Abstract
Although family history of premature coronary artery disease (CAD) confers increased risk of CAD, the magnitude of this increase beyond that expected from the risk factors incorporated in the Framingham Risk Equation (FRE), remains unknown. We prospectively determined the accuracy of the FRE 10-year incident CAD events prediction in initially healthy siblings of patients with documented premature CAD. We recruited 784 siblings (30–59 years) of 449 patients hospitalized with CAD < 60 years of age (1983 – 1995). We compared the estimated 10-year incidence of total CAD events by the sex-specific FREs at baseline, to the observed incidence at 10-years of follow-up. In men, the 10-year actual CAD event rate was 20%, only half of which was predicted by the FRE (12 % vs 20%, p<0.001). In women, the observed CAD event rate was 7.1% (p < 0.001 vs. men), modestly but not significantly greater than the 6.3% predicted by the FRE (p= 0.34). Thus, there was a significant 66.6% excess risk in men, and a nonsignificant 12.7% excess risk in women beyond the risk predicted by the FRE for total CAD events. The FRE and its known classical risk factor profile failed to accurately predict total incident 10-year CAD events in individuals with a sibling history of premature CAD, most particularly in men. In conclusion, in families with a history of premature CAD, the excess risk observed cannot be attributed to traditional risk factors, suggesting a major role for as yet undetermined genetic and other susceptibility factors.
Keywords: coronary disease, risk factors, epidemiology
Introduction
In 2004, >147,000 deaths due to cardiovascular disease occurred in the U.S population under 65 years of age.1 About 60% of premature coronary artery disease (CAD) clusters in families.2 The risk of premature CAD in first degree relatives is notably higher than in the general population,3–5 the absolute risk being highest in siblings, about 3 times that of the general population.3,4,6–11 While this increased sibling risk was recently examined by the Framingham Heart Study,12 the extent to which the CAD predictive equations developed by the Framingham Heart Study13–17 accurately predict disease in persons with a family history of premature CAD remains unknown. Thus, we applied the 1998 Wilson et al. Framingham Risk Equation (FRE)14 for total CAD events to predict incident CAD over 10 years in apparently healthy siblings of patients hospitalized with premature CAD events < 60 years of age, and recalibrated the equations to be population-specific using standard methods developed by the Framingham Heart Study.18 We then compared the 1998 Wilson et al. FRE14 predicted 10-year total predicted CAD event rates with those observed in the sibling population.
Methods
The study was approved by the Johns Hopkins Institutional Review Board. Caucasian and African American probands with premature (< age 60) CAD events (sudden death or myocardial infarction [26.4%], coronary artery bypass surgery [35.0%], coronary angioplasty [28.6%], or anginal symptoms with a ≥ 50% lesion in one or more vessels by coronary angiography [9.1%]) were identified at the time of event from 10 Baltimore area hospitals from 1983 to 1996. CAD events associated with aortic stenosis, cardiac transplantation, chronic glucocorticosteroid therapy, chest irradiation, or cocaine intoxication were excluded. Their siblings < 60 years of age and apparently free of CAD were recruited for screening. Siblings with known autoimmune disease, taking chronic glucorticosteroids, or who had any life-threatening diseases (e.g., AIDS, cancer) with a life expectancy of <5 years were also excluded.
Participants gave informed consent prior to screening. During screening, information regarding demographics was obtained from interviewer-verified self-administered questionnaires. Medical history, including current medication use, was obtained by a physician or nurse, and a physical examination was performed by a cardiologist. All measures of risk factors were identical or nearly identical to those measured in the Framingham Heart Study.14 Any reported cigarette smoking within one month or expired carbon monoxide ≥ 8 ppm on 2 readings were considered current smoking. Venous blood was obtained after a 12-hour overnight fast. Total and high density lipoprotein (HDL) cholesterol, triglycerides, and glucose were measured in the Johns Hopkins Analytical Chemistry Laboratory using standardized methods (coefficient of variation for total cholesterol <3%). Low density lipoprotein (LDL) cholesterol was calculated if triglyceride levels were < 400 mg/dL.19 Diabetes mellitus was defined as a self-reported physician’s diagnosis, current insulin or hypoglycemic medication use, and/or a measured fasting glucose ≥ 126 mg/dl. Blood pressure was measured using methods described by the Joint National Committee on the Detection, Evaluation, and Treatment of High Blood Pressure,20 and the average of three resting measures was used. Hypertension was defined as resting blood pressure ≥ 140/90 mmHg and/or current antihypertensive medication use.
The Wilson et al 1998 FRE scoring system provides estimates of 10-year absolute risks for total CAD.14 For calculating the risk using this model, we followed the same sex-specific FRE14 scoring system based on categories of risk for baseline levels of total cholesterol and blood pressure, smoking, diabetes mellitus and age as for the Framingham Heart Study.14 Total cholesterol categories (in mg/dL) were: (1) <160, (2) 160–199, (3) 200–229, (4) 230–239, (5) 240–279, (6) ≥280. HDL categories (in mg/dL) were: (1) < 35, (2) 35–44, (3) 45–49, (4) 50–59, (5) ≥60. Blood pressure (BP) categories (in mmHg) were: (1) diastolic BP < 80 and systolic BP < 120, (2) diastolic BP = 80–84 or systolic BP = 120–129, (3) diastolic BP = 85–89 or systolic BP = 130–139, (4) diastolic BP = 90–99 or systolic BP = 140–159, (5) diastolic BP > 100 or systolic BP > 160. Classification was by the highest value for either the systolic or diastolic blood pressure.
Siblings were contacted periodically by trained telephone interviewers for follow-up from 1993–2005 and completed a standardized health status and cardiovascular disease event questionnaire. Medical records were obtained for all siblings reporting a CAD event, any possibly related diagnosis, related diagnostic procedure (exercise test, coronary calcium scan, thallium imaging, stress echocardiography, or coronary angiography) or related therapeutic procedure (including percutaneous coronary intervention or coronary bypass surgery). For this study, total events included all CAD events as defined in the Framingham Heart Study, using the same classifications and definitions.14,21 Total CAD events thus included angina pectoris, myocardial infarction, or coronary heart disease death. Event adjudication was done using a manner similar to the Framingham Heart Study.14,21 Medical records were independently reviewed by two cardiologists and one cardiovascular epidemiologist. Whenever there was disagreement in adjudication, an External Adjudication Committee consisting of at least one non-study cardiologist from Johns Hopkins and a cardiologist from another institution reviewed the event and determined the final event classification using the standardized coding schema.
All analyses were sex-specific. Demographic and risk factor characteristics of the cohort were tabulated according to methods similar to those used in the Framingham Heart Study.14 The number of total CAD events expected within 10-years in siblings based on the FRE was calculated as a sum of proportions with its standard error. This expectation was compared against the actual events observed in the sibling cohort with binomial standard error. In preliminary survival analysis, we examined the necessity for including a family-specific frailty to account for different baseline hazard functions in family clusters. There was no statistically significant within-family frailty, thus models without frailty are presented.
We compared 10-year cumulative incidence in siblings with their baseline Framingham 10-year predicted risk using the techniques recommended by the Framingham Heart Study by D’Agostino et al.18 A Cox regression analysis was fitted to the data using the 1998 FRE risk factors as independent variables.14 The coefficient estimates and their standard errors were compared against the coefficients from the standard FRE.14 For these comparisons, we used p < 0.10 as the critical value for significance testing, as recommended by D’Agostino et al.18 Calibration analysis was done using the Hosmer Lemeshow χ2 statistic. The observed and expected numbers of events predicted by the FRE were calculated for every decile of risk. The χ2 statistic was considered significant at χ2 > 20 (p < 0.01) as suggested.18 Also, as recommended by the Framingham Heart Study whenever the FRE is applied to a different population, the FRE was recalibrated specifically to the sibling population.18 Recalibration involved the estimation of two parameters: (1) the mean survival, and (2) the G-statistic. The mean survival was calculated as (1- the observed cumulative 10-year incidence). The G-statistic is a population-specific constant used for the calculation of cumulative survival from the relative hazard function. This method has been used to validate the predictive capacity of the FRE in different ethnic groups.18
Results
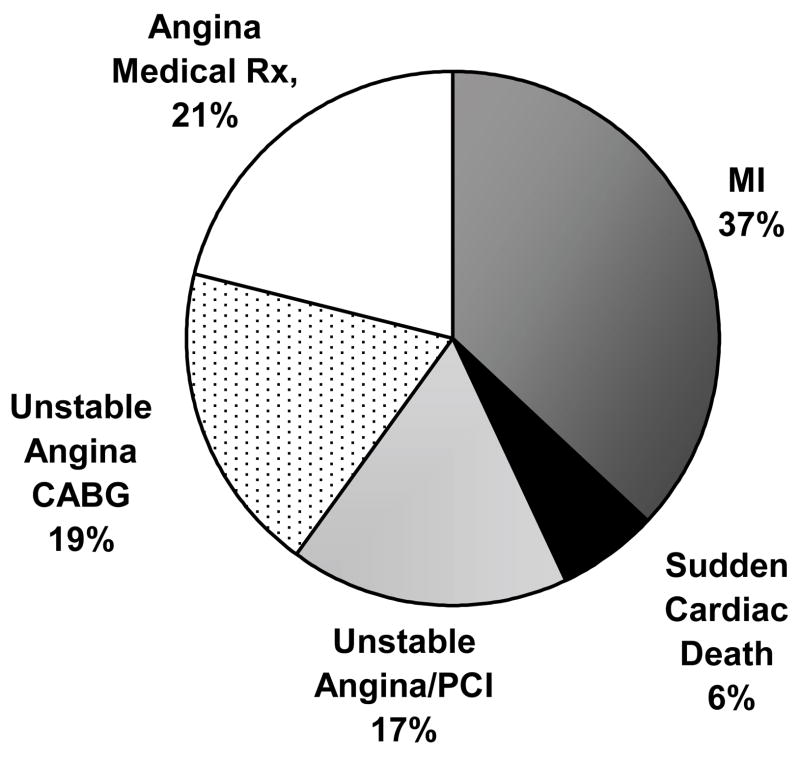
The baseline characteristics of the sibling cohort are shown in Table 1. The sample contained slightly more men. Both sexes were similar to the mean age of the Framingham population in whom the 1998 FRE was calculated (48.6±11.7 years in men and 49.8±12.0 years in women).14 The average total and LDL cholesterol levels were higher than optimal by the current national guidelines.22 There was a high prevalence of hypertension in both sexes, about a third of siblings smoked cigarettes, while a small percentage was diabetic. Over 10 years, 108 events among 784 siblings represented CAD event rates of 20% in men and 7.1% in women. African American men had lower 10-year CAD incidence (10.9%) than Caucasian men (21.0%), p = 0.11. Event rates were much lower in women, with no difference by race, 7.1% in women in both race groups. “Hard CAD events”, i.e., sudden cardiac death and myocardial infarction constituted 42% of events in men and 50% of events in women (Figure 1). Further, 37% of men and 31% of women had unstable angina with revascularization, viz. coronary artery bypass surgery (53% of all revascularizations) or a percutaneous coronary intervention. There were 23 medically treated diagnoses of angina (17% of events in men and 11% of events in women). These were diagnosed by their own physicians and this classification required at least one major coronary vessel with > 50% stenosis on angiography, symptoms, and an abnormal noninvasive test for ischemia. The absolute number of events was lower overall in African Americans, but the distribution of the types of events was similar (in men 40%, and in women 66% of all CAD were “hard events”). Race was neither a confounder nor an effect modifier in any analysis.
Table 1.
Sample characteristics: The Johns Hopkins Sibling Study
| Variable | (Men N=404) | (Women N=380) |
|---|---|---|
| Age (years) | 45.2 ± 7.3 | 46.1 ± 7.4 |
| Black race | 46 (11.3%) | 85 (22.4%) |
| Total cholesterol (mg/dL) | 232.8 ± 42.1 | 232.3 ± 52.7 |
| High Density Lipoprotein cholesterol (mg/dL) | 44.6 ± 12.8 | 56.3 ± 12.1 |
| Low Density Lipoprotein cholesterol (mg/dL) | 149.9 ± 49.4 | 155.1 ± 41.9 |
| Triglycerides (mg/dL) | 104 [74, 161] | 135 [95, 218] |
| Systolic blood pressure (mmHg) | 131.7 ± 16.8 | 134.5 ± 13.8 |
| Diastolic blood pressure (mmHg) | 82.6 ± 9.9 | 86.9 ± 9.4 |
| Hypertension (≥140/90 mmHg) or antihypertensive medication use | 161 (42.4%) | 193 (47.8%) |
| Current smoker | 127 (31.3%) | 132 (34.7%) |
| Diabetes mellitus | 26 (6.4%) | 23 (6.1%) |
Number (%), mean ± SD, or median [interquartile range]
Figure 1.
Percentage of coronary artery disease events comprising the total 10-year coronary disease events, N=108 events
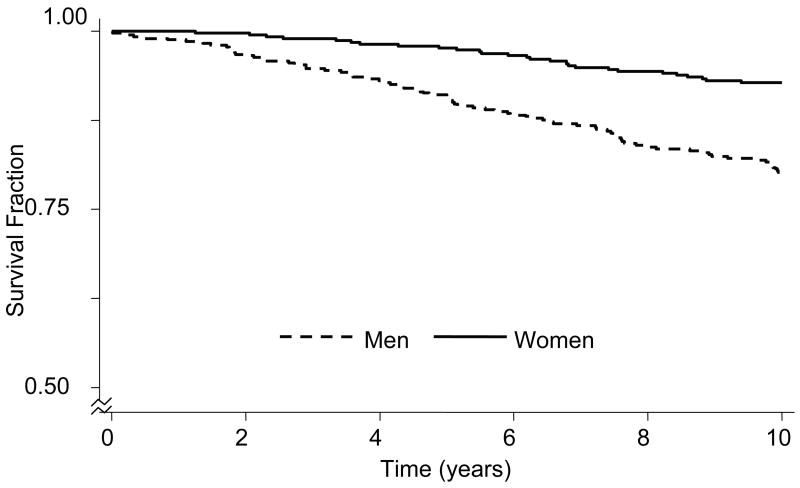
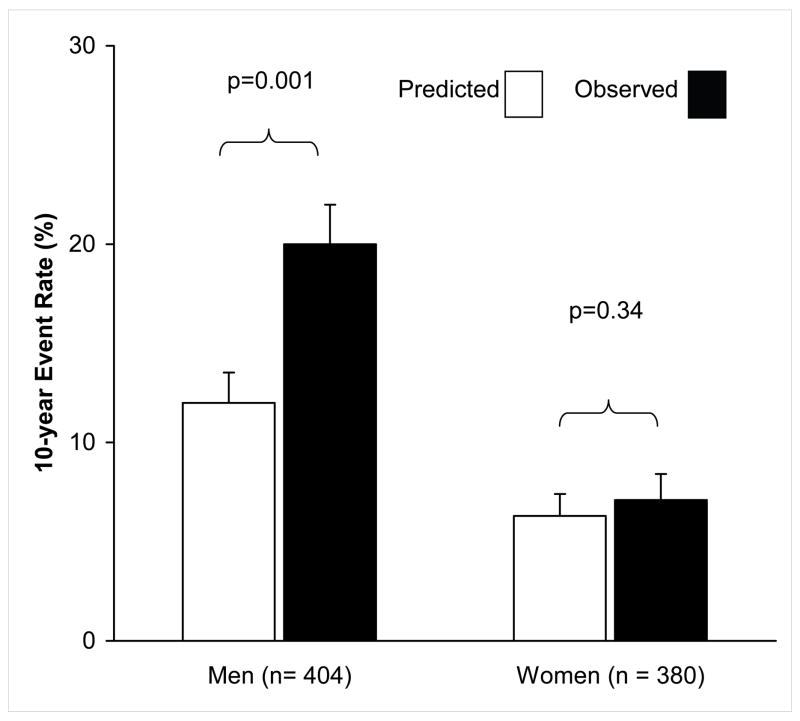
The CAD-event free survival fraction over 10-years is shown by sex on Kaplan-Meier curves in Figure 2. The median time to event (Figure 1) from screening was 5.9 years in men and 6.8 years in women. Event-free survival from the time of screening in men was 99.2% at 1 year, 92.7% at 5 years, and 86.4% at 10 years. At 10 years the CAD-event free survival for women of 92.9% was the same as that for men at 3.6 years. Figure 3 shows the 10-year 1998 FRE-predicted total CAD event incidence in men and women in the Sibling cohort, along with the actual observed 10-year cumulative incidence. The observed total CAD event rate in men was more than double that of women, p < 0.001. The observed incidence of all CAD was 20% in men, nearly double that predicted by their baseline FRE risk, which was 12%, p = 0.001. The observed incidence among women was 7.1%, slightly higher than that predicted by the 1998 FRE (6.3%), although this was not statistically significant, p = 0.34. Thus, there was a statistically significant 66.5% observed CAD excess risk over that predicted by the 1998 FRE in men, and a 12.5% nonsignificant excess risk in women over that predicted by the FRE. The coefficients of the Cox proportional hazards models predicting observed events in siblings and the coefficients incorporated in the 1998 FRE14 risk scoring prediction from baseline are shown in Table 2. In men, the categories of diastolic blood pressure of 90–99 mmHg and systolic blood pressure of 140–159 mmHg, and very low HDL cholesterol (< 35 mg/dL) differed significantly (p<0.1) from the published 1998 FRE coefficients. Both of these predictors were associated with a lower relative hazard of events than the FRE. Among women, very high total cholesterol (≥280 mg/dL) demonstrated a greater relative hazard for predicting observed events among siblings as compared to the relative hazard for the Framingham cohort based on the FRE, p=0.06.14 The relative hazards of all other parameters were similar in the sibling study as in the 1998 FRE.
Figure 2.
Kaplan-Meier survival curves over 10-year follow-up, by sex, n=404 men, 380 women
Figure 3.
The predicted 10-year event rate using the Framingham Risk Equation and observed the 10-year coronary artery disease event rate in the sibling cohort by sex.
Table 2.
Cox multivariate regression analysis coefficients for the risk factor categories used in the Framingham Risk Equation estimated from the sibling cohort compared with those published for the Framingham cohort (1998)
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Variable | Siblings (N=404) | Framingham (N=2489) | p | Siblings (N=380) | Framingham (N=2856) | p |
| Age (years) | 0.058±0.017 | 0.048±0.005 | 0.583 | 0.071±0.431 | 0.338±0.075 | 0.543 |
| Age2 (years2) | -* | -* | -* | −0.000±0.005 | −0.003±0.001 | 0.593 |
| Diastolic Blood Pressure < 80 and Systolic Blood Pressure < 120 mmHg | 0.930±0.639 | −0.002±0.194 | 0.164 | −0.413±0.890 | −0.534±0.257 | 0.896 |
| Diastolic Blood Pressure = 80–84 or Systolic Blood Pressure = 120–129 mmHg | REF | REF | REF | REF | ||
| Diastolic Blood Pressure = 85–89 or Systolic Blood Pressure = 130–139 mmHg | 1.091±0.507 | 0.283 ±0.172 | 0.131 | 0.399±0.643 | −0.068±0.232 | 0.494 |
| Diastolic Blood Pressure = 90–99 or Systolic Blood Pressure= 140–159 mmHg | 1.153±0.488 | 0.522±0.159 | 0.049 | −0.080±0.653 | 0.263±0.205 | 0.617 |
| Diastolic Blood Pressure > 100 or Systolic Blood Pressure > 160 mmHg | 1.502±0.543 | 0.619±0.174 | 0.122 | 1.106±0.664 | 0.466±0.219 | 0.360 |
| Total Cholesterol < 160 mg/dL | −0.039±0.771 | −0.659±0.320 | 0.458 | REF ** | −0.261±0.535 | NS*** |
| Total Cholesterol = 160–199 mg/dL | REF | REF | REF | |||
| Total Cholesterol = 200–239 mg/dL | 0.065±0.354 | 0.177±0.133 | 0.766 | 1.975±1.067 | 0.208±0.209 | 0.104 |
| Total Cholesterol = 240–279 mg/dL | 0.561±0.349 | 0.505±0.148 | 0.884 | 0.244±0.220 | 1.139±1.172 | 0.453 |
| Total Cholesterol >280 mg/dL | 0.575±0.366 | 0.657±0.194 | 0.842 | 2.616±1.072 | 0.535±0.236 | 0.058 |
| High Density Lipoprotein cholesterol < 35 mg/dL | −0.202±0.362 | 0.497± 0.177 | 0.083 | −0.854±1.115 | 0.843±0.248 | 0.137 |
| High Density Lipoprotein cholesterol 35–44 mg/dL | −0.118±0.317 | 0.243±0.167 | 0.313 | 0.389±0.525 | 0.378±0.192 | 0.985 |
| High Density Lipoprotein cholesterol 45–49 mg/dL | REF | REF | - | 0.115±0.637 | 0.198±0.212 | 0.902 |
| High Density Lipoprotein cholesterol 50–59 mg/dL | −0.258±0.367 | −0.051±0.188 | 0.617 | REF | REF | - |
| High Density Lipoprotein ≥ 60 mg/dL | −0.702±0.517 | −0.487±0.243 | 0.706 | −0.458±0.602 | −0.430±0.184 | 0.964 |
| Current smoker | 0.343±0.258 | 0.523±0.105 | 0.516 | 0.801±0.435 | 0.292±0.143 | 0.266 |
| Diabetes mellitus | 0.505±0.369 | 0.428±0.178 | 0.851 | 1.098±0.555 | 0.596±0.214 | 0.399 |
The quadratic term for age is not included for men in the Framingham Risk Equation
There were no events in women with TC < 160 mg/dL in the sibling cohort, so this coefficient cannot be calculated. Thus women with TC < 160 mg/dL and TC 160–199 are pooled as the reference category.
One sample test using 95% confidence intervals with null hypothesis that coefficient = 0 (because these women are included in reference category for the sibling cohort analysis)
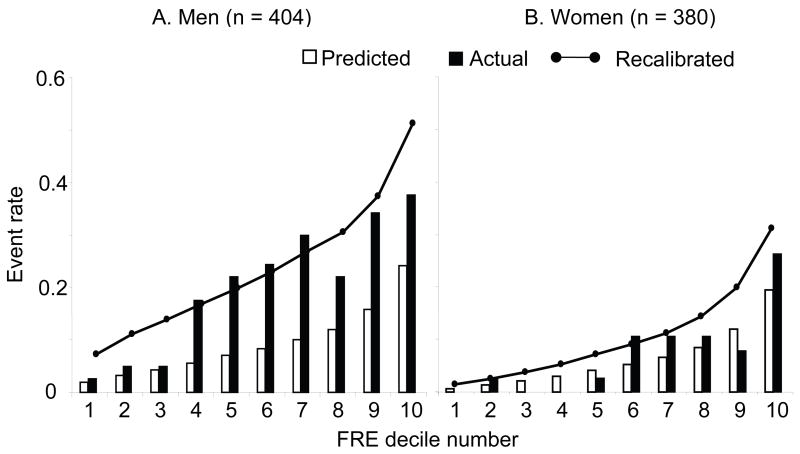
The average risk for each decile of the baseline 1998 FRE is plotted along with the actual observed number of total incident CAD events within each decile. (Figure 4) The Hosmer-Lemeshow χ2 statistic for women was 8, indicating adequate fit, while for men the statistic was 75 (20 is the maximal threshold for a good fit18). Thus the FRE was a poor fit for predicting actual events in men, most notably above the 3rd decile of 1998 FRE predicted risk, or in the highest risk subsets. The 1998 FRE prediction was reasonably close to, but still somewhat higher than actual events when the baseline predicted FRE was < 6.8% in men. After recalibration of the FRE,18 the Hosmer-Lemeshow χ2 statistic was 9 in men (with the recalibration constants: G-statistic - 3.07; average 10-year survival - 0.802), showing improvement in the average overall fit of the 1998 FRE-predicted model to the actual events, although there was no improvement in fit in women (with the recalibration constants: G-statistic – 10.02; average 10-year survival - 0.929). While the average overall fit is improved, the recalibration line plotted on Figure 4 shows that the recalibrated FRE prediction in men overestimates incident CAD events in the lowest and highest risk groups and underestimates observed events in the intermediate risk groups. Thus, recalibration only improves the average prediction in men but cannot be applied to any single risk group accurately. Recalibration in women overall was close to the observed and the original predictions of CAD and did not on average improve the model fit to the original FRE (Hosmer-Lemeshow χ2 = 9), as shown on the recalibrated prediction line in Figure 4.
Figure 4.
Comparison of predicted and observed coronary artery disease event rate by deciles of baseline Framingham 10-year risk, by sex. FRE Baseline Deciles in men: 1st: ≤4.0%, 2nd: 4.1–5.803%, 3rd: 5.804–6.81%, 4th: 6.85–8.41%, 5th: 8.42–10.00% 6th: 10.01–11.70%, 7th: 11.72–14.00%, 8th: 14.08–16.41%, 9th: 16.43–21.86%, 10th: ≥22.16%. FRE Baseline Deciles in women: 1st: ≤1.0%, 2nd: 1.31–1.63%, 3rd: 1.66–2.50%, 4th: 2.53–3.56%, 5th: 3.58–4.58% 6th: 4.62–5.67%, 7th: 5.70–7.31%, 8th: 7.32–9.74%, 9th: 9.97–13.76%, 10th: ≥14.16%. The predicted event rate for each baseline FRE decile was the mean FRE within that decile. The observed rate was the actual cumulative event rate at 10-years within each decile. Using the higher cohort-specific average event rate and the cohort-specific offset for mean cohort risk characteristics (G-statistic), a recalibrated Framingham score was calculated. The nodes on the solid line represent the mean recalibrated Framingham risk score for each decile.
Discussion
Our 10-year prospective study indicates that the 1998 FRE used to calculate 10-year predicted risk of total CAD events14 markedly underestimates the observed incidence of total CAD events in initially healthy young brothers of patients with documented premature CAD. However, in women, where the number of incident CAD rate is quite low over 10 years in this cohort, our findings indicate that the FRE derived from the Framingham Heart Study cohort14 slightly and not significantly underestimated the observed 10-year risk of CAD. Framingham investigators have previously applied recalibration methods for the FRE to verify the applicability to different populations including African Americans, Native Americans, Japanese Americans, and Hispanic men.18 Among siblings, though the FRE recalibration improved the average prediction of total CAD events in the entire cohort of men, it markedly overestimated observed events at the lowest and highest deciles of baseline FRE, while underestimating risk in the middle of the range. Recalibration appears to represent a statistical manipulation possible only after the cumulative incidence is determined in a population. Thus it does not improve the true estimate of risk for any individual or any particular risk classification. Thus, it is reasonable to conclude that the 1998 FRE and any recalibration recommended is not adequate to predict 10-year risk of total incident CAD in a population with a sibling history of premature CAD, and further, that the true risk is significantly higher than would be expected based on traditional risk factor profiles. Indeed, in the Framingham Offspring Study’s 8-year prospective examination of middle-aged Caucasian adults (mean age 57 years), siblings of an individual with CAD compared to subjects whose siblings did not have CAD, had a risk factor-adjusted relative risk of 1.45 (95% CI, 1.10–1.91).12 This suggests an excess risk of 45% that was not explained by traditional 1998 FRE risk factor categories. However, only whites were included and the follow-up was of a shorter duration. Our study extends the observation of increased sibling risk to a broader population.
The National Cholesterol Education Program’s Adult Treatment Panel III Guidelines emphasize the prediction of only “hard events” (myocardial infarction and sudden death) to classify 10-year CAD risk.23 However, it is important to predict total CAD events in individuals with a family history who are at far higher risk in 10 years than the general population. As many as half of family members in whom actual risk of incident CAD is high would not qualify for either preventive chemoprophylaxis or lifestyle changes under current guidelines. This is a function both of the fact that the global risk estimation, which is used to set thresholds and goals for therapy, does not address the importance of the many “non-hard” but definite CAD events that are observed in premature CAD families, and the fact that CAD events occur far in excess of what would be predicted based on traditional risk factors alone.
The majority of events (78%) in both men and women were myocardial infarctions, sudden cardiac deaths, or were of such symptomatic and anatomic severity that they required revascularization procedures. In the 1998 Framingham analysis14 the proportion of hard events among total events varied between 33% (baseline age 30–34 years) and 81% (baseline age 55–59 years) in men, and between 50% (baseline age 40–44 years) and 58% (baseline age 55–59 years) in women. Thus the high incidence of events we observed in male siblings at an average age of 45 ± 7 years cannot be attributed to a larger number of stable medically managed angina pectoris diagnoses among this population as compared to the population of the 1998 Framingham analyses.14 The time to event after screening was sufficiently long that the excess event rate was unlikely to result from intensive scrutiny immediately following screening.
One of the limitations of this study is that a smaller number of events may have occurred in women because they were not at the same “risk age” for CAD events as men. Had we extended the age to 65 years for identifying female probands or had female siblings been 10 years older than male siblings at baseline, more women in this cohort may have suffered events. Longer follow-up is needed to determine if the modest FRE underestimation bias persists, is magnified, or disappears as female siblings age. For both sexes, a specific proband ascertainment age (<60 years) may misclassify the biological substrate, with some high familial risk CAD expressing later, and some sporadic CAD expressing earlier than the threshold. This would likely lead to a bias towards no difference by sibling history. Further, any baseline prediction of CAD events in a cohort cannot take into account changes in medication and risk behaviors after the predictive calculation, and averages the secular trend over the cohort. However, this may be more relevant in preventive practice where an individual’s future trajectory is unknown. Finally, the number of African-Americans did not allow for race-specific recalibration. However, recalibration was shown to be not necessary in African-Americans compared to whites.18
There are many possible explanations for the excess CAD risk observed in premature CAD families. Novel phenotypic coronary disease risk factors,24–26 and those as yet undiscovered, may cause premature CAD to cluster in families and play a greater role than in the general population. Additionally, genetic susceptibility almost certainly plays a role in families with premature disease.27,28 In addition, these families may experience unknown shared environmental factors that cause the disease to occur at an earlier than usual age. This study provides a clear mandate to investigate genetic and, environmental factors as well as gene-environment interactions that may cause accelerated premature CAD to cluster in families, particularly in siblings.
Acknowledgments
This research was supported by the NIH Grants NR02241, R01 HL49762, R01 HL59684, and the Johns Hopkins University School of Medicine General Clinical Research Center, NIH grant M01 RR00052 (National Institutes of Health, Bethesda, MD, USA).
The authors thank Drs. Gary Gerstenblith and Steven P. Schulman for their assistance in the conduct of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Williams RR. Understanding genetic and environmental risk factors in susceptible persons. West J Med. 1984;141:799–806. [PMC free article] [PubMed] [Google Scholar]
- 3.Rissanen AM. Familial occurrence of coronary heart disease: effect of age at diagnosis. Am J Cardiol. 1979;44:60–66. doi: 10.1016/0002-9149(79)90251-0. [DOI] [PubMed] [Google Scholar]
- 4.Rissanen AM, Nikkila EA. Identification of the high-risk groups in familial coronary heart disease. Atherosclerosis. 1984;53:37–46. doi: 10.1016/0021-9150(84)90103-5. [DOI] [PubMed] [Google Scholar]
- 5.Horne BD, Camp NJ, Muhlestein JB, Cannon-Albright LA. Identification of excess clustering of coronary heart diseases among extended pedigrees in a genealogical population database. Am Heart J. 2006;152:305–311. doi: 10.1016/j.ahj.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 6.Persson B, Johansson BW. The Kockum study: twenty-two-year follow-up. Coronary heart disease in a population in the south of Sweden. Acta Med Scand. 1984;216:485–493. [PubMed] [Google Scholar]
- 7.Williams RR, Hunt SC, Heiss G, Province MA, Bensen JT, Higgins M, Chamberlain RM, Ware J, Hopkins PN. Usefulness of cardiovascular family history data for population-based preventive medicine and medical research (the Health Family Tree Study and the NHLBI Family Heart Study) Am J Cardiol. 2001;87:129–135. doi: 10.1016/s0002-9149(00)01303-5. [DOI] [PubMed] [Google Scholar]
- 8.Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330:1041–1046. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- 9.Silberberg JS, Wlodarczyk J, Fryer J, Robertson R, Hensley MJ. Risk associated with various definitions of family history of coronary heart disease. The Newcastle Family History Study II. Am J Epidemiol. 1998;147:1133–1139. doi: 10.1093/oxfordjournals.aje.a009411. [DOI] [PubMed] [Google Scholar]
- 10.Ciruzzi M, Schargrodsky H, Rozlosnik J, Pramparo P, Delmonte H, Rudich V, Piskorz D, Negri E, Soifer S, La Vecchia C. Frequency of family history of acute myocardial infarction in patients with acute myocardial infarction. Argentine FRICAS (Factores de Riesgo Coronario en America del Sur) Investigators. Am J Cardiol. 1997;80:122–127. doi: 10.1016/s0002-9149(97)00304-4. [DOI] [PubMed] [Google Scholar]
- 11.Bertuzzi M, Negri E, Tavani A, La Vecchia C. Family history of ischemic heart disease and risk of acute myocardial infarction. Prev Med. 2003;37:183–187. doi: 10.1016/s0091-7435(03)00094-x. [DOI] [PubMed] [Google Scholar]
- 12.Murabito JM, Pencina MJ, Nam BH, D’Agostino RB, Sr, Wang TJ, Lloyd-Jones D, Wilson PW, O’Donnell CJ. Sibling cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults. JAMA. 2005;294:3117–123. doi: 10.1001/jama.294.24.3117. [DOI] [PubMed] [Google Scholar]
- 13.Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83:356–362. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- 14.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 15.Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation. 2002;105:310–315. doi: 10.1161/hc0302.102575. [DOI] [PubMed] [Google Scholar]
- 16.Hingorani AD, Vallance P. A simple computer program for guiding management of cardiovascular risk factors and prescribing. BMJ. 1999;318:101–105. doi: 10.1136/bmj.318.7176.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joint British recommendations on prevention of coronary heart disease in clinical practice. British Cardiac Society, British Hyperlipidaemia Association, British Hypertension Society, endorsed by the British Diabetic Association. Heart. 1998;80(Suppl 2):S1–S29. [PMC free article] [PubMed] [Google Scholar]
- 18.D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20.The 1980 report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1980;140:1280–1285. [PubMed] [Google Scholar]
- 21.Kannel WB, Wolf PA, Garrison RJ. Monograph Section 34: Some Risk Factors Related to the Annual Incidence of Cardiovascular Disease and Death Using Pooled Repeated Biennial Measurements: Framingham Heart Study, 30-Year Followup. Springfield, MA: National Technical Information Service; 1987. pp. 1–459. [Google Scholar]
- 22.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 23.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 24.Reinhard W, Holmer SR, Fischer M, Gloeckner C, Hubauer U, Baessler A, Mayer B, Schunkert H, Riegger GA, Hengstenberg C. Association of the metabolic syndrome with early coronary disease in families with frequent myocardial infarction. Am J Cardiol. 2006;97:964–967. doi: 10.1016/j.amjcard.2005.10.063. [DOI] [PubMed] [Google Scholar]
- 25.Boyar A. Creating a web application that combines Framingham risk with Electron Beam CT Coronary Calcium Score to calculate a new event risk. J Thorac Imaging. 2006;21:91–96. doi: 10.1097/01.rti.0000185141.27738.63. [DOI] [PubMed] [Google Scholar]
- 26.Scheuner MT, Whitworth WC, McGruder H, Yoon PW, Khoury MJ. Familial risk assessment for early-onset coronary heart disease. Genet Med. 2006;8:525–531. doi: 10.1097/01.gim.0000232480.00293.00. [DOI] [PubMed] [Google Scholar]
- 27.Shiffman D, Ellis SG, Rowland CM, Malloy MJ, Luke MM, Iakoubova OA, Pullinger CR, Cassano J, Aouizerat BE, Fenwick RG, Reitz RE, Catanese JJ, Leong DU, Zellner C, Sninsky JJ, Topol EJ, Devlin JJ, Kane JP. Identification of four gene variants associated with myocardial infarction. Am J Hum Genet. 2005;77:596–605. doi: 10.1086/491674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer B, Erdmann J, Schunkert H. Genetics and heritability of coronary artery disease and myocardial infarction. Clin Res Cardiol. 2006;96:1–7. doi: 10.1007/s00392-006-0447-y. [DOI] [PubMed] [Google Scholar]