Abstract
We analyzed pooled data from two comparable randomized placebo-controlled clinical trials of bupropion pharmacotherapy for smoking cessation for which data on DRD2 Taq1A genotype were available. A total of 722 smokers across the two trials were randomized to 10 weeks of sustained-release bupropion hydrochloride or placebo. General estimating equation analysis demonstrated a significant gene × drug interaction (B=0.87, SE=0.34, p=.009). Smokers with the A2/A2 genotype using bupropion were more than three times as likely, relative to placebo, to be abstinent at end of treatment (35.2% vs. 15.1%; OR=3.25, 95% CI 2.00–5.28) and at 6 months of follow-up (26.7% vs. 12.2%; OR=2.81, 95% CI 1.66–4.77), which was attenuated by 12 months (16.3% vs. 10.7%; OR=1.70, 95% CI 0.95–3.05). We found no significant benefit of bupropion relative to placebo on smoking cessation outcomes at any time point in participants with A1/A1 or A1/A2 genotypes. These data suggest that bupropion may be effective for smoking cessation only in a subgroup of smokers with the DRD2 Taq1 A2/A2 genotype.
Introduction
Despite major recent public health gains, fewer than 10% of smokers who attempt to quit remain abstinent after years of follow-up (Yudkin et al., 2003), and among persistent smokers, approximately half, or 5 million annually worldwide, die prematurely (Peto et al., 1996). Bupropion, nicotine replacement therapy (NRT), and varenicline are the three first-line, FDA-approved, pharmacological interventions for smoking cessation (Foulds, 2006; Schnoll & Lerman, 2006). Despite the efficacy of these interventions, 70%–80% of patients relapse within 12 months of attempting to quit smoking (Hurt et al., 1997; Jorenby et al., 1999). Therefore, attention has focused increasingly on the prospect of tailored medicine as a means of identifying subgroups of smokers most likely to respond to specific pharmacotherapies, thereby increasing smoking cessation efficacy (Munafò, Shields, Berrettini, Patterson, & Lerman, 2005).
Approximately 50% of the variance in risk for nicotine dependence is attributable to genetic factors (Li, Cheng, Ma, & Swan, 2003). Bupropion has demonstrated dopamine and norepinephrine reuptake and nicotinic acetylcholine receptor antagonist properties (Ascher et al., 1995; Fryer & Lukas, 1999). Given the well-established role of the mesocorticolimbic dopamine reward system in nicotine dependence (Balfour, 2002; Laviolette & van der Kooy, 2004), genes influencing dopamine and bupropion pharmacodynamics and pharmacokinetics are biologically plausible candidates for studying pharmacogenetic response for smoking cessation (Lerman et al., 2006; Lerman & Niaura, 2002).
Of the likely hundreds of candidate genes believed to influence nicotine dependence (Sullivan et al., 2004), the most widely studied genetic variant in pharmacogenetic smoking cessation studies is the DRD2 Taq1A polymorphism, which was recently found to be present in a newly discovered tyrosine kinase gene (ANKK1) located 3′ to the DRD2 gene (Neville, Johnstone, & Walton, 2004). The Taq1A variant may alter the function of the nearby DRD2 gene and has been reported to affect dopamine receptor D2 availability in postmortem striatal samples (Blum et al., 1990). Evidence from in vivo studies indicates an association between the A1 allele and lower mean relative glucose metabolic rate in dopaminergic regions in the human brain (Noble, Gottschalk, Fallon, Ritchie, & Wu, 1997) and low striatal receptor density (Jonsson et al., 1999).
A moderating effect of Taq1A genotype on treatment response has been observed in only one of three published pharmacogenetic studies (David et al., 2007), despite qualitative observations in the other two studies of higher abstinence rates among those using bupropion possessing the DRD2 Taq1 A2/A2 genotype compared with A1/A1 or A1/A2 genotypes (Lerman et al., 2003; Swan et al., 2003).
To establish whether the DRD2 Taq1A polymorphism moderates treatment response to bupropion for smoking cessation, we conducted a pooled analysis of data from two comparable pharmacogenetic clinical trials (David et al., in press; Lerman et al., 2003). This analysis provided greater statistical power and allowed a more accurate estimate of the effect of DRD2 Taq1A genotype on response to bupropion. The other published pharmacogenetic investigation of DRD2 Taq1A genotype and bupropion efficacy (Swan et al., 2005) was an open-label clinical effectiveness study lacking a placebo-control group. This study could not be in our pooled analyses given our aim of analyzing the relative efficacy (compared with placebo) of bupropion according the DRD2 Taq1A genotype.
Method
Procedure
Recruitment methods and study outcomes for both studies have been described elsewhere (David et al., 2007; Lerman et al., 2003). Participants were adult smokers (aged 18–65 years), without medical contra-indications to bupropion. Participants were screened for DSM-IV disorders (American Psychiatric Association, 1994) using the Structured Clinical Interview for DSM (SCID nonpatient version; First, Spitzer, Gibbon, & Williams, 1989) and excluded for Axis I disorders, with the exception of nicotine dependence.
Both studies were double-blind, randomized, placebo-controlled clinical trials of sustained-release bupropion hydrochloride for smoking cessation, with comparable study protocols, conducted at two sites: Washington, DC, and Buffalo, NY (Study 1: n=555), and Brown University (Study 2: n=524). All participants received 10 weeks of either placebo or bupropion (150 mg/day for the first 3 days, then 300 mg/day) plus standardized behavioral group counseling. Target quit dates were set for 1 week following initiation of drug or placebo. Only participants of European ancestry were included in the pooled analysis (n=722) to minimize population stratification. In Study 1, Lerman and colleagues did not observe any significant DRD2 × bupropion interactions on smoking outcomes. In a subset of smokers who were enrolled in a randomized, double-blind, placebo-controlled trial of bupropion (291 of 524 enrolled in trial [56%]; Study 2; Brown et al., 2007), David and colleagues evaluated the moderating effects of three candidate gene variants (dopamine transporter SLC6A3 3′ variable number of tandem repeats, cytochrome P450 2B6 CPY2B6 C1459T, and DRD2 Taq1A polymorphisms) and observed a significant DRD2 × bupropion interaction. Smokers possessing the A2/A2 genotype were more than three times as likely to be abstinent at 6 months of follow-up compared with placebo, but there was virtually no difference in bupropion and placebo efficacy for those with A1/A1 or A1/A2 genotypes (David et al., 2007). However, statistical significance was demonstrated only at the 6-month follow-up.
DNA extraction and polymerase chain reaction techniques and assays for the DRD2 Taq1A polymorphism have been described previously (Lerman et al., 1999), validated by confirming a polymorphic inheritance in seven human family lines. The DRD2 Taq1A genotype was classified as the presence or absence of the A1 allele (A1/A1 or A2/A1 vs. A2/A2).
Measures
Demographics and nicotine dependence
Self-reported sex, ancestry, and age were assessed at a baseline interview. Nicotine dependence severity was assessed with the Fagerström Test for Nicotine Dependence (validated, six-item self-reported measure of nicotine dependence; Heatherton, Kozlowski, Frecker, & Fagerström, 1991).
Smoking cessation
Point-prevalence abstinence, defined as 7-day biochemically verified abstinence, validated using salivary cotinine levels (≤15 ng/ml) by a gas–liquid chromatography method (Feyerabend & Russell, 1990), was assessed at 10 weeks (end of treatment at 9 weeks post target quit date; EOT) and at 6-month and 12-month follow-ups (Society for Research on Nicotine and Tobacco, 2002).
Data analyses
We used generalized estimating equations (GEEs; Zeger & Liang, 1986) to analyze biochemically verified 7-day point-prevalence abstinence at EOT, 6 months, and 12 months (Society for Research on Nicotine and Tobacco, 2002), including sex and FTND scores as covariates, and the linear effect of time, prior to entering main effects. All tests of interactions included lower-order interactions and were conducted in R (www.r-project.org/) using GEEPACK (Halekoh, Hojsgaard, & Yan, 2006) with the Logit link function and an unstructured correlation matrix specified.
Based on a genotype relative risk (Cardon, Idury, Harris, Witte, & Elston, 2000) of 3, as observed for the DRD2 Taq1A polymorphism (David et al., in press; Lerman et al., 2003), for 2 df tests of genotypes, we calculated that a sample size of approximately 400 for one diallelic marker was necessary to achieve 80% power at an alpha of .05.
Results
Characteristics of participants
The pooled study sample consisted of 722 participants of European ancestry (53% female), comprising 431 participants from Study 1 and 291 participants from Study 2. Baseline characteristics (sex, age, ancestry, treatment allocation, cigarettes/day, FTND score, and loss to follow-up) were similar across studies (Table 1), although participants in Study 2 reported somewhat higher FTND scores (6 vs. 5, p<.05) and smoked more cigarettes per day (25 vs. 22, p<.05). A total of 369 participants received bupropion and 353 received placebo. The mean age of participants was 45 years (SD=12), and mean cigarette consumption was 23 cigarettes/day (SD=10).
Table 1.
Characteristics of study participants.
| Variable | Study 1: Georgetown/Buffalo (N=431) | Study 2: Brown University (N=291) | p value for differencea |
|---|---|---|---|
| Treatment condition | |||
| Bupropion | 233 (54.0%) | 136 (46.7%) | .55 |
| Placebo | 198 (46.0%) | 155 (53.3%) | |
| Age (years) | 44.6 (SD=11.4) | 45.5 (SD=10.7) | .34 |
| Sex (female) | 234 (54.3%) | 148 (50.9%) | .12 |
| Cigarettes/dayb | 21.6 (SD=9.3) | 24.6 (SD=9.9) | <.001 |
| Fagerström Test for Nicotine Dependence scoreb | 5.1 (SD=2.1) | 6.3 (SD=1.9) | <.001 |
| DRD2 Taq1A genotype | |||
| A1/A1 | 28 (6.5%) | 22 (7.5%) | .54 |
| A1/A2 | 155 (36.0%) | 107 (36.8%) | |
| A2/A2 | 248 (57.5%) | 162 (55.7%) | |
Note. Values are numbers of subjects with percentages or means with standard deviations.
Chi-square tests for dichotomous variables, Student t tests for continuous variables, and one-way analysis of variance for the categorical variable (DRD2 Taq1A genotype).
Data missing for 4 participants (Study 1) and 1 participant (Study 2).
Data missing for 5 participants (Study 1) and 2 participants (Study 2).
Genotype frequencies (A1/A1: n=50, 6.9%; A1/A2: n=262, 36.3%, or A2/A2: n=410, 56.8%) did not deviate significant from Hardy-Weinberg equilibrium in either the individual or pooled samples (p values>.46). Genotype was not associated with any socio-demographic variable, treatment group assignment, or nicotine dependence severity (p values >.22).
Genotype and smoking cessation
Given significant associations with rates of abstinence, we included sex (B=−0.44, SE=0.16, p<.008), cigarettes/day (B=−0.02, SE=0.01, p<.05), and study site (B=0.26, SE=0.10, p<.02) as covariates in all analyses examining the associations between main effects and continuous abstinence at the three follow-up assessments. Participant characteristics (including abstinence rates at EOT by genotypic stratum) are presented in Table 2.
Table 2.
Characteristics and outcomes by genotype.
| DRD2 Taq1 genotype | A1/A1 (n=50) | A1/A2 (n=262) | A2/A2 (n=410) | All participants (n=722) |
|---|---|---|---|---|
| Age (years) | 48.2 (SD=10.6) | 44.8 (SD=11.2) | 44.7 (SD=11.1) | 45.0 (11.2) |
| Sex (male) | 25 (50.0%) | 130 (49.6%) | 185 (45.1%) | 340 (47.1%) |
| Cigarettes/daya | 25.1 (SD=11.5) | 22.8 (SD=9.2) | 22.6 (SD=9.7) | 22.9 (SD=9.7) |
| Fagerström Test for Nicotine | 6.1 (SD=1.9) | 5.4 (SD=2.1) | 5.7 (SD=2.1) | 5.6 (SD=2.1) |
| Dependence scoreb | ||||
| Abstinence (end of treatment) | 12 (24.0%) | 79 (30.2%) | 102 (24.9%) | 193 (26.7%) |
| Abstinence (6 months) | 10 (20.0%) | 54 (20.6%) | 79 (19.3%) | 143 (19.8%) |
| Abstinence (12 months) | 7 (14.0%) | 35 (13.4%) | 55 (13.4%) | 97 (13.4%) |
Note. Values are means with standard deviations, except for sex and abstinence (number of subjects and percentage).
Data missing for 5 participants.
Data missing for 7 participants.
The test of main effects suggested higher rates of abstinence among individuals who received bupropion (B=0.74, SE=0.17, p<.001) compared with placebo. Although we did not find a main effect of DRD2 genotype on abstinence (B=−0.12, SE=0.17, p>.40), we did find a significant two-way interaction between DRD2 genotype and bupropion (B=0.87, SE=0.34, p=.009). Moreover, the three-way interaction between DRD2 genotype, bupropion, and the linear effect of time in the same model, with all lower-order interactions, was not significant (p>.24), suggesting that the rate of relapse over time was not different across DRD2 genotype groups in the bupropion and placebo conditions.
The adjusted odds ratios for cessation were for A2/A2: OR=3.25 (95% CI 2.00–5.28) at EOT, OR=2.81 (95% CI 1.66–4.77) at 6 months, and OR=1.70 (95% CI 0.95–3.05) at 12 months, and for A1/A1 or A1/A2: OR=1.15 (95% CI 0.58–2.29) at EOT, OR=1.19 (95% CI 0.68–2.08) at 6 months, and OR=1.09 (95% CI 0.57–2.11) at 12 months (abstinence rates demonstrated in Figure 1). Abstinence rates for each study site individually and combined stratified by treatment group and DRD2 genotype are indicated in Table 3.
Figure 1.
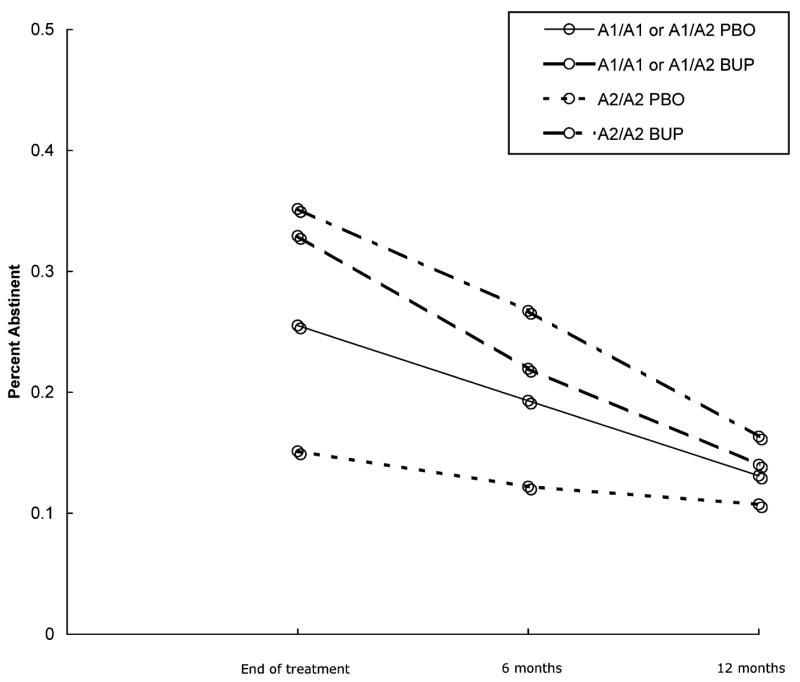
Smoking cessation outcomes by treatment condition and DRD2 Taq1A genotype. Rate of biochemically verified 7-day point-prevalence abstinence at end of treatment and at 6- and 12-month follow-up assessments grouped by DRD2 Taq1 genotype and treatment condition (A1/A1 or A1/A2 on placebo: solid; A1/A1 or A1/A2 on bupropion: dashed; A2/A2 on placebo (PBO): dotted; A2/A2 on bupropion (BUP): dotted and dashed).
Table 3.
Smoking cessation outcomes by treatment condition and DRD2 Taq1A genotype.
| Percent abstinent (number of subjects/sample)
|
|||||
|---|---|---|---|---|---|
| Treatment condition | DRD2 Taq1A genotype | Assessment of abstinence | Study 1 (N=431)* | Study 2 (N=291) | Pooled data (N=722)* |
| Bupropion | A1/A1 or A1/A2 | End of treatment | 25.7 (26/101) | 44.4 (28/63) | 32.9 (54/164) |
| Placebo | 25.3 (20/79) | 25.8 (17/66) | 25.5 (37/145) | ||
| Bupropion | A2/A2 | 29.5 (38/129) | 45.2 (33/73) | 35.2 (71/202) | |
| Placebo | 12.1 (14/116) | 19.1 (17/89) | 15.1 (31/205) | ||
| Bupropion | A1/A1 or A1/A2 | 6 months | 20.8 (21/101) | 23.8 (15/63) | 22.0 (36/164) |
| Placebo | 12.7 (10/79) | 27.3 (18/66) | 19.3 (28/145) | ||
| Bupropion | A2/A2 | 20.9 (27/129) | 37.0 (27/73) | 26.7 (54/202) | |
| Placebo | 10.3 (12/116) | 14.6 (13/89) | 12.2 (25/205) | ||
| Bupropion | A1/A1 or A1/A2 | 12 months | 11.9 (12/101) | 17.5 (11/63) | 14.0 (23/164) |
| Placebo | 8.9 (7/79) | 18.2 (12/66) | 13.1 (19/145) | ||
| Bupropion | A2/A2 | 14.7 (19/129) | 19.2 (14/73) | 16.3 (33/202) | |
| Placebo | 8.6 (10/116) | 13.5 (12/89) | 10.7 (22/205) | ||
Note. Frequencies for participants at both study sites who reported continuous abstinence with biochemically verified 7-day point prevalence, grouped by treatment condition using intention-to-treat criteria and DRD2 Taq1A genotype.
Smoking outcomes data missing on 6 individuals in Study 1 (3 each for the A1/A1 or A1/A2 group, and for the A2/A2 group).
Discussion
The results of our pooled analysis of the combined data from two clinical trials suggest that DRD2 Taq1A genotype influences response to bupropion for smoking cessation. The observation that the DRD2 × bupropion interaction was significant, independent of the linear effect of time, is novel, and suggests that the lack of statistical significance of this interaction in previous pharmacogenetic analyses was the result of insufficient statistical power to detect the interaction. The sample size supported testing one gene × treatment interaction, prompting selection of the only polymorphism for which replication data were available, to minimize alpha inflation (Wacholder, Chanock, Garcia-Closas, El Ghormli, & Rothman, 2004). Although viable candidate genes are found throughout the pathway involved in dopamine synthesis, reuptake, receptor function, and degradation, all of which need to be thoroughly evaluated in studies of sufficient sample size to examine multiple genetic loci, we focused our examination on the DRD2 Taq1A polymorphism. However, to have sufficient statistical power to conduct a pharmacogenetic analyses of multiple loci, clinical trials of much larger magnitude (5,000 subjects or more) should be performed (Cardon et al., 2000).
We had not anticipated observing associations between DRD2 Taq1A genotype and FTND score or cigarettes per day, two phenotypes associated with genetic variation in cytochrome alpha 4 nicotine acetylcholine receptor (Feng et al., 2004; Li et al., 2005) and p450 2A6 (Malaiyandi et al., 2006; O’Loughlin et al., 2004; Schoedel, Hoffmann, Rao, Sellers, & Tyndale, 2004) genes, respectively. Given the lack of consistency in the literature with regard to smoking-related trait phenotypes (Munafò, Clark, Johnstone, Murphy, & Walton, 2004), the Taq1A polymorphism has emerged as a more reliable pharmacogenetic endophenotype candidate gene, given apparent moderating effects on bupropion and NRT. A recent meta-analysis (Munafò et al., 2004) that did not find evidence of association between the Taq1A polymorphism and smoking cessation defined cessation as a comparison of current smokers and ex-smokers identified in case–control studies, rather than cessation within a cohort of treatment-seeking smokers over time. These phenotypes may reflect differing underlying processes and certainly are not informative with respect to pharmacogenetic gene × treatment interactions.
The DRD2 A2/A2 genotype has been associated with increased receptor density (Jonsson et al., 1999). Recent evidence that the Taq1A variant alters an amino acid in the ANKK1 protein kinase gene, near the DRD2 locus (Neville et al., 2004), does not rule out an effect on the DRD2 gene. Data from the HapMap project reveal that the variant is in linkage disequilibrium with other variants in the DRD2 gene, but not with variants in the ANKK1 gene. Thus additional functional variants affecting DRD2 expression may be contributing to the observed association with response to bupropion, as was demonstrated for the DRD2 -141Cins/del polymorphism, a functional variant associated with increased transcriptional efficiency of the DRD2 gene, in a separate published report of Study 1 (Lerman et al., 2006).
Another, more speculative possibility is that ANKK1 may exert an effect on dopaminergic neurotransmission itself. The function of many proteins can be influenced or regulated by a process of phosphorylation of key amino acid residues within the protein, which could influence factors such as the affinity of the protein for ligands that bind to it, such as dopamine to its receptor or its transporter in this instance, and could also influence other aspects of activity. If this were to be so, it might explain how a polymorphism in a gene that was not the receptor itself might relate to dopaminergic activity (D. J. K Balfour, personal communication, June 23, 2006). Data do not currently exist to test this possibility directly.
A similar pattern of pharmacogenetic efficacy has emerged for two polymorphisms associated with D2 receptor activity. Johnstone and colleagues (2004) found that the DRD2 A1/A1 or A1/A2 genotype was associated with relatively greater efficacy of nicotine replacement therapy patch for smoking cessation compared with A2/A2 genotypes, an effect opposite to what is reported here for bupropion efficacy. Similarly, Lerman and colleagues (2006) reported that a genetic variant (DRD2 -141Cins) portending greater expression of the DRD2 gene was associated with greater bupropion efficacy while the low-activity variant (-141Cdel) was associated with greater NRT (patch and nasal spray) efficacy. A tentative pattern is therefore emerging, albeit based on a small number of studies, whereby genotypes associated with reduced D2 receptor availability or function predict better outcomes with NRT, while those associated with normal receptor expression or function predict a better response to bupropion (Lerman et al., 2006).
Mechanistic explanations for these observations require speculation but have focused on differences in the neurological sites of action for both drugs and the notion that, for bupropion to exert its pharmacodynamic actions, relatively “unaltered” striatal D2 receptor density might be required while NRT might actually be treating withdrawal symptoms in individuals who have relatively reduced striatal dopaminergic tone (David et al., 2007; Lerman et al., 2006). Indeed, we have demonstrated previously that only individuals with A2/A2 genotypes appear to experience reductions in nicotine withdrawal symptoms and that DRD2 genotype may exert its effects on smoking cessation via craving reduction in individuals using bupropion (David et al., 2007; David et al., 2003).
Additional research, in future trials of markedly larger size, is needed to examine potential moderating effects of sex, whether these effects exist in other populations (e.g., on the basis of ancestry; Munafò, Lerman, Niaura, Shields, & Swan, 2005; Munafò, Shields et al., 2005) and to examine the complex biopsychosocial and ethical implications of genetically tailored therapies for smoking cessation (Marteau & Lerman, 2001). However, if the studies reporting evidence from pharmacogenetic evaluations of NRT and bupropion efficacy are replicated, individuals who may be bupropion nonresponders—in part because of their DRD2 Taq1A genotypes, may fortuitously derive greater efficacy from the use of NRT.
Acknowledgments
This study was funded in part by U.S. Public Health Service grants HL32318, CA63562, DA08511, CA84719, CA84718, and DA14276-04 and by GlaxoSmithKline, Inc.
References
- American Psychiatric Assocation. Diagnostics and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E, Sulser F. Bupropion: A review of its mechanism of antidepressant activity. Journal of Clinical Psychiatry. 1995;56:395–401. [PubMed] [Google Scholar]
- Balfour DJ. Neuroplasticity within the mesoaccumbens dopamine system and its role in tobacco dependence. Current Drug Targets Central Nervous System and Neurological Disorders. 2002;1:413–421. doi: 10.2174/1568007023339076. [DOI] [PubMed] [Google Scholar]
- Blum K, Noble EP, Sheridan PJ, Montgomery A, Ritchie T, Jagadeeswaran P, Nogami H, Briggs AH, Cohn JB. Allelic association of human dopamine D2 receptor gene in alcoholism. The Journal of the American Medical Association. 1990;263:2055–2060. [PubMed] [Google Scholar]
- Brown RA, Niaura R, Lloyd-Richardson EE, Strong DR, Kahler CW, Abrantes AM, Abrams D, Miller IW. Bupropion and cognitive behavioral treatment for depression in smoking cessation. Nicotine & Tobacco Research. 2007;9:721–730. doi: 10.1080/14622200701416955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon LR, Idury RM, Harris TJ, Witte JS, Elston RC. Testing drug response in the presence of genetic information: Sampling issues for clinical trials. Pharmacogenetics. 2000;10:503–510. doi: 10.1097/00008571-200008000-00003. [DOI] [PubMed] [Google Scholar]
- David SP, Brown RA, Papandonatos GD, Kahler CW, Lloyd-Richardson EE, Munafò MR, Shields PG, Lerman C, Strong D, McCaffery J, Niaura R. Pharmacogenetic clinical trial of sustained-release bupropion for smoking cessation. Nicotine & Tobacco Research. 2007;9:821–823. doi: 10.1080/14622200701382033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Niaura R, Papandonatos GD, Shadel WG, Burkholder GJ, Britt DM, Day A, Stumpff J, Hutchison K, Murphy M, Johnstone E, Griffiths SE, Walton RT. Does the DRD2-Taq1 A polymorphism influence treatment response to bupropion hydrochloride for reduction of the nicotine withdrawal syndrome? Nicotine & Tobacco Research. 2003;5:935–942. doi: 10.1080/14622200310001615295. [DOI] [PubMed] [Google Scholar]
- Feng Y, Niu T, Xing H, Xu X, Chen C, Peng S, Wang L, Laird N, Xu X. A common haplotype of the nicotine acetylcholine receptor alpha 4 subunit gene is associated with vulnerability to nicotine addiction in men. American Journal of Human Genetics. 2004;75:112–121. doi: 10.1086/422194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyerabend C, Russell MA. A rapid gas–liquid chromatographic method for the determination of cotinine and nicotine in biological fluids. Journal of Pharmacy and Pharmacology. 1990;42:450–452. doi: 10.1111/j.2042-7158.1990.tb06592.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM III Axis I disorders, research version, non-patient version. New York: New York State Psychiatric Institute; 1989. [Google Scholar]
- Foulds J. The neurobiological basis for partial agonist treatment of nicotine dependence: Varenicline. International Journal of Clinical Practice. 2006;60:571–576. doi: 10.1111/j.1368-5031.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Lukas RJ. Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine. Journal of Pharmacology and Experimental Therapeutics. 1999;288:88–92. [PubMed] [Google Scholar]
- Halekoh U, Hojsgaard S, Yan J. The R package GEEPACK for generalized estimating equations. Journal of Statistical Software. 2006;15:1–9. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, Croghan IT, Sullivan PM. A comparison of sustained-release bupropion and placebo for smoking cessation. The New England Journal of Medicine. 1997;337:1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Yudkin PL, Hey K, Roberts SJ, Welch SJ, Murphy MF, Griffiths SE, Walton RT. Genetic variation in dopaminergic pathways and short-term effectiveness of the nicotine patch. Pharmacogenetics. 2004;14:83–90. doi: 10.1097/00008571-200402000-00002. [DOI] [PubMed] [Google Scholar]
- Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, Sedvall GC. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Molecular Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. The New England Journal of Medicine. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: Bridging the gap from molecules to behaviour. Nature Reviews Neuroscience. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- Lerman C, Caporaso NE, Audrain J, Main D, Bowman ED, Lockshin B, Boyd NR, Shields PG. Evidence suggesting the role of specific genetic factors in cigarette smoking. Health Psychology. 1999;18:14–20. doi: 10.1037//0278-6133.18.1.14. [DOI] [PubMed] [Google Scholar]
- Lerman C, Jepson C, Wileyto EP, Epstein LH, Rukstalis M, Patterson F, Kaufmann V, Restine S, Hawk L, Niaura R, Berrettini W. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: Results of two randomized clinical trials. Neuropsychopharmacology. 2006;31:231–242. doi: 10.1038/sj.npp.1300861. [DOI] [PubMed] [Google Scholar]
- Lerman C, Niaura R. Applying genetic approaches to the treatment of nicotine dependence. Oncogene. 2002;21:7412–7420. doi: 10.1038/sj.onc.1205801. [DOI] [PubMed] [Google Scholar]
- Lerman C, Shields PG, Wileyto EP, Audrain J, Hawk LH, Jr, Pinto A, Kucharski S, Krishnan S, Niaura R, Epstein LH. Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health Psychology. 2003;22:541–548. doi: 10.1037/0278-6133.22.5.541. [DOI] [PubMed] [Google Scholar]
- Li MD, Beuten J, Ma JZ, Payne TJ, Lou XY, Garcia V, Duenes AS, Crews KM, Elston RC. Ethnic- and gender-specific association of the nicotinic acetylcholine receptor alpha4 subunit gene (CHRNA4) with nicotine dependence. Human Molecular Genetics. 2005;14:1211–1219. doi: 10.1093/hmg/ddi132. [DOI] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Malaiyandi V, Lerman C, Benowitz NL, Jepson C, Patterson F, Tyndale RF. Impact of CYP2A6 genotype on pretreatment smoking behaviour and nicotine levels from and usage of nicotine replacement therapy. Molecular Psychiatry. 2006;11:400–409. doi: 10.1038/sj.mp.4001794. [DOI] [PubMed] [Google Scholar]
- Marteau TM, Lerman C. Genetic risk and behavioural change. British Medical Journal. 2001;322:1056–1059. doi: 10.1136/bmj.322.7293.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Clark T, Johnstone E, Murphy M, Walton R. The genetic basis for smoking behavior: A systematic review and meta-analysis. Nicotine & Tobacco Research. 2004;6:583–597. doi: 10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Lerman C, Niaura R, Shields AE, Swan GE. Smoking cessation treatment: Pharmacogenetic assessment. Current Opinion in Molecular Therapeutics. 2005;7:202–208. [PubMed] [Google Scholar]
- Munafò MR, Shields AE, Berrettini WH, Patterson F, Lerman C. Pharmacogenetics and nicotine addiction treatment. Pharmacogenomics. 2005;6:211–223. doi: 10.1517/14622416.6.3.211. [DOI] [PubMed] [Google Scholar]
- Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: A novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Human Mutation. 2004;23:540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- Noble EP, Gottschalk LA, Fallon JH, Ritchie TL, Wu JC. D2 dopamine receptor polymorphism and brain regional glucose metabolism. American Journal of Medical Genetics. 1997;74:162–166. [PubMed] [Google Scholar]
- O’Loughlin J, Paradis G, Kim W, DiFranza J, Meshefedjian G, McMillan-Davey E, Wong S, Hanley J, Tyndale RF. Genetically decreased CYP2A6 and the risk of tobacco dependence: A prospective study of novice smokers. Tobacco Control. 2004;13:422–428. doi: 10.1136/tc.2003.007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R, Lopez AD, Boreham J, Thun M, Heath C, Jr, Doll R. Mortality from smoking worldwide. British Medical Bulletin. 1996;52:12–21. doi: 10.1093/oxfordjournals.bmb.a011519. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Lerman C. Current and emerging pharmacotherapies for treating tobacco dependence. Expert Opinion on Emerging Drugs. 2006;11:429–444. doi: 10.1517/14728214.11.3.429. [DOI] [PubMed] [Google Scholar]
- Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14:615–626. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- Society for Research on Nicotine and Tobacco. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale BM, van den Oord E, Miles MF, Neale MC, Bulik CM, Joyce PR, Straub RE, Kendler KS. Candidate genes for nicotine dependence via linkage, epistasis, and bioinformatics. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;126:23–36. doi: 10.1002/ajmg.b.20138. [DOI] [PubMed] [Google Scholar]
- Swan GE, McAfee T, Curry SJ, Jack LM, Javitz H, Dacey S, Bergman K. Effectiveness of bupropion sustained release for smoking cessation in a health care setting: A randomized trial. Archives of Internal Medicine. 2003;163:2337–2344. doi: 10.1001/archinte.163.19.2337. [DOI] [PubMed] [Google Scholar]
- Swan GE, Valdes AM, Ring HZ, Khroyan TV, Jack LM, Ton CC, Curry SJ, McAfee T. Dopamine receptor DRD2 genotype and smoking cessation outcome following treatment with bupropion SR. The Pharmacogenomics Journal. 2005;5:21–29. doi: 10.1038/sj.tpj.6500281. [DOI] [PubMed] [Google Scholar]
- Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N. Assessing the probability that a positive report is false: An approach for molecular epidemiology studies. Journal of the National Cancer Institute. 2004;96:434–442. doi: 10.1093/jnci/djh075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin P, Hey K, Roberts S, Welch S, Murphy M, Walton R. Abstinence from smoking eight years after participation in a randomised controlled trial of nicotine patch. British Medical Journal. 2003;327:28–29. doi: 10.1136/bmj.327.7405.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]


