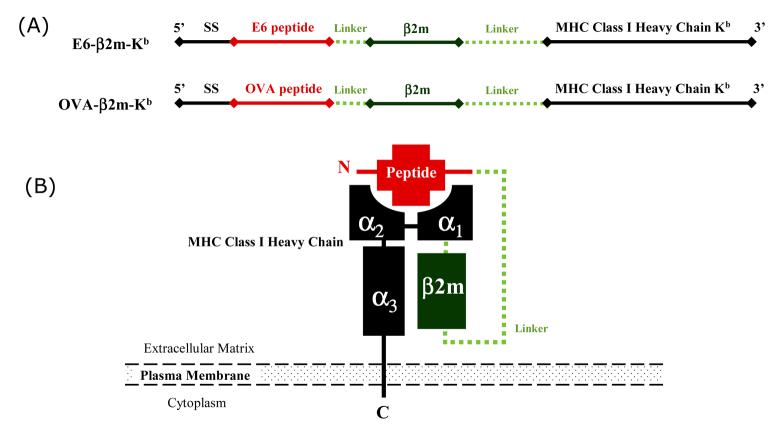
Using the gene gun delivery system, Huang, et al. have introduced an innovative strategy to enhance DNA vaccine potency using a DNA construct encoding the single chain trimer (SCT) molecule which consists of a murine MHC class I heavy chain, β2-microglobulin and an immunogenic peptide (see Figure 1). The main advantage of this strategy employing DNA vaccine encoding SCT is that the vaccine peptide chosen for expression may be optimized for maximal immunogenicity by circumventing the endogenous antigen processing and presentation mechanisms [7]. Huang, et al. have chosen an immunodominant antigenic peptide derived from HPV-16 oncoprotein E6. HPV-16 E6 is constantly expressed in cervical cancer cells and responsible for the malignant transformation of the cervical cancer and thus represents a feasible target antigen for therapeutic HPV DNA vaccine development. Huang, et al. showed that vaccination of mice with the SCT-E6 DNA construct resulted in significantly high levels of E6-specific CD8+ T cell-mediated immune responses and potent antitumor effects against E6-expressing tumors [8].
Figure 1. Schematic representations of the DNA vaccine constructs and our single chain trimer.
A) Chimeric DNA constructs encoding single chain trimers of E6 peptide (aa49-57) or OVA peptide (aa257-264) linked to β2m and an H2-Kb-restricted MHC class I molecule. B) Diagram depicting the structure of the single chain trimer molecule.
Another strategy to enhance the DNA vaccine potency is to prolong the life of DNA-transfected dendritic cells (DCs). We previously demonstrated that inhibition of apoptotic processes in DNA-transfected DCs using co-administration of DNA vaccines with DNA encoding antiapoptotic proteins led to prolonged life of transfected DCs, resulting in enhanced antigen-specific T cell-mediated immune responses [9]. Our data strongly suggested that perpetuating the survival of antigen-expressing DCs constitutes a compelling strategy for enhancing antigen presentation by DCs to T cells and amplifying the vaccine-directed T cell immune responses (for review, see [4]).
In the current study, we hypothesized that DNA employing SCT strategy in conjunction with a strategy to prolong the life of DCs would further enhance DNA vaccine potency. Our rationale is based on the fact that both strategies modify the properties of DCs through two completely different mechanisms, which generate the opportunity to combine these two vaccines. We found that intradermal vaccination of mice with a DNA vaccine encoding SCT-E6 with Bcl-xL DNA resulted in significantly better E6-specific T cell-mediated immune responses compared to vaccination with SCT-E6 DNA with non-functional mutant Bcl-xL (mtBcl-xL) DNA. Furthermore, the observed enhanced E6-specific CD8+ T cell-mediated immune responses translated into more potent anti-tumor effects against a luciferase-expressing E6-positive tumor (TC-1/Luciferase). We also observed that the co-administration of SCT-E6 DNA with Bcl-xL DNA led to significantly better survival in mice challenged with TC-1/Luciferase tumor cells compared to co-administration of SCT-E6 DNA with mtBcl-xL DNA. Our findings thus suggest that the effectiveness of a DNA vaccine encoding a SCT specific for the HPV-16 E6 antigen can be further enhanced by combining the vaccine with an anti-apoptotic modality to prolong the lifespan of DCs. The clinical implications of this study are discussed.
Materials and Methods
Plasmid DNA constructs and DNA preparation
The plasmids encoding the single-chain trimer DNA vaccines (pIRES-E6-β2m-Kb and pIRES-OVA-β2m-Kb) have been described previously,[7, 8] as have the plasmids encoding the anti-apoptotic proteins (pSG5-Bcl-xL and pSG5-mtBcl-xL) [9-11]. Figure 1 depicts the single-chain trimer constructs used in this study. DNA plasmids were amplified in Escherichia coli DH5α and purified as described previously using an endotoxin-free plasmid purification kit (Qiagen, Valencia, CA).
Cells
The HPV-16 E6-expressing murine tumor model TC-1 has been described previously [12]. Briefly, TC-1 cells were generated by cotransformation of primary C57BL/6 mice lung epithelial cells with HPV-16 E6, E7, and an activated ras oncogene. TC-1/Luciferase was generated by transduction of TC-1 cells with viral particles packaged (in Phoenix retroviral producer cells) with a Luciferase/Thy1.1 expression construct on the pMSCVpuro backbone (a kind gift from Hyam Levitsky). Following transduction and subsequent culture, puromycin-resistant cells were stained with Thy1.1-specific phycoerythrin-conjugated monoclonal antibodies and sorted by flow cytometry to isolate high Thy1.1-expressing cells. TC-1/Luciferase cells were cultured in RPMI 1640 containing 10% fetal bovine serum, and 0.4mg/ml G418.
Mice
Female C57BL/6 mice (6-8 weeks old) were purchased from the National Cancer Institute (Frederick, MD) and kept in the oncology animal facility of the Johns Hopkins Medical Institute (Baltimore, MD). All animal procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals.
DNA vaccination
DNA-coated gold particles were prepared according to a previously described protocol.[13] DNA-coated gold particles were delivered to the shaved abdominal region of C57BL/6 mice (5 per group) using a helium-driven gene gun (BioRad, Hercules, CA) with a discharge pressure of 400 psi. Mice were immunized with 1μg of pIRES-E6-β2m-Kb or pIRES-OVA-β2m-Kb in combination with 1μg of pSG5-Bcl-xL or pSG5-mBcl-xL for a final 2 μg/bullet. Three days after the first vaccination, the mice received two subsequent boost vaccinations with the same dose at 3-day intervals.
Intracellular cytokine staining and flow cytometry analysis
Pooled splenocytes from the vaccinated mice were harvested one week after the last vaccination. Cell surface CD8 marker staining, intracellular IFN-γ staining and flow cytometric analysis were performed as previously described [13]. Briefly, five million pooled splenocytes from each vaccination group were incubated with 1μg/ml of E6 aa49-57 or OVA aa257-264 peptide in the presence of 1μg/ml of GolgiPlug (BD Pharmingen) at 37°C overnight. Cells were then washed with FACScan buffer and stained with phycoerythrin-conjugated monoclonal rat anti-mouse CD8 antibody, followed by intracellular cytokine staining with FITC-conjugated IFN-γ antibodies using the Cytofix/Cytoperm kit according to the manufacturer's instructions (BD Pharmingen, San Diego, CA). Analysis was performed on a Becton-Dickinson FACScan with CELLQuest software (Becton Dickinson Immunocytometry System, Mountain View, CA).
Bioluminescent cytotoxicity assay
Splenocytes were harvested from mice of each vaccination group one week after the last vaccination. Five million pooled splenocytes from each group were incubated with 1μg/ml of E6 aa49-57 or OVA aa257-264 peptide and 20 units/ml IL-2 for five days, then added in several effector:target ratios to TC-1/Luciferase target cells previously plated in 96-well plates. Following 8 hours of incubation, media was drawn from the wells, briefly centrifuged to remove cells and cell debris, and quantitatively assayed in quadruplicate for luciferase activity by addition of Steady-Glo reagent (Promega, Madison, WI) in light-resistant black plastic 96-well plates. Visualization and signal quantitation of luciferase activity was performed using the Xenogen IVIS system and Living Image software package (Xenogen Corp., Alameda, CA).
In vivo tumor treatment experiment
Mice (five per group) were challenged with 1×104 TC-1/Luciferase tumor cells / mouse by tail vein injection to simulate hematogenous spread of tumors. DNA vaccine treatments (1μg / mouse) were co-administered with plasmids encoding functional or non-functional Bcl-xL (1μg / mouse) 3 days after tumor challenge, followed by two boosters of the same dose at 4-day intervals. For two months following vaccine treatment, metastatic tumor growth in the lungs was periodically visualized under general anesthesia using the Xenogen IVIS system and quantitated following bioluminescent detection using Living Image software package (Xenogen Corp., Alameda, CA). Mice were observed regularly for signs of morbidity (cachexia, failure to groom, decreased social interaction with cage-mates), and euthanized when overtly moribund.
Statistical Analysis
All data expressed as means ± standard deviation (SD) are representative of at least two different experiments. The statistical significance of group differences were made using a Student's t-test to generate p-values. Differences in survival between experimental groups were analyzed using the Kaplan-Meier approach.
Results
Co-administration of SCT-E6 DNA with Bcl-xL DNA significantly increases the number of E6-specific CD8+ T cells in vaccinated mice
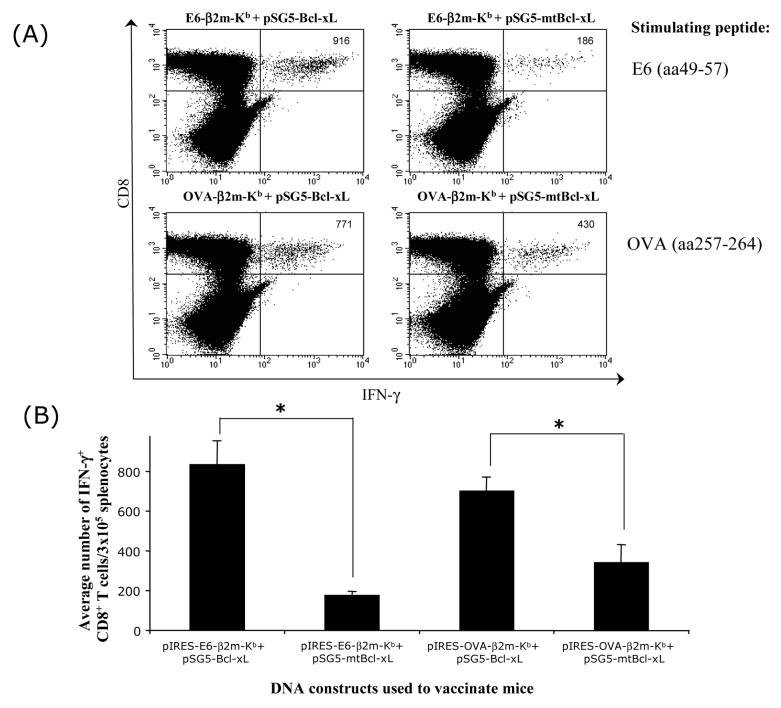
We have previously demonstrated that mice vaccinated with the DNA construct encoding SCT-E6 (pIRES-E6-β2m-Kb) were capable of generating appreciable numbers of E6-specific CD8+ T cell immune responses.[8] In order to evaluate if the employment of an anti-apoptotic strategy will further enhance the ability of the SCT-E6 DNA vaccine to generate E6-specific CD8+ T-cell immune responses, we vaccinated C57BL/6 mice intradermally with pIRES-E6-β2m-Kb or pIRES-OVA-β2m-Kb SCT DNA (illustrated in Figure 1) in combination with pSG5-Bcl-xL or pSG5-mtBcl-xL DNA which encode the antiapoptotic protein, Bcl-xL and its non-functional mutated form, mtBcl-xL respectively [9]. As shown in Figure 2, co-administration of pIRES-E6-β2m-Kb with pSG5-Bcl-xL, resulted in significantly higher numbers of E6-specific CD8+ IFN-γ+ T cells as compared to co-administration of pIRES-E6-β2m-Kb and pSG5-mtBcl-xL. No OVA-specific CD8+ T cell immune response was observed in mice vaccinated with pIRES-E6-β2m-Kb co-administered with pSG5-Bcl-xL or pSG5-mtBcl-xL (data not shown). In contrast, co-administration of pIRES-OVA-β2m-Kb with pSG5-Bcl-xL, resulted in significantly higher numbers of OVA-specific CD8+ IFN-γ+ T cells, as compared to co-administration of pIRES-OVA-β2m-Kb with pSG5-mtBcl-xL. No E6-specific CD8+ immune response was observed in mice vaccinated with pIRES-OVA-β2m-Kb co-administered with pSG5-Bcl-xL or pSG5-mtBcl-xL (data not shown). Thus, our data suggest that the potency of SCT DNA vaccines administered by gene gun in generating antigen-specific CD8+ T cell immune responses is greatly enhanced by employing Bcl-xL DNA.
Figure 2. Flow cytometry analysis of the E6- or OVA-specific CD8+ T cell responses in mice vaccinated with combinations of SCT DNA and Bcl-xL or mtBcl-xL DNA vaccines.
C57BL/6 mice were vaccinated intradermally via gene gun with pIRES-E6-β2m-Kb or pIRES-OVA-β2m-Kb combined with pSG5-Bcl-xL or pSG5-mtBcl-xL. Splenocytes obtained from vaccinated mice were cultured with E6 peptide (aa49-57) or OVA peptide (aa257-264) overnight, followed by analysis for CD8 and intracellular IFN-γ staining by flow cytometry. A) Representative flow cytometry data showing the number of IFN-γ-producing E6- or OVA-specific CD8+ T cells in vaccinated mice. Data shown are representative of two separate analyses. B) Bar graph depicting the average number of IFN-γ+ CD8+ T cells from each group of vaccinated mice (*p<0.05).
Splenocytes from mice vaccinated with SCT-E6 DNA and Bcl-xL DNA generate significantly increased cytotoxic activity against TC-1/Luciferase target cells
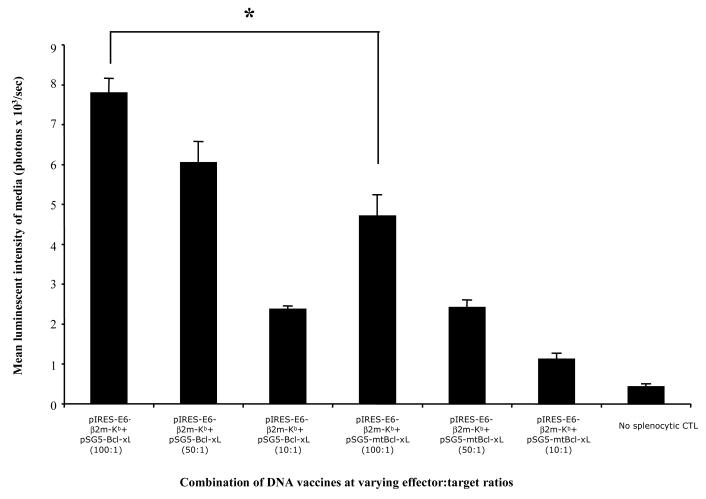
To further evaluate the cytolytic activities of splenocytes derived from mice vaccinated with the combinations of DNA vaccines against E6-expressing tumor cells, we performed a cytoxicity assay using splenocytes from vaccinated mice as described in the Materials and Methods. Since the cell culture media did not contain any significant intrinsic luciferase enzymatic activity, the only source of luciferase enzyme in the assay originated from lysed TC-1/Luciferase target cells. Thus, measures of bioluminescent activity of the supernatant serve as a direct indicator of target cell lysis by effector cells. As shown in Figure 3, splenocytes isolated from mice vaccinated with pIRES-E6-2m-Kb DNA in combination with pSG5-Bcl-xL demonstrated significantly more cytolytic activity against TC-1/Luciferase target cells compared to splenocytes isolated from mice vaccinated with pIRES-E6-β2m-Kb DNA in combination with pSG5-mtBcl-xL (*p<0.001). Thus, our data suggests that the greater numbers of E6-specific CD8+ T cells generated by mice vaccinated with SCT-E6 DNA and Bcl-xL DNA lead to increased cytolytic activity in vitro against E6-expressing tumor cells.
Figure 3. CTL assay with luciferase-expressing tumor cells using T cells from the various vaccinated mice groups.
Splenocytes from immunized mice were harvested as previously described in Figure 2 and stimulated with E6 peptide (aa49-57) for 120hr, then added in several effector:target ratios to TC-1/Luciferase target cells previously plated in 96-well plates. Following 8hr incubation, media was drawn from the wells, briefly centrifuged, and quantitatively assayed for luciferase activity using the Steady-Glo reagent (Promega, Madison, WI). Visualization and quantitation was performed using Xenogen IVIS system and Living Image software package (Xenogen Corp., Alameda, CA) as a readout for target cell lysis (*p<0.001).
Co-administration of SCT-E6 DNA with Bcl-xL DNA generates potent anti-tumor effects against E6-expressing TC-1/Luciferase tumors in C57BL/6 mice
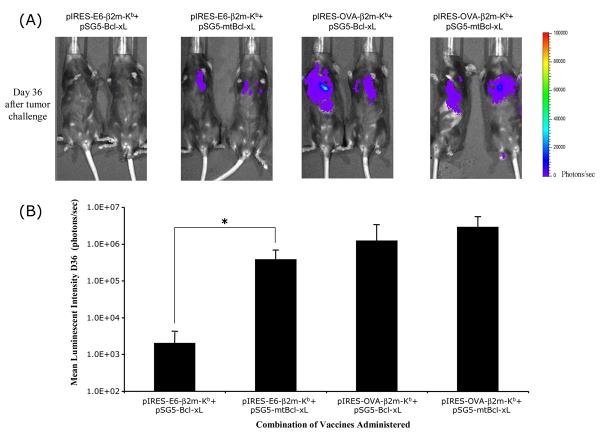
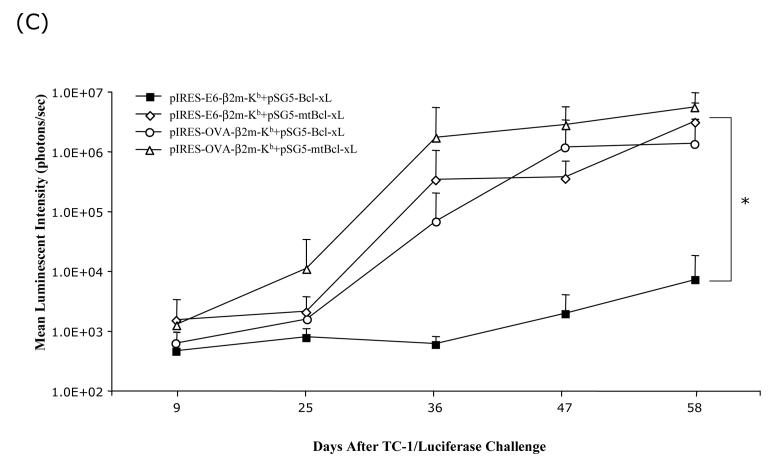
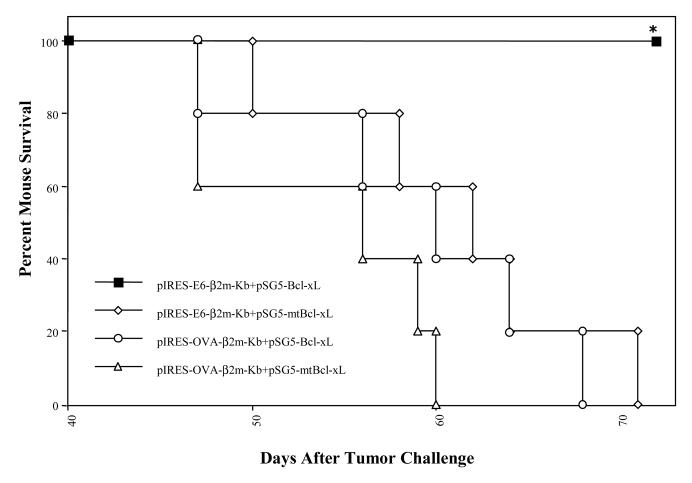
To evaluate if the co-administration of SCT-E6 DNA with Bcl-xL DNA can lead to better anti-tumor effects, we performed in vivo tumor treatment experiments. C57BL/6 mice were challenged with 1×104 TC-1/Luciferase tumor cells/mouse by tail vein injection. Intravenous administration of TC-1 tumor cells has been shown to lead to lung tumor nodules [14]. The presence of TC-1/Luciferase tumor nodules in tumor-challenged mice was monitored using non-invasive bioluminescence imaging systems. In general, the luminescence intensity correlated with tumor load, as we have previously shown [15]. Tumor-challenged mice were treated with the pIRES-E6-β2m-Kb or pIRES-OVA-β2m-Kb DNA co-administered with pSG5-Bcl-xL or pSG5-mtBcl-xL DNA via gene gun 3 days after tumor challenge. The presence of lung tumor nodules was monitored on a regular basis by Xenogen imaging for a period of two months. In Figure 4A, we see representative pictures from luminescent imaging of tumor-challenged mice treated with the various DNA constructs on day 36 after tumor challenge. We observed significant inhibition of tumor growth in mice treated with pIRES-E6-B2m-Kb co-administered with pSG5-Bcl-xL DNA compared to mice treated with pIRES-E6-B2m-Kb co-administered with pSG5-mtBcl-xL DNA. In contrast, tumor-challenged mice treated with pIRES-OVA-B2m-Kb co-administered with pSG5-Bcl-xL DNA or pSG5-mtBcl-xL DNA failed to control tumor growth. Quantification of the luciferase activity in tumor-challenged mice is shown in Figure 4B. We also characterized the kinetic changes of tumor growth in tumor-challenged mice following treatment with the various DNA vaccines over a period of two months. As shown in Figure 4C, co-administration of SCT-E6 DNA in conjunction with Bcl-xL DNA greatly improved the potency of the SCT-E6 DNA vaccine in treating established tumors over time. The inhibition of tumor growth in tumor-challenged mice treated with the DNA vaccines also translated into better survival in tumor-challenged mice. In Figure 5, we see that all of the mice treated with the pIRES-E6-β2m-Kb co-administered with pSG5-Bcl-xL DNA survived two months after tumor challenge, compared to mice of the other treatment groups, which exhibited significant mortality over the same time period. Taken together, our results suggest that tumor-challenged mice treated by co-administration of pIRES-E6-β2m-Kb with pSG5-Bcl-xL DNAs leads to significantly improved anti-tumor effects and long-term survival in treated mice.
Figure 4. In vivo tumor treatment experiment in challenged mice vaccinated with combinations of the SCT and Bcl-xL DNA vaccines.
C57BL/6 mice were intravenously challenged with 1×104 TC-1/Luciferase cells/mouse by tail-vein injection. Three days after tumor challenge, mice were treated with combinations of SCT-E6 or OVA DNA with Bcl-xL or mtBcl-xL DNA vaccines three times at 3-day intervals. Mice were imaged using the Xenogen IVIS Imaging System Series 200. Bioluminescence signals were acquired for one minute. (A) Images of representative tumor-challenged mice vaccinated with combinations of SCT and Bcl-xL DNA vaccines from day 36 after tumor challenge. (B) Bar graphs depicting the luminescent intensities of lung tumors in tumor-challenged mice on day 36 after tumor challenge (*p<0.05). (C) Line graph depicting the luminescent intensities of lung tumors in tumor-challenged mice, which represent tumor volume, over time.
Figure 5. Survival analysis of challenged mice vaccinated with combinations of the SCT and Bcl-xL DNA vaccines.
C57BL/6 mice were intravenously challenged with 1×104 TC-1/Luciferase cells/mouse by tail-vein injection. Three days after tumor challenge, mice were treated with combinations of SCT-E6 or OVA DNA with Bcl-xL or mtBcl-xL DNA vaccines three times at 3-day intervals. Kaplan-Meier survival analysis of tumor-challenged mice treated with the various DNA vaccine combinations (*p<0.05).
Discussion
In the current study, we found that intradermal vaccination of mice with a DNA construct encoding SCT-E6 with DNA encoding Bcl-xL vaccines resulted in a significantly better E6-specific T cell-mediated immune response compared to vaccination with SCT-E6 DNA with DNA encoding non-functional mutant Bcl-xL (mtBcl-xL). Furthermore, the observed enhanced E6-specific T cell-mediated immune responses translated into better anti-tumor effects against a luciferase-expressing E6-positive tumor (TC-1/Luciferase) as analyzed using a non-invasive bioluminescent monitoring system. We also observed that the co-administration of SCT-E6 DNA vaccine with DNA encoding Bcl-xL led to significantly better survival in mice challenged with TC-1/Luciferase tumor cells compared to co-administration of SCT-E6 DNA vaccine with DNA encoding mutant Bcl-xL. Our findings confirm our hypothesis that the potency of the DNA vaccine encoding SCT specific for HPV-16 E6 antigen can be further enhanced by combining the vaccine with an anti-apoptotic modality to prolong the lifespan of DCs.
One important concern for the employment of a DNA vaccine encoding an SCT encoding an antigenic peptide such as E6 (aa49-57) is that tumor cells may evade antigen-specific CD8+ T cell immune attack by the mutation of the HPV-16 E6 cytotoxic T-lymphocyte (CTL) epitope, so called “antigenic drift”[16]. This situation is more likely to occur if the location of the CTL epitope is not at a crucial functional domain of the E6 protein. If such a mutation occurs on the E6 CTL epitope in E6-expressing tumors, the DNA vaccines employing the SCT technology may be unable to control the tumor. One potential approach to avoid such a pitfall is to consider using combinations of different DNA vaccines employing the SCT strategy that target CTL epitopes of different HPV antigens. For example, it is now clear that HPV E7 oncoprotein also serves as an ideal target for therapeutic HPV vaccine development (for review, see [17]). The murine MHC class I (H2-Db)-restricted HPV-16 E7 CTL epitope has been identified to be located at aa49-57, which provides us an opportunity to generate a DNA vaccine encoding SCT targeting E7 CTL epitope. In doing so, a combination of SCT DNA vaccines targeting E6 and E7 CTL epitopes may circumvent the potential problem of “antigenic drift” of CTL epitopes. Furthermore, with continued identification of other HPV CTL epitopes, we may be able to develop a better regimen of HPV DNA vaccines to avoid the undesirable effects of mutations on the CTL epitope of the tumor antigens.
Although the SCT technology can be used to elicit robust peptide-specific CD8+ T cell immune responses, it is not effective in activating CD4+ T helper cells. It is now clear that CD4+ T cells play a crucial role in the generation of CD8+ T effector and memory T-cell populations (for review, see [18]). Thus, it will be desirable to design an immunization regimen that utilizes DNA vaccines employing the SCT technology in conjunction with DNA vaccines capable of activating CD4+ T helper cells. We have recently shown that the DNA vaccine employing either the SCT strategy or an intracellular targeting strategy in conjunction with a strategy to enhance antigen-specific CD4+ T cell immune responses were capable of improving antigen-specific CD8+ T cell immune responses in vaccinated mice [19]. Thus, vaccination with a DNA vaccine encoding a CD4+ helper T cell epitope together with a DNA vaccine encoding the SCT technology provide an opportunity to generate both CD8+ T cell and CD4+ helper T cell immune responses, enhancing the long-term protective immunity by developing a reservoir of antigen-specific memory T cells.
Our study has significant implications for clinical translation. For eventual clinical application, it is important to seek the best combination of multiple strategies that modify the properties of DCs through different mechanisms to generate the best vaccine potency. Indeed, immunization of mice with DNA vaccines employing the SCT strategy, a strategy to prolong the life of DCs, and a strategy to enhance the numbers of antigen-specific CD4+ T cells was able to generate the highest number of antigen-specific CD8+ T cells in vaccinated mice compared to immunization with DNA vaccines employing only two of these strategies to modify DCs (Huang, et al., unpublished observation). Thus, the combination of the various strategies to modify the properties of DCs via different but complementary mechanisms may result in the most potent DNA vaccine. Further explorations of the various combinations of strategies to modify the properties of DCs will facilitate future clinical translation of the DNA vaccines.
Acknowledgements
We would like to thank Archana Monie and Talia Hoory for help with preparation of the manuscript. This work was supported by 1 RO1 CA114425□01 and Cervical Cancer SPORE Program (P50 CA098252) of the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boyd D, Hung CF, Wu TC. DNA vaccines for cancer. IDrugs. 2003;6(12):1155–64. [PubMed] [Google Scholar]
- 2.Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927–74. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 3.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–48. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 4.Tsen SW, Paik AH, Hung CF, Wu TC. Enhancing DNA vaccine potency by modifying the properties of antigen-presenting cells. Expert Rev Vaccines. 2007;6(2):227–39. doi: 10.1586/14760584.6.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2(10):1122–8. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 6.Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J Exp Med. 1998;188(6):1075–82. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu YY, Netuschil N, Lybarger L, Connolly JM, Hansen TH. Cutting edge: single-chain trimers of MHC class I molecules form stable structures that potently stimulate antigen-specific T cells and B cells. J Immunol. 2002;168(7):3145–9. doi: 10.4049/jimmunol.168.7.3145. [DOI] [PubMed] [Google Scholar]
- 8.Huang CH, Peng S, He L, Tsai YC, Boyd DA, Hansen TH, et al. Cancer immunotherapy using a DNA vaccine encoding a single-chain trimer of MHC class I linked to an HPV-16 E6 immunodominant CTL epitope. Gene Ther. 2005;12(15):1180–6. doi: 10.1038/sj.gt.3302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim TW, Hung CF, Ling M, Juang J, He L, Hardwick JM, et al. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. The Journal of clinical investigation. 2003;112(1):109–17. doi: 10.1172/JCI17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng EH, Levine B, Boise LH, Thompson CB, Hardwick JM. Bax-independent inhibition of apoptosis by Bcl-XL. Nature. 1996;379(6565):554–6. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 11.Kim TW, Hung CF, Boyd D, Juang J, He L, Kim JW, et al. Enhancing DNA vaccine potency by combining a strategy to prolong dendritic cell life with intracellular targeting strategies. J Immunol. 2003;171(6):2970–6. doi: 10.4049/jimmunol.171.6.2970. [DOI] [PubMed] [Google Scholar]
- 12.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer research. 1996;56(1):21–6. [PubMed] [Google Scholar]
- 13.Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer research. 2000;60(4):1035–42. [PubMed] [Google Scholar]
- 14.Ji H, Chang EY, Lin KY, Kurman RJ, Pardoll DM, Wu TC. Antigen-specific immunotherapy for murine lung metastatic tumors expressing human papillomavirus type 16 E7 oncoprotein. Int J Cancer. 1998;78(1):41–5. doi: 10.1002/(sici)1097-0215(19980925)78:1<41::aid-ijc8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Hung CF, Tsai YC, He L, Coukos G, Fodor I, Qin L, et al. Vaccinia virus preferentially infects and controls human and murine ovarian tumors in mice. Gene Ther. 2007;14(1):20–9. doi: 10.1038/sj.gt.3302840. [DOI] [PubMed] [Google Scholar]
- 16.Bai XF, Liu J, Li O, Zheng P, Liu Y. Antigenic drift as a mechanism for tumor evasion of destruction by cytolytic T lymphocytes. The Journal of clinical investigation. 2003;111(10):1487–96. doi: 10.1172/JCI17656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roden RB, Ling M, Wu TC. Vaccination to prevent and treat cervical cancer. Hum Pathol. 2004;35(8):971–82. doi: 10.1016/j.humpath.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–40. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 19.Hung CF, Tsai YC, He L, Wu TC. DNA Vaccines Encoding Ii-PADRE Generates Potent PADRE-specific CD4(+) T-Cell Immune Responses and Enhances Vaccine Potency. Mol Ther. 2007;15(6):1211–9. doi: 10.1038/sj.mt.6300121. [DOI] [PMC free article] [PubMed] [Google Scholar]