Abstract
Background
In experimental animal models of irritable bowel syndrome (IBS) and human studies, peripheral kappa opioid agonists have been demonstrated to decrease sensation to colonic distention.
Aims
To compare the effects of the kappa opioid agonist, asimadoline, and placebo on episodes of abdominal pain in patients with IBS.
Methods
Following a two-week run–in period, 100 patients with IBS were randomized (3:2 ratio) to receive asimadoline, up to 1 mg four times daily, or placebo for four weeks under double-blind. Pain was scored by daily diary using a 100 mm visual analog scale. During pain episodes, patients recorded the pain severity, took study medication, and recorded pain score 2 hours later. Primary endpoint was the average reduction in pain severity 2 hours after treatment.
Results
The average pain reduction 2 hours post-treatment was not significantly different between the groups. Post-hoc analyses suggest asimadoline was effective in mixed IBS (p=0.003, unadjusted), but may be worse in diarrhea-predominant IBS (p=0.065 unadjusted). Anxiety score was modestly reduced by asimadoline (p=0.053). No significant adverse effects were noted.
Conclusions
An on-demand dosing schedule of asimadoline was not effective in reducing severity of abdominal pain in IBS. Further studies in visceral pain and IBS appear warranted.
Keywords: visceral pain, kappa opioid, functional gastrointestinal disorders
INTRODUCTION
Irritable bowel syndrome (IBS) is a commonly occurring functional gastrointestinal disorder affecting approximately 14% of the general population (1) and is associated with high health care costs and decreased quality of life (2). Effective treatment for abdominal pain in IBS has remained elusive. Although the pathophysiology of IBS is incompletely understood, visceral hypersensitivity is thought to have an important role (3,4). The neurotransmitters involved in control of visceral sensation include: norepinephrine, serotonin, tachykinins, calcitonin gene related peptide and opiates.
Three major opioid receptors, μ, δ and κ, are known to modulate visceral nociception (5). Opioid agonists that are approved by regulatory agencies for use in routine patient management are predominantly active on μ receptors. They are, however, addictive and have peripheral and central adverse effects that make them inappropriate for medium or long term clinical use in patients with IBS. In contrast, asimadoline is a diarylacetamide kappa opioid receptor agonist that binds preferentially to κ-opioid receptors (6), which are involved in the perception of visceral pain (7). This compound has a very low distribution to the brain (8). In animal studies, CNS-mediated adverse reactions were seen at doses 50 to 600 times higher than doses associated with analgesia, and no opiate-like physical dependence has been observed in treatment for up to 8 weeks with asimadoline in humans (9). In animal models, asimadoline has been shown to reduce sensation responses to gastric and colon distention (10).
In previous pharmacodynamic studies conducted in healthy human volunteers, we have shown that asimadoline has a good safety profile. Asimadoline decreased colonic tone during fasting and gas perception to colonic distention particularly at moderate distension pressures (between ~18 and 28 mmHg); however, asimadoline did not inhibit colonic compliance, postprandial colonic contraction, or transit (11). In the same study, asimadoline also increased nutrient drink intake, suggesting a reduction in upper gut sensation without affecting upper gut transit. Separately, Delgado-Aros et al. also demonstrated that a single 1.5 mg dose of asimadoline decreased satiation and postprandial fullness without affecting gastric volume in females (12). In patients with irritable bowel syndrome, acute dosing of 0.5 mg asimadoline was associated with decreased pain perception over a range of distending pressures in the colon without affecting the colonic compliance (13). These effects suggest that this κ-opioid agonist may have a role in treatment of pain associated with functional gut disorders without significant effects on bowel function, given the absence of any significant effects on small bowel or colonic transit.
In the present study, we investigated the effects of asimadoline on abdominal pain and discomfort and other symptoms in female IBS patients during a four week trial with an on-demand dosing schedule. The on-demand schedule was selected in view of the recognition that abdominal symptoms in IBS, including pain and discomfort, are not present continuously and that there are substantial symptom free periods (13) when patients may prefer not receiving any treatment.
MATERIALS AND METHODS
Study Population
The study was conducted between January 2005 and April 2006. A total of 155 patients with irritable bowel syndrome were recruited from the local referral population. Inclusion criteria included non-pregnant, non breast-feeding females between the ages of 18 and 65; diagnosis of IBS by Rome II criteria; absence of alarm symptoms; acceptable method of contraception; and abdominal pain or discomfort of at least 40 mm on a 100 mm visual analog scale (VAS) for at least 4 of the 14 days in the run-in period, but no more than 60 mm on the VAS on more than 10 days of the 14-day run-in period. Exclusion criteria included hypersensitivity to asimadoline or opioid agonists; alcoholics or substance abuse, previous gastrointestinal surgery (except cholecystectomy, appendectomy or hysterectomy); structural or metabolic conditions that affect the gastrointestinal system; clinically significant abnormal laboratory values at screening visit; unable to withdraw medications that alter gastrointestinal transit, that inhibit CYP 3A4 and 2D6, benzodiazepines or any analgesics.
The protocol was approved by the Mayo Institutional Review Board, and written informed consent was obtained from all participants prior to enrollment in the study.
Study Design
This was a randomized, parallel group, double-blind, placebo controlled study evaluating the effects of a flexible dose of asimadoline (0.5 mg p.r.n. up to 1.0 mg q.i.d. for four weeks) or identical appearing placebo on improvement of pain and gastrointestinal symptoms in participants with IBS. Sixty subjects were randomized to on-demand treatment with asimadoline, and forty subjects were randomized to placebo.
Study Medication
Asimadoline is a highly selective, peripherally active κ agonist. The medication (in 0.5mg dose per tablet) and identical matching placebo were provided by Merck kGaA, Darmstadt, Germany. After oral administration of asimadoline, the pharmacokinetics suggest a Tmax of approximately one hour, and a Thalf of approximately 2–3 hours (21).
Study Protocol
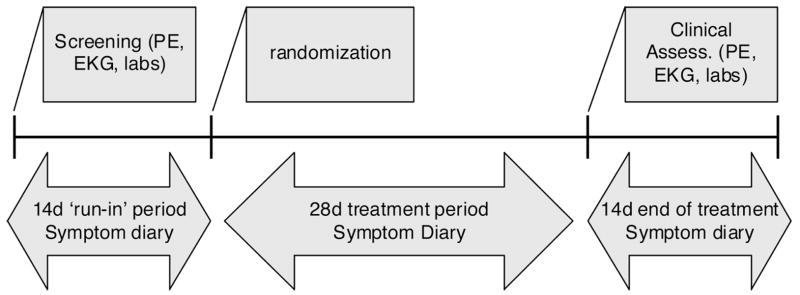
At the screening visit, an interview, physical examination, EKG, standard laboratory tests, Bowel Disease Questionnaire [BDQ (14)], Hospital Anxiety and Depression Scale [HADS (15)] and Quality of Life questionnaire [IBS QoL (16)] were obtained, and patients received diaries to record daily pain and other IBS symptoms. After a 2-week run-in period to establish symptoms at baseline, patients meeting all entry criteria were randomized and received study medication or placebo for the double blind treatment period of four weeks’ duration. Allocation of treatment was concealed. Participants returned to the study center after two weeks of treatment and underwent EKG and standard clinical laboratory tests. The patients returned after four weeks of treatment and underwent EKG, standard clinical laboratory tests, filled BDQ, HADS and IBS QoL questionnaires, and returned daily diaries recording pain and bowel function were collected.
During the double-blind treatment period, patients were instructed to fill in the rating of pain severity on the VAS in the diary whenever they felt pain of at least moderate intensity the first time during a day, before taking the first dose of study medication for that day. Two hours after the intake of the medication, the pain severity was rated again on the 100 mm VAS. If the pain was adequately controlled for the rest of the day, this was recorded in the diary. If the pain was not adequately controlled, the patients were allowed to re-medicate (up to two 0.5mg tablets), but not earlier than four hours after the previous dose. Patients were allowed to take up to two tablets at a time, up to four times a day.
Bowel movement frequency, consistency [Bristol Stool Form Scale (17)], daily ease of passage [7 point adjectival scale (18)], and daily adequate relief (19) of IBS pain and discomfort (a binary global endpoint) were also collected.
Safety Monitoring
Adverse effects were monitored at the study site daily, and patients were given telephone numbers to contact the study investigators to report any side effects. A follow-up visit was held 2 weeks after the end of the double-blind treatment period to follow-up any abnormalities observed in the previous visits and to undergo a physical examination and repeat BDQ.
Experimental Protocol
The experimental protocol is shown in Figure 1.
Figure 1.
Experimental protocol
Data Analysis
The primary endpoint for analysis was the average reduction in pain severity 2 hours after first dose on each day that the participant experienced at least moderate pain. In essence, we assessed the change in pain intensity before versus two hours after the first dose on each day the subject recorded a pain level of at least 30 mm on the VAS and took medication. For each of these days, the pain reduction of the first dose was calculated: Pain Intensity Difference (PID) = Pain0h − Pain2h. The average pain reduction on these days was calculated by: first summing up all the PIDs over the treatment period and dividing this result by the number of days with pain when medication was used. Secondary endpoints were daily frequency of bowel movements (number), daily consistency of bowel movements (Bristol stool scale), daily ease of passage (7 point adjectival scale), daily adequate relief of IBS pain and discomfort (global endpoint, analyzed as both a continuous proportion of days per subject and as a discrete nominal response, >50% of days with adequate relief), and also the BDQ, HADS, and irritable bowel syndrome quality of life (IBS-QoL) scores were assessed at the beginning and end of the study.
Statistical Analysis
The treatment effects on the primary and secondary endpoints were assessed using an analysis of covariance (ANCOVA) incorporating relevant covariates (e.g. age, baseline pain severity VAS score, baseline proportion of days with pain and baseline HAD anxiety score for the analysis of pain intensity differences). Secondary analyses were also examined incorporating the IBS subtype according to predominant bowel function as a covariate along with a treatment by subtype interaction term in the ANCOVA model. For the primary analysis endpoints an intent-to-treat (ITT) paradigm was followed imputing missing values for an endpoint using the corresponding overall (subjects with non-missing values) mean value. A consequent adjustment in the error degrees of freedom for the ANCOVA model was made by subtracting one degree of freedom for each missing value imputed in order to obtain an appropriate residual error variance for testing treatment effects. Summary values are reported as median (IQR) or mean (±SE) as indicated.
RESULTS
Demographics and Baseline Characteristics
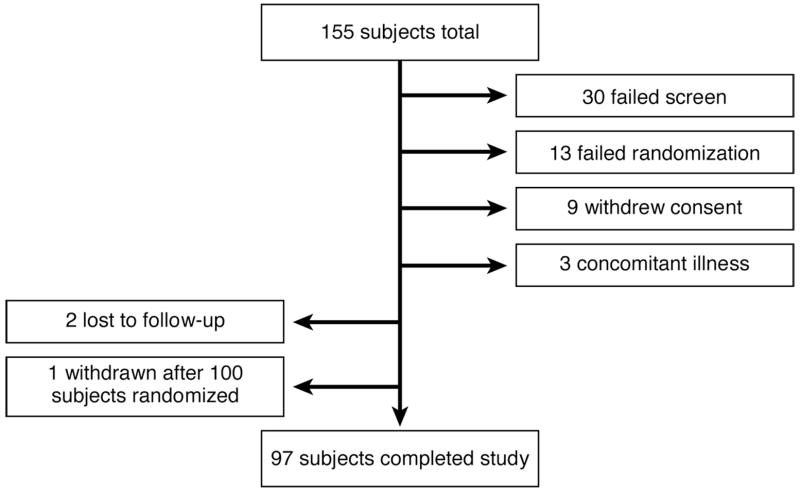
The trial flow is shown in Figure 2.
Figure 2.
Trial flow
Table 1 summarizes characteristics of the subjects entering the four week active part of the trial. The treatment group and the placebo group were comparable for age, IBS subtype, gastrointestinal symptoms, anxiety and depression scores and IBS-related quality of life scores. Based on the baseline BDQ characterization of symptoms and patient history, there were 39 with mixed bowel pattern (IBS-M), 27 with constipation-predominant (IBS-C), and 33 with diarrhea-predominant (IBS-D), and one subject could not be classified. Patients were approximately equally randomized to the different treatments, except that there were somewhat more IBS-D randomized to asimadoline relative to placebo.
Table 1.
| A. Demographics of participants | |||
|---|---|---|---|
| Overall (n=100) | Asimadoline (n=60) | Placebo (n=40) | |
| Age (y), mean ± SE | 38 ± 1 range 18–67 | 39 ± 2 range 18–67 | 36 ± 2 range 18–66 |
| IBS subtype† | |||
| Constipation-predominant | 27(27%) | 14(24%) | 13(32%) |
| Diarrhea-predominant | 33(33%) | 22(37%) | 11(28%) |
| Mixed bowel pattern | 39(39%) | 23(39%) | 16(40%) |
| B. Baseline symptoms (median and IQR or mean ± SEM) | |||
| Overall | Asimadoline | Placebo | |
| Bowel movement frequency | 1.68 ± 0.11 | 1.66 ± 0.13 | 1.70 ± 0.20 |
| Stool consistency | 3.92 ± 0.12 | 4.06 ± 0.15 | 3.71 ± 0.20 |
| Pain (VAS 100mm) | 37.2 (27.1–49.5) | 37.2 (29.6–51.5) | 37.7 (23.8–44.6) |
| Bloating (VAS 100mm) | 35.3 (26.1–49.1) | 34.8 (27.2–49.7) | 40.7 (22.8–47.5) |
| Flatulence (VAS 100mm) | 31.0 (21.7–45.7) | 31.8 (25.2–43.4) | 28.6 (17.9–47.2) |
| Urgency (VAS 100mm) | 25.1 (15.0–38.4) | 26.1 (16.2–40.4) | 22.2 (14.0–38.2) |
| Severity of most intense pain episode of day (0–4) | 1.6 (1.3–2.0) | 1.6 (1.4–2.0) | 1.6 (1.0–2.0) |
One subject could not be classified.
Characteristics of Pain and Intake of Treatment during the 4-Week Trial
Table 2 shows that, for the asimadoline and placebo groups respectively, the median (IQR) proportionate number of days with pain was 40% (24%–58%) and 36% (23%–50%), and the proportion of days with pain when medication was used was 97% (87%–100%) and 100% (82%–100%). The number of tablets used during the first episode of pain on days with pain was 1.62 (1.18–1.83) for asimadoline and 1.43 (1.08–1.88) for the placebo group.
Table 2.
Pain Characteristics during Treatment (median, IQR)
| Asimadoline | Placebo | |
|---|---|---|
| Days with pain | 10.5 (6.0–15.0) | 9.5 (6.5–14.0) |
| Proportion of days with pain | 40% (24%–58%) | 36% (23%–50%) |
| Proportion of days with pain when medication was used | 97% (87%–100%) | 100% (82%–100%) |
| Number of tablets used per day with pain | 1.62 (1.18–1.83) | 1.43 (1.08–1.88) |
The baseline pain severity score was used as a covariate in the analysis of the delta pain scores and was not significant. On the other hand, the proportion of days with pain was significant, such that higher proportions of days with pain tended to be associated overall with smaller changes in pain scores during treatment.
Effect of Treatment on Episodes of IBS Pain
The assessment of the effect of treatment on episodes of pain reflects the primary endpoint in the study. The average reduction in pain severity on 100 mm VAS within two hours after first dose was not significantly different between the two treatment groups (Table 3). In addition, the proportion of days with adequate relief was not significantly different between treatment groups. Also, the proportion of subjects in each group reporting adequate relief on greater than 50% of days with pain was similar (32.5% on placebo vs. 33.3% on Asimadoline). Other effects of treatment on stool consistency, HAD scores and QoL are summarized in Table 3.
Table 3.
| A. Symptoms during Treatment (median, IQR, unless otherwise stated) | ||||||
|---|---|---|---|---|---|---|
| Overall | Asimadoline | Placebo | ||||
| Δ Pain intensity after 2 h, VAS, mm | 26.5 (15.3, 41.8) | 27.9 (17.5, 44.5) | 23.5 (13.3, 34.5) | |||
| Proportion (%) of days with adequate relief of symptoms (mean ± SE) | 36 ± 4% | 36 ± 5% | 35 ± 6% | |||
| Mean daily bowel movement frequency (mean ± SE) | 1.68 ± 0.11 | 1.66 ± 0.13 | 1.70 ± 0.20 | |||
| Mean stool consistency (mean ± SE) | 3.92 ± 0.12 | 4.06 ± 0.15 | 3.71 ± 0.20 | |||
| B. Baseline symptoms (median and IQR or mean ± SEM) | ||||||
| Overall | Asimadoline | Placebo | ||||
| Baseline | Post-treatment | Baseline | Post-treatment | Baseline | Post-treatment | |
| HAD score
Anxiety (mean±SE) Depression (mean±SE) |
5.1 ± 0.3 2.0 ± 0.2 |
5.3 ± 0.3 1.9 ± 0.2 |
4.7 ± 0.3 2.0 ± 0.3 |
4.5 ± 0.4 1.6 ± 0.3 |
5.8 ± 0.4 2.0 ± 0.3 |
6.5 ± 0.6 2.2 ± 0.3 |
| IBS QoL score (mean± SE) | 25.8 ± 1.3 | 22.20 ± 1.4 | 26.0 ± 1.8 | 20.9 ± 1.7 | 25.5± 1.9 | 24.2 ± 2.4 |
Effect of On-Demand Study Medication on Abdominal Pain in IBS Subgroups According to Predominant Bowel Pattern (Table 4a, b, and c)
Table 4.
| A. Bowel function at baseline based on predominant bowel function by questionnaire | ||||||
|---|---|---|---|---|---|---|
| IBS-C | IBS-D | IBS-M | ||||
| Age (mean ± SE) | 36.9±1.9 | 39.3±2.5 | 37.2±2.0 | |||
| Mean # daily stools | 1.3±0.2 | 2.1±0.2 | 1.6±0.2 | |||
| Mean stool form | 3.3±0.3 | 4.6±0.2 | 3.8±0.2 | |||
| Mean ease of passage | 4.1±0.1 | 4.7±0.1 | 4.3±0.1 | |||
| Mean proportion stools with complete evacuation | 0.31±0.05 | 0.60±0.05 | 0.40±0.05 | |||
| B. Change in bowel function on treatment compared to baseline based on predominant bowel function | ||||||
| IBS-C | IBS-D | IBS-M | ||||
| Asimadoline | Placebo | Asimadoline | Placebo | Asimadoline | Placebo | |
| Δ mean # stools | −0.1±0.1 | −0.2±0.2 | −0.3±0.1 | −0.1±0.1 | 0.0±0.1 | −0.3±0.15 |
| Δ mean stool form | 0.0±0.2 | 0.3±0.2 | −0.1±0.2 | 0.0±0.2 | 0.2±0.2 | 0.0±0.2 |
| Δ mean ease of passage | −0.1±0.1 | −0.1±0.1 | −0.1±0.1 | 0.0±0.1 | 0.0±0.1 | 0.0±0.1 |
| C. Symptoms during treatment in IBS subgroups by predominant bowel pattern (mean ±SE; * p=0.003, unadjusted; **p=0.065, unadjusted) | ||||||
| IBS-C | IBS-D | IBS-M | ||||
| Asimadoline | Placebo | Asimadoline | Placebo | Asimadoline | Placebo | |
| Number | 14 | 13 | 22 | 11 | 23 | 16 |
| Δ Pain intensity (mm) | 23.8±4.0 | 20.9±3.9 | 25.0±2.9 | 37.5±6.5** | 37.8±3.7* | 22.0±3.0 |
| Proportion of days with adequate relief of symptoms | 0.11±0.07 | 0.18±0.07 | 0.45±0.08 | 0.50±0.11 | 0.46±0.08 | 0.37±0.10 |
The IBS-C subgroup appeared to have the higher pain scores. There were no significant differences in the change in pain intensity (p=0.538) or proportion of days with adequate relief of symptoms (p=0.86) between treatment groups overall when adjusting for predominant bowel pattern.
However a significant treatment by IBS subtype interaction was detected (p=0.004) for delta pain intensity scores. In IBS-D subjects, the delta pain scores (pre-post) were greater for subjects assigned to placebo than in those assigned to asimadoline, suggesting greater pain relief on placebo. This post-hoc analysis suggested a worse response to asimadoline compared to placebo (unadjusted p=0.065) in IBS-D. In IBS-C, the changes in pain scores on placebo and asimadoline were similar. In contrast, there were larger changes in pain scores for IBS-M subjects assigned to asimadoline relative to placebo treatment. The unadjusted pair-wise comparison indicated a significant difference for IBS-M subjects (p=0.003) indicating asimadoline was significantly better in reducing pain scores than placebo in IBS-M.
Days with Adequate Relief of Pain in IBS Subtypes
Overall (i.e. pooled across both treatments), IBS-C subjects had substantially smaller proportions of days with adequate relief than the other two subtypes. Thus, an overall main effect of IBS subgroup on the proportion of days with relief was detected (p=0.001). However, there were no treatment effects, and no treatment by IBS subgroup interactions were detected for the proportion of days with adequate relief.
Effect of Asimadoline Based on Number of Tablets Ingested On-Demand by Patients
The overall median number of tablets used during the treatment period (both treatment groups) was 14.5 (IQR=8.0 to 24.5): placebo median 13.5 (IQR=9.5 to 19.5), asimadoline median 16.5(IQR=8.0 to 25.5). Splitting the data at the median (i.e. >14.5), the median (IQR) delta pain score was 23.4 (14.8–34.3) in placebo patients taking ≤14.5 tabs and 26.2 (11.1–35.1) in placebo patients taking >14.5 tabs. The mean (±SE) proportions (%) of days with adequate relief in placebo patients were 34.7 (±7.3) and 34.6 (±9.3) respectively.
The corresponding mean delta pain scores in patients taking asimadoline were 30.8 (18.0–46.3) and 26.4 (16.4–37.9), and the mean (±SE) proportion (%) adequate relief days were 38.5 (±8.1) vs. 34.8 (±6.1).
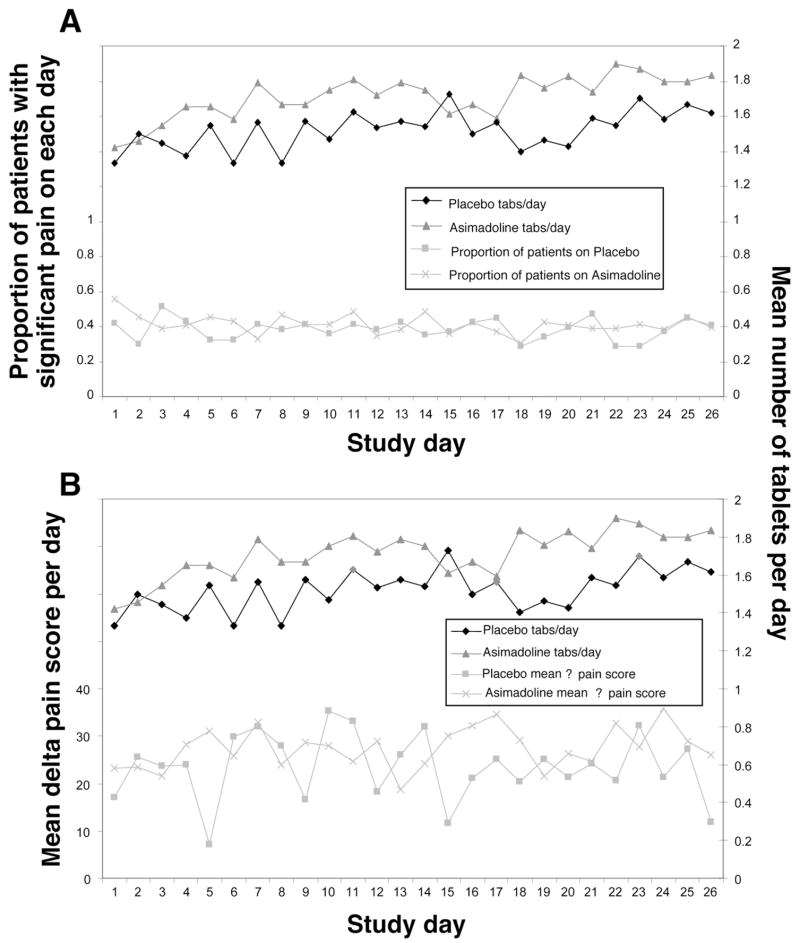
Figure 3a illustrates the mean (per day during treatment) proportion of subjects reporting moderate or worse pain and the mean number of tablets taken for the initial pain episode on each day by subjects in each treatment arm. Similarly, Figure 3b illustrates the mean delta pain intensity scores per day in those subjects reporting pain on each day by treatment group and, again, the corresponding mean number of tablets taken. Note that the specific subjects reporting pain on each day vary and, thus, the broken lines are not intended to represent the same subjects over the course of the treatment period, but merely to illustrate that these responses did not change appreciably over the course of the treatment period.
Figure 3.
Figure 3A Plots of the number of tablets taken in each treatment group and the change in pain score reported each day for those experiencing pain. Note there is no trend to change the number of tablets taken in this on demand study, nor any trend on the change in efficacy as measured by the change in pain score each day.
Figure 3B Plots of the number of tablets taken in each treatment group and the proportion of patients with significant pain on each day of the treatment phase. Note there is no trend to change the number of tablets taken in this on demand study, nor any trend on the proportion experiencing significant pain on each day.
Anxiety and Depression Response
Anxiety score (Table 3B) was modestly affected by asimadoline treatment (p=0.053), but the numerical differences are small. There was no significant difference in depression scores.
Quality of Life
Tables 3B and 5 summarize baseline and post-treatment overall quality of life scores for the entire treatment group as well as the groups subdivided by predominant bowel function. There were no significant effects of treatment on quality of life in the whole IBS group or the subgroups based on predominant bowel function.
Table 5.
Baseline and post-treatment overall quality of life scores for entire treatment group and for groups subdivided by predominant bowel function
| IBS-C | IBS-D | IBS-M | ||||
|---|---|---|---|---|---|---|
| Asimadoline | Placebo | Asimadoline | Placebo | Asimadoline | Placebo | |
| Number (base/post-Rx) | 14/12 | 13/10 | 21/18 | 11/8 | 23/18 | 15/13 |
| QoL baseline | 17.4 ± 3.0 | 20.6 ± 3.0 | 27.9 ± 3.0 | 28.3 ± 4.1 | 29.4 ± 3.0 | 27.7 ± 2.8 |
| QoL post-Rx | 14.9 ± 2.5 | 18.7 ± 3.4 | 25.2 ±2.8 | 29.4 ±6.1 | 20.5 ± 2.8 | 25.3 ± 3.4 |
Adverse Events
There were no serious clinical or laboratory adverse events. Adverse events reported more than three times were grouped into the categories shown in Table 6 and were similar in each group, except for the larger number of gastrointestinal events reported in the placebo group.
Table 6.
Adverse events (%)
| Asimadoline | Placebo | |
|---|---|---|
| Musculoskeletal | 6.67% | 5.00% |
| Central and peripheral nervous system | 48.33% | 50.00% |
| Psychiatric | 15.00% | 12.50% |
| Gastrointestinal | 16.67% | 30.00% |
| Genitourinary | 1.67% | 5.00% |
| Respiratory | 33.33% | 37.50% |
| Body as a whole | 15.00% | 5.00% |
DISCUSSION
In this phase IIb study, on-demand administration of the selective kappa-opioid agonist, asimadoline, did not significantly alter average reduction in severity of abdominal pain, or any of the secondary endpoints related to bowel patterns, compared to placebo treatment. However, stool frequency and consistency were essentially within what would be considered the normal range for each treatment group and an effect would, therefore, not be anticipated if asimadoline serves to “normalize” abnormal bowel function. The study design was novel and therefore there is no track record for on-demand drug treatment for irritable bowel syndrome or the primary endpoint as measured here using a VAS based on a diary (change in pain severity). As such, the sample size is primarily conducive to exploratory observations or hypothesis generation, rather than hypothesis testing. Nevertheless, there are a number of interesting findings in the post-hoc analyses related to subgroups based on predominant bowel function, as discussed below. These data suggest there may also be a beneficial effect overall, but this was not associated with a greater proportion treated with asimadoline reporting either adequate relief on a daily basis or improved quality of life over the 4-week treatment period.
The novel design of on-demand treatment was chosen because it was considered that, in the absence of a treatment that definitively corrects the mechanism or natural history of IBS (20), the study should focus on targeting the relief of symptoms. It is known that IBS symptoms wax and wane, and each pain episode may be exacerbated only for a few days (14). Therefore, it seemed appropriate to attempt to develop a treatment for the relief of IBS pain that does not require persistent treatment, since this requires intake of treatment at times when the patient is relatively or completely pain-free.
The study was performed on a group of patients with a relatively homogenous and moderate severity of abdominal pain, between 40 and 60 mm on a 100 mm visual analog scale for at least 4 of 14 days at baseline. This level of pain severity was pre-specified and included in the eligibility criteria to ensure that participants had sufficient pain symptoms to be able to demonstrate an effect and to avoid excessively high baseline pain levels, which could have led to subsequent confounding by regression to the mean. Conversely, excessively low baseline levels could have potentially confounded the study interpretation by a floor effect.
Heterogeneity of participants was also reduced by studying only female subjects because data available from animal studies indicates that there are gender related differences in the analgesic effect of asimadoline (21). The dose of 0.5 mg asimadoline, 1–2 tablets qid prn, was chosen based on previous studies of the effects of asimadoline on the gastrointestinal tract in healthy volunteers (11). In the study by Delgado-Aros et al., asimadoline was administered at doses of 0.15, 0.5 and 1.5 mg bid for 9 days. It was found that gas scores in response to colonic distention of moderate intensity (8 and 16 mmHg above baseline operating pressures that averaged 12 mmHg) were decreased with the 0.5 mg dose (11). In contrast, in the same pharmacodynamic study, the higher dose of asimadoline tested (1.5 mg) was associated with increased gas and pain perception (12). The hyperalgesia associated with high doses of asimadoline has been previously observed in animals and humans (21). This hyperalgesia effect is thought to be consistent with the dual modulatory mechanisms of opioids proposed by Crain and Shen (22). In the present study, however, patients self-administered less medication on average than was used in the Delgado-Aros et al. study [(11) 1 mg per day versus 0.7–0.8 mg in current study], and this may have potentially impacted efficacy of the medication.
The number of tablets taken in the two treatment arms was similar; moreover, the response was not significantly different based on intake of more than or less than 14.5 tablets. In contrast, there was some suggestion that there is less relief with increasing medication use in asimadoline treated subjects, but about the same relief (or slightly better) with increasing medication in placebo subjects. However, this requires further study in future trials.
The post-hoc analysis showed greater change in pain score with asimadoline versus placebo in IBS patients with mixed bowel function. This observation is of interest and contrasts with the lack of effect in IBS-D in IBS-C. The numerical improvement in anxiety scores with asimadoline is also hypothesis generating and requires confirmation.
The experimental design used here does not exclude the potential benefit of this agent or class of compound if administered daily over the medium or longer term. We performed a post-hoc analysis to determine whether there was a difference in reported outcomes, such as days with adequate relief of IBS symptoms as a function of the number of days with treatment, or the number of tablets actually taken. It was intended for such an analysis to reflect, in part, the potential of chronic treatment in these patients. Those analyses also did not show a clear effect of asimadoline relative to placebo. Clearly, a formal study of chronic treatment is essential to address the longer term effects and potential of asimadoline to relieve pain in IBS.
Consistent with the reports on the lack of deleterious effect of asimadoline on gastrointestinal or colonic transit or postprandial motility and tone (11), there were no significant effects on bowel function or adverse effects to suggest motor effects of asimadoline, at least in individuals with bowel function in the normal range. It is also important to note that the questionnaire data and history review concurred with the information on bowel function collected in the run-in period using the daily diaries, suggesting that the categorization of patients in subgroups by responses on the baseline BDQ was appropriate based on actual run-in observations.
Previous trials of another selective kappa receptor agonist, fedotozine, in IBS showed that, at the highest dose tested, fedotozine was marginally effective in relieving maximal daily pain and abdominal bloating compared to placebo (23). However, the study had a high drop-out rate, and further development of fedotozine in the United States seems to have been halted due to inconsistent clinical response (24). It is possible that peripheral kappa opioid antagonists may be more effective in the presence of pain associated with inflammation, because there is evidence to suggest that kappa opioid receptors may have their most significant role in modulating nociception associated with inflammation (25). In general, inflammation is not a major component in IBS, though subclinical inflammation may be evident in some patients.
It is also possible that the pain symptoms are predominately mediated by the central rather than the peripheral nervous system, and therefore exclusively targeting peripheral receptors, as with asimadoline which penetrates the CNS poorly, may be unsatisfactory (26). Despite the results in IBS with kappa opioid receptor agonists, it remains important to better elucidate the mechanisms of visceral pain in IBS in order to provide better treatment for IBS pain, given the impact of pain on overall well-being and quality of life in IBS.
In summary, the present study provides interesting experience on study design with on-demand therapy for abdominal pain in IBS. Though the drug, asimadoline, appears ineffective for relief of abdominal pain episodes in IBS, the post-hoc data analysis showing apparent efficacy (p=0.003, unadjusted) in IBS with mixed bowel function and, possibly, anxiety suggests that this medication or class deserves further study in treatment of IBS, either in on-demand or consistent treatment.
Acknowledgments
The study was funded by Merck kGaA and Tioga Pharmaceuticals. Dr. Camilleri is funded in part by grants RO1- DK54681 and K24-DK02638 from National Institutes of Health. We thank Cindy Stanislav, secretary, and Dr. Allen Mangel for assistance. This publication is dedicated to the memory of the study coordinator, Nancy Sullivan, who died only 2 months after the completion of the treatment phase of the last (100th) patient in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hungin AP, Chang L, Locke GR, Dennis EH, Barghout V. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther. 2005;21:1365–1375. doi: 10.1111/j.1365-2036.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- 2.Hulisz D. The burden of illness of irritable bowel syndrome: current challenges and hope for the future. J Managed Care Pharmacy. 2004;10:299–309. doi: 10.18553/jmcp.2004.10.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holtmann G, Goebell H, Talley NJ. Functional dyspepsia and irritable bowel syndrome – is there a common pathophysiologic basis? Am J Gastroenterol. 1997;92:954–959. [PubMed] [Google Scholar]
- 4.Trimble KC, Farouk R, Pryde A, Douglas S, Heading RC. Heightened visceral sensation in functional gastrointestinal disease is not site specific evidence for a generalized disorder of gut sensitivity. Dig Dis Sci. 1995;40:1607–1613. doi: 10.1007/BF02212678. [DOI] [PubMed] [Google Scholar]
- 5.Satoh M, Minami M. Molecular pharmacology of the opioid receptors. Pharmacol Ther. 1995;68:343–364. doi: 10.1016/0163-7258(95)02011-x. [DOI] [PubMed] [Google Scholar]
- 6.Gottschlich R, Krug M, Barber A, Devant RM. Kappa-opioid activity of the four stereoisomers of the peripherally selective kappa-agonists, EMD 60,400 and EMD 61,753. Chirality. 1994;6:685–689. doi: 10.1002/chir.530060814. [DOI] [PubMed] [Google Scholar]
- 7.Simonin F, Gaveriaux-Ruff C, Befort K, Matthes H, Lannes B, Micheletti G, Mattei MG, Charron G, Bloch B, Kieffer B. Kappa-opioid receptor in humans: cDNA and genomic cloning, chromosomal assignment, functional expression, pharmacology, and expression pattern in the central nervous system. Proc Natl Acad Sci USA. 1995;92:7006–7010. doi: 10.1073/pnas.92.15.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barber A, Bartoszyk GD, Bender HM, Gottschlich R, Greiner HE, Harting J, Mauler F, Minck KO, Murray RD, Simon M. A pharmacological profile of the novel, peripherally-selective kappa- opioid receptor agonist, EMD 61753. Br J Pharmacol. 1994;113:1317–1327. doi: 10.1111/j.1476-5381.1994.tb17142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asimadoline (EMD 61 753) Investigators Brochure. Darmstadt: E. Merck; 2001. [Google Scholar]
- 10.Burton MB, Gebhart GF. Effects of κ-opioid receptor agonists on responses to colorectal distention in rats with and without acute colonic inflammation. J Pharmacol Exp Ther. 1998;285:707–715. [PubMed] [Google Scholar]
- 11.Delgado-Aros S, Chial HJ, Camilleri M, Szarka LA, Weber FT, Jacob J, Ferber I, McKinzie S, Burton DD, Zinsmeister AR. Effects of a κ-opioid agonist, asimadoline, on satiation and GI motor and sensory functions in humans. Am J Physiol. 2003;284:G558–G566. doi: 10.1152/ajpgi.00360.2002. [DOI] [PubMed] [Google Scholar]
- 12.Delgado-Aros S, Chial HJ, Cremonini F, Ferber I, McKinzie S, Burton DD, Camilleri M. Effects of asimadoline, a κ-opioid agonist, on satiation and postprandial symptoms in health. Aliment Pharmacol Ther. 2003;18:507–514. doi: 10.1046/j.1365-2036.2003.01670.x. [DOI] [PubMed] [Google Scholar]
- 13.Delvaux M, Beck A, Jacob J, Bouzamondo H, Weber FT, Frexinos J. Effect of asimadoline, a kappa opioid agonist, on pain induced by colonic distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20:237–246. doi: 10.1111/j.1365-2036.2004.01922.x. [DOI] [PubMed] [Google Scholar]
- 14.Hahn B, Watson M, Yan S, Gunput D, Heuijerjans J. Irritable bowel syndrome, symptom patterns: frequency, duration, and severity. Dig Dis Sci. 1998;43:2714–2718. doi: 10.1023/a:1026663613695. [DOI] [PubMed] [Google Scholar]
- 15.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 16.Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400–411. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 17.Heaton KW, Ghosh S, Braddon FE. How bad are the symptoms and bowel dysfunction of patients with the irritable bowel syndrome? A prospective, controlled study with emphasis on stool form. Gut. 1991;32:73–79. doi: 10.1136/gut.32.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coulie B, Szarka LA, Camilleri M, Burton DD, McKinzie S, Stambler N, Cedarbaum JM. Recombinant human neurotrophic factors accelerate colonic transit and relieve constipation in humans. Gastroenterology. 2000;119:41–50. doi: 10.1053/gast.2000.8553. [DOI] [PubMed] [Google Scholar]
- 19.Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035–1040. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- 20.Andresen V, Camilleri M. Irritable bowel syndrome: recent and novel therapeutic approaches. Drugs. 2006;66:1073–1088. doi: 10.2165/00003495-200666080-00004. [DOI] [PubMed] [Google Scholar]
- 21.Machelska H, Pfluger M, Weber W, Piranvisseh-Volk M, Daubert JD, Dehaven R, Stein C. Peripheral effects of the kappa-opioid agonist EMD 61753 on pain and inflammation in rats and humans. J Pharmacol Exp Ther. 1990;290:354–361. [PubMed] [Google Scholar]
- 22.Crain SM, Shen KF. Opioids can evoke direct receptor-mediated excitatory effects on sensory neurons. Trends Pharmacol Sci. 1990;11:77–81. doi: 10.1016/0165-6147(90)90322-y. [DOI] [PubMed] [Google Scholar]
- 23.Dapoigny M, Abitbol JL, Fraitag B. Efficacy of peripheral kappa agonist fedotozine versus placebo in treatment of irritable bowel syndrome. A multicenter dose-response study. Dig Dis Sci. 1995;40:2244–2249. doi: 10.1007/BF02209014. [DOI] [PubMed] [Google Scholar]
- 24.Callahan M. Irritable bowel syndrome neuropharmacology: a review of approved and investigational compounds. J Clin Gastroenterol. 2002;35:S58–S67. doi: 10.1097/00004836-200207001-00011. [DOI] [PubMed] [Google Scholar]
- 25.Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995;332:1685–1690. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- 26.Pasricha PJ. “Kapping” visceral pain in patients with irritable bowel syndrome: does it work? Gastroenterology. 1996;111:531–533. doi: 10.1053/gast.1996.v111.agast961110531. [DOI] [PubMed] [Google Scholar]