Abstract
Background & Aims
Heme oxygenase-1 (HO-1) is an antioxidant defense and key cytoprotective enzyme, which is repressed by Bach1. MicroRNA-122 (miR-122) is specifically expressed and highly abundant in human liver and required for replication of hepatitis C virus (HCV) RNA. This study was to assess whether a specific miR-122 antagomir down-regulates HCV protein replication and up-regulates HO-1.
Methods
We transfected antagomir of miR-122, 2′-O-methyl-mimic miR-122, or non-specific-control antagomir (NSCA) into wild type Huh-7 cells or Huh-7 stably replicating HCV subgenomic core-NS3 (CNS3 replicon cells), or NS3-5B (9–13 replicon cells).
Results
Antagomir of miR-122 reduced the abundance of HCV-RNA by 64% in CNS3, and by 84% in 9–13 cells. In contrast, transfection with 2′-O-methlyl-mimic miR-122 increased HCV levels up to 2.5-fold; transfection with NSCA did not change the level of HCV. Antagomir of miR-122 also decreased Bach1 and increased HO-1 mRNA levels in CNS3, 9–13, and WT Huh-7 cells. Increasing HO-1 by silencing Bach1 with 50 nM Bach1-siRNA or by treatment with 5 μM cobalt protoporphyrin or heme (known inducers of HO-1) decreased HCV RNA and protein by 50% in HCV replicon cells.
Conclusions
Down-regulation of HCV replication using an antagomir targeted to miR-122 is effective, specific, and selective. Increasing HO-1, by silencing the Bach1 gene or by treatment with cobalt protoporphyrin or heme, decreases HCV replication. Thus, miR-122 plays an important role in the regulation of HCV replication and HO-1/Bach1 expression in hepatocytes. Down-regulation of miR-122 and up-regulation of HO-1 may be new strategies for anti-HCV intervention and cytoprotection.
Keywords: Bach1, hepatitis C, heme oxygenase-1, Huh-7, miR-122
Hepatitis C virus (HCV) is a major cause of chronic liver disease, with an estimated 170 million people infected world-wide and approximately 4 million infected in the USA (1). Chronic hepatitis C (CHC) is the major cause for liver transplantations in the USA (2). Currently, there is no approved, effective vaccine for HCV, and current treatments are expensive, unpleasant, and relatively ineffective. Thus, there is a pressing need for improved therapeutic intervention. The mechanisms underlying HCV-associated liver injury are imperfectly understood. Several lines of evidence indicate that increased oxidative stress is an important pathogenetic mechanism in CHC (3–9). HCV core and nonstructural proteins have been shown to cause transcriptional modulation as either trans-activators or trans-suppressors of cellular signaling pathways through interference with intracellular oxidation/reduction reactions (10–12). The HCV core protein alters mitochondrial function and results directly in an increase in the cellular abundance of reactive oxygen species (ROS) with consequent increases in cellular lipid peroxidation (13–16). These results provide new insight into how chronic HCV infection leads to hepatic injury, and provide a firm theoretical basis for the investigation of antioxidant and cytoprotective therapies in this important disease.
HO-1 is a key cytoprotective enzyme and the rate-limiting enzyme involved in heme degradation to bilirubin, carbon monoxide (CO), and iron (17–20). Multiple lines of evidence support the idea that biliverdin, bilirubin, and CO contribute to the physiological functions of HO-1, including anti-oxidative, anti-inflammatory, anti-proliferative, and anti-apoptotic effects (21–27). HO-1 is widely distributed in tissues and robustly induced in many cells by its substrate heme (28–30) and by numerous stressful stimuli such as ultraviolet radiation, heat shock, inflammation, hypoxia, and various oxidative agents (31–37). The physiological function of HO-1 has been confirmed by observations in HO-1 knockout mice (38; 39) and in one child, the product of a consanguineous mating, with severe genetic deficiency of HO-1 activity (40; 41). Hence, the induction of HO-1 is one of the important events in cellular response to pro-oxidative and pro-inflammatory insults, and also provides potent cytoprotective and anti-oxidative effects as assessed in various models in vitro and in vivo (21–27). Recent work by us and others indicates that Bach1, a known heme-binding protein (42–44), forms heterodimers with small proteins of the Maf family, and that these heterodimers repress transcription of the HO-1 gene by binding to the heme responsive elements (HeRE) in the 5′-UTR of the HO-1 promoter. We more recently reported that heme-mediated induction of HO-1 gene expression occurs via Bach1 and Nrf2 (a basic leucine zipper transcription factor) in human Huh-7 cells. By using Bach1-siRNA to silence the Bach1 gene, we demonstrated that up-regulation of HO-1 gene expression by heme was markedly increased in these cells (45).
MicroRNAs (miRNA) are a class of small RNA molecules involved in regulation of translation and degradation of mRNAs (46). The miRNAs typically interact with sequences within the 3′ non-coding region (NCR) of target mRNAs. Certain miRNAs are expressed ubiquitously, whereas others are expressed in a highly tissue-specific manner (47; 48). miR-122 is expressed at high levels in the liver with more than 50,000 copies per cell, where it constitutes 70% of the total miRNA population (47; 49; 50). Although it was first thought that miRNA acted solely to down-regulate gene expression (51; 52), recent results indicate that these small RNA’s, in fact, produce both up- and down-regulation of different genes (53–55). Thus, their effects are multidimensional, and we still have much to learn about their global effects on cells. To study the biochemistry and biological function of miRNA, miRNA inhibitors and mimics can be designed based on known or predicted miRNA sequences. Recently, Jopling et al reported that miR-122 has two predicted binding sites in the HCV genome (53). The putative miR-122 binding site in the 5′-NCR of HCV genome is required for miR-122 to affect HCV RNA abundance. Surprisingly, they failed to find that the putative 3′-NCR miR-122 binding site affected HCV RNA replication. They reported that miR-122 neither affects HCV RNA stability nor RNA translation, but more likely facilitates replication of the viral RNA. The possibility is not ruled-out that miR-122 and its antagomir also act indirectly, for example, by leading to up-regulation of non-HCV genes, which, in turn, alter HCV gene expression.
Recently we reported that the expression of hepatic HO-1 mRNA is increased in HCV replicon cells (56). In this study, we demonstrate that a chemically synthesized oligonucleotide, with sequence complementary to miR-122 (miR-122 antagomir), markedly inhibited HCV RNA replication in two Huh-7-derived HCV replicon cell lines. The antagomir up-regulated the expression of the HO-1 gene in Huh-7 wild type cells, and in Huh-7 cells expressing HCV proteins. Knockdown of the Bach1 gene, using Bach1-siRNA, increased HO-1 and decreased HCV replication in both replicon cell lines.
Materials and Methods
Materials
The human hepatoma cell line, Huh-7, was purchased from the Japan Health Research Resources Bank (Osaka, Japan). Ferric (Fe+3)-protoporphyrin IX•Cl (hemin) and cobalt protoporphyrin (CoPP) were from Porphyrin Products (Logan, UT). Goat anti-human GAPDH antibody was purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Mouse anti-hepatitis C core protein was purchased from Affinity BioReagents, Inc (Golden, CO). Mouse anti-HCV NS5a was purchased from Virogen (Plantation, FL). Rabbit anti-human HO-1 antibody was purchased from Stressgen (Victoria, BC Canada). ECL-Plus was purchased from Amersham Biosciences Corp (Piscataway, NJ). Chemically synthesized antagomir of miR-122, non-specific control antagomir (NSCA), 2′-O-methyl-mimic miR-122, and Bach1-siRNA were purchased from Dharmacon (Lafayette, CO). iScript Reaction Mix and SYBR Green Mix were purchased from Bio-Rad Laboratories (Hercules, CA). CNS3 and 9-13 replicon cells were gifts from Dr. R. Bartenschlager (University of Heidelberg, Heidelberg, Germany).
Cell Cultures
Huh-7 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) as previously reported (57; 58). CNS3, R-Luc, and 9–13 cells were cultured with additional antibiotics (10 μg/ml Zeocin, 5 μg/ml Zeocin, or 1 mg/ml G418), respectively. CNS3 cells stably express HCV subgenomic proteins core-NS3 and were generated by transduction of WT Huh-7 cells with a retroviral vector (pRV-CNS3-IGZ) carrying an expression cassette for HCV core-NS3 region encompassing nucleotide positions 342–4040 of the Con1 isolate (Fig 1A) (56; 59). Clone 9–13 carries a stably replicating HCV subgenomic (NS3-5B) replicon (Fig. 1A) (60). The appropriate control for CNS3 and 9–13 is R-Luc, which is a stable Huh-7 cell line that expresses a Renilla luciferase reporter protein due to transduction with the retroviral vector pRV-R-Luc-IGZ.
Figure 1. Organization of the HCV constructs CNS3 and 9–13.
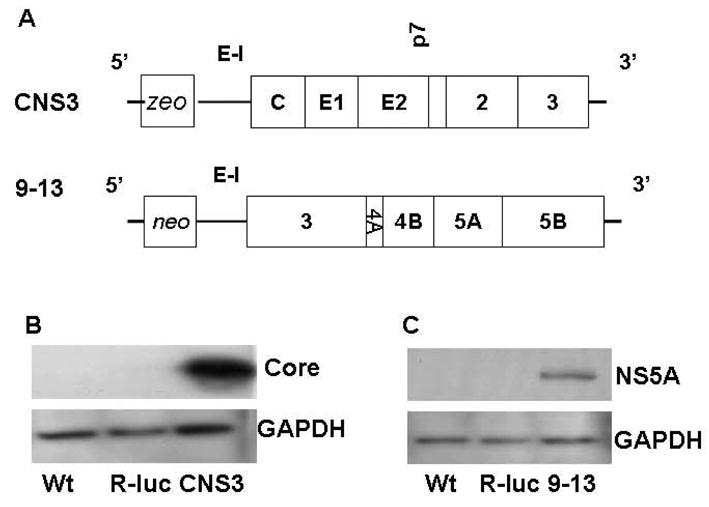
(A). Open reading frames (thick boxes) are flanked by the 5′- and 3′-UTRs (thin boxes). CNS3 is the Huh-7 cell line that expresses a zeo resistance gene (zeo), the IRES of the encephalomyocarditis virus (E–I), which directs translation of HCV sequences from core, envelop (E1 and E2), up to the aminoterminal domain of NS3. Replicon 9–13 is composed of HCV-IRES, the neomycin phosphotransferase (neo) gene, E–I, HCV sequences from NS3 through NS5B, and the 3′-NTR. Panels B and C are Western blot analysis of the HCV core, NS5A and GAPDH proteins from CNS3 and 9–13 replicon cell lines. 50 μg of protein were separated by 4–15% SDS-polyacrylamide gel, transferred to a PVDF membrane, and probed with anti-HCV core, anti-NS5A, and GAPDH specific antibodies. Representative results from one of three experiments are shown.
Transfections
Huh-7 WT, CNS3, 9–13 cells were transfected with antagomir of miR-122, 2′-O-methyl-mimic miR-122, Bach1-siRNA, or NSCA as described previously (57; 58). In brief, cells were plated the day before transfections and grown to 50% confluence in 12-well plates. 0–50 nM antagomir of miR-122, 2′-O-methyl-mimic miR-122, Bach1-siRNA, or NSCA were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. Transfected cells were grown for 24–72 hours in a 37 °C incubator with 5% CO2, after which, cells were harvested. Total RNA and protein were extracted, and stored at −80°C for subsequent quantitative RT-PCR or Western Blot measurements. Each treatment used at least triplicate samples.
Quantitative RT-PCR
Total RNA from treated cells was extracted and quantitative RT-PCR was performed as described before (45). Primers used for HCV core, HO-1, Bach1, and GAPDH quantitative PCR were described before (45; 56; 58). Primers used for NS5A were: forward primer, 5′-CGGACGTAGCAGTGCTCACTTC; reverse primer, 5′-CGGAAGCTGGGATGGTCAAAC. We included samples without template as negative controls, which, as expected produced negligible signals (Ct values > 35). Standard curves of HCV core, NS5A, HO-1, Bach1, and GAPDH were constructed with results of parallel PCR reactions performed on serial dilutions of a standard DNA (from one of the controls). Fold-change values were calculated by comparative Ct analysis after normalizing for the quantity of GAPDH mRNA.
Western Blot
Protein preparation and Western blots were as described before (45). In brief, total proteins (30–50 μg) were separated on 4–15% gradient SDS-polyacrylamide gels (Bio-Rad). After electrophoretic transfer onto PVDF membrane (Bio-Rad), membranes were blocked for 1 hour in PBS containing 5% nonfat dry milk and 0.1% Tween-20, and then incubated for 1 hour with primary antibody at room temperature. The dilutions of the primary antibodies were as follows: 1:500 for the NS5A antibody, and 1:2000 for anti-HCV core, and anti-GAPDH antibodies. The membranes were then incubated for 1 hour with horseradish peroxidase-conjugated secondary antibodies (dilution 1:10,000). Finally, the bound antibodies were visualized with the ECL-Plus chemiluminescence system according to the manufacturer’s protocol. A Kodak 1DV3.6 computer-based imaging system (Eastman-Kodak, Rochester, NY) was used to measure the relative optical density of each specific band obtained. Data are expressed as percentage of the control (corresponding to the value obtained with non-transfected cells), which were assigned a value of one.
Statistical analyses of data
Experiments were repeated at least three times with similar results. Except for Western blots, all experiments included at least triplicate samples for each treatment group. Representative results from single experiments are presented. Statistical analyses were performed with JMP 4.0.4 software (SAS Institute, Cary, NC). Differences in mean values were assessed by analysis-of-variance techniques (ANOVA), with the Tukey-Kramer correction for multiple pairwise comparisons, or Dunnett’s test versus a control. Values of P <0.05 were considered significant.
Results
Down-regulation of HCV RNA and protein expression by antagomir of miR-122 in CNS3 and 9–13 replicon cells
miR-122 is specifically expressed and highly abundant in the human liver, where it constitutes 70% of the total miRNA population (47; 50). There are two predicted binding sites for miR-122 in HCV viral 3′ and 5′ NCRs (53; 55). To assess down-regulation of antagomir of miR-122 on HCV expression, Huh-7 cells stably replicating HCV subgenomic (NS3-5B) replicon were used (Fig. 1A). First, we compared the two cell lines stably replicating HCV core-NS3 or NS3-NS5B proteins with wild type Huh-7 cells and cells not expressing HCV proteins (R-Luc) as negative control (Fig. 1B&C). Next, we determined whether miR-122 inactivation would alter the abundance of HCV RNA replication in CNS3 and 9–13 replicon cells. To inactivate miR-122, CNS3 and 9–13 cells were transfected with 0–50 nM of chemically synthesized antagomir of miR-122 for 48 or 72 hours. The level of HCV mRNA in the cells without miR-122 antagomir was set equal to 1. The miR-122 antagomir reduced the abundance of HCV core RNA by 64% in CNS3 (Fig 2A, P<0.01), and by 84% in 9–13 replicon cells (Fig 2B, P<0.01). The decreases were dose-dependent and time-related. Exposure of HCV replicon to the miR-122 antagomir for 72 hours produced a greater decrease of HCV RNA expression than exposure for 48 hours (Fig. 2C&D, P<0.05).
Figure 2. An antagomir of miR-122 reduces HCV RNA abundance in dose- and time-dependent manner in HCV replicon cells.
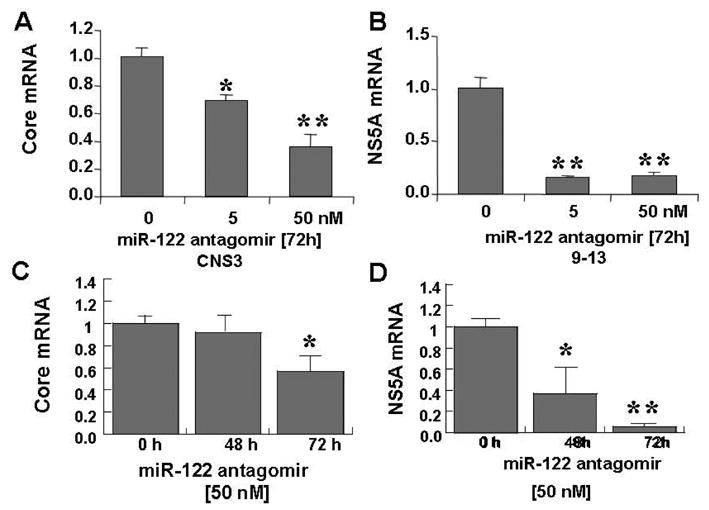
Chemically synthesized miR-122 antagomir was introduced into CNS3 and 9–13 replicon cells by Lipofectamine 2000-mediated transfection, and total RNA was extracted 48 or 72 h later. qRT-PCR analysis of HCV core (A) and NS5A (B) mRNAs of cells treated with the indicated dose of miR-122 antagomir for 72 h as shown. Time-courses of replication of HCV core (C) and NS5A (D) treated with 50 nM of miR-122 antagomir are shown. Normalized values of miRNA in absence of miR-122 antagomir were set equal to 1. Data are presented as means ± SE from three samples. * differs from no treatment or zero time, p<0.05, ** differs from no treatment or zero time, p< 0.01.
Using this chemically synthesized antagomir of miR-122, we also successfully down-regulated expression of NS5A protein in 9–13 replicon cells. As little as 5 nM of miR-122 markedly decreased HCV NS5A protein expression (Fig. 3). Thus, miR-122 plays a role to down-regulate HCV replication in both of the HCV replicon cell lines tested.
Figure 3. An antagomir of miR-122 reduces HCV protein abundance in HCV replicon cells.
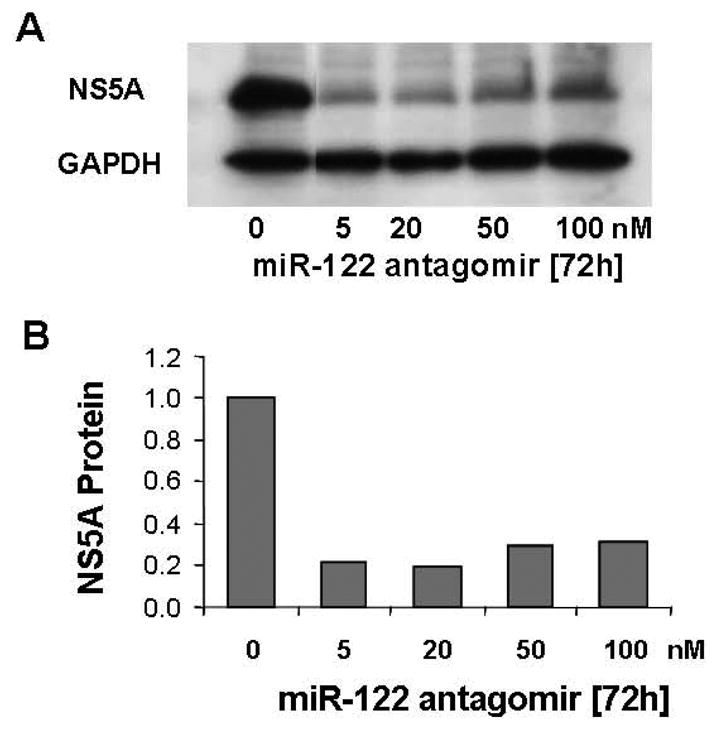
Chemically synthesized miR-122 antagomir (50 nM) was introduced into 9–13 cells using Lipofectamine 2000-mediated transfection, and total protein was extracted 72 h later. Western blot analysis of HCV NS5A protein treated with the indicated doses of miR-122 antagomir is shown in panel A. 50 μg of protein were separated by 4–15% SDS-polyacrylamide gel, transferred to a PVDF membrane, and probed with anti-NS5A and GAPDH specific antibodies. Representative results from one of three experiments are shown. The relative NS5A protein amounts normalized to GAPDH, the invariant control, are shown in panel B. No miR-122 antagomir was set equal to 1.
Specific regulation of HCV RNA levels with miR-122 antagomir in 9–13 replicon cells
To confirm the specificity of gene silencing by the antagomir of miR-122, we tested HCV NS5A mRNA expression from the cells transfected with a non-specific control antagomir (NSCA), which is a 2′-O-melthylated randomized oligomer. We found that there were no significant reductions of HCV mRNA levels following transfections with this NSCA, when compared to cells that were not transfected (Fig. 4). To further assess whether miR-122 is required for HCV RNA expression, we transfected 50 nM 2′-O-methylated-mimic-miR-122, which contained a near perfectly matched sequence to the liver specific miR-122, into 9–13 replicon cells for 72 hours. As expected, mimic-miR-122 up-regulated HCV mRNA (1.6-fold) expression in 9–13 replicon cells. 50 nM antagomir of miR-122 at 72 hours significantly down-regulated NS5A mRNA levels by approximately 70% (P<0.05), when compared to non-treated cells. Thus, silencing of the HCV RNA replication using a chemically synthesized antagomir targeted to miR-122 was effective, specific, and selective. A similar result was found in CNS3 cells (data not shown).
Figure 4. An antagomir of miR-122 specifically down-regulates HCV mRNA in 9–13 cells.
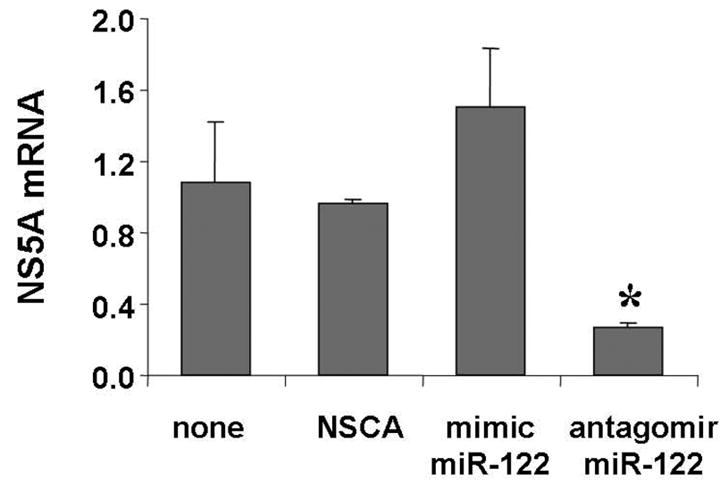
50 nM of non-specific control antagomir (NSCA), 2-O-methylated mimic miR-122 (mimic miR-122), and chemically synthesized miR-122 antagomir were introduced into 9–13 cells by Lipofectamine 2000-mediated transfection. Total RNA were extracted and the levels of HCV NS5A mRNAs were measured by qRT-PCR. No miRNA treatment was set equal to 1. Data are presented as means ± SE from three samples. * differs from none or other treatments, p<0.05.
An antagomir to miR-122 regulates Bach1 and HO-1 mRNA in Huh-7 cells
By computational analysis, we found at least one predicted miR-122 binding site located in the Bach1 3′UTR (816 bp from transcription start site). No predicted miR-122 binding sites were found in the HO-1 or Nrf2 genes. To determine whether miR-122 regulates the expression of HO-1/Bach1 in hepatocytes, we employed antagomir of miR-122 to inactivate miR-122 in Huh-7 WT and 9–13 cells. Transfection of 50 nM miR-122 antagomir into 9–13 cells for 72 hours significantly down-regulates the levels of Bach1 mRNAs by 53% (Fig. 5A, P<0.05) and up-regulates the levels of HO-1 mRNA 2.2-fold as expected (Fig. 5B, P<0.05). Antagomir of miR-122 (5–50 nM) also significantly up-regulates HO-1 mRNA levels 2.5-fold in Huh-7 wild type cells (data not shown). These results establish that miR-122 regulates hepatic HO1 and Bach1 gene expression.
Figure 5. An antagomir to miR-122 regulates Bach1 and HO-1 mRNAs in 9–13 cells.
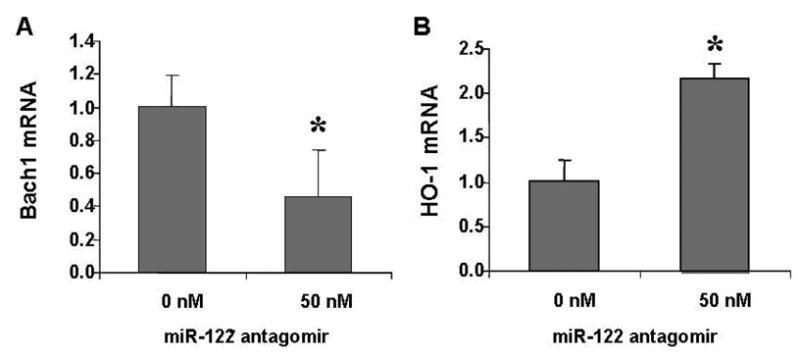
The miR-122 antagomir was transfected into 9–13 replicon cells by Lipofectamine 2000. Total RNA was extracted 72 hours later. HO-1 mRNA or Bach1 levels were quantified by qRT-PCR. A) Levels of Bach1 mRNA were down-regulated significantly in HCV replicon cells transfected with miR-122 antagomir. B) HO-1 mRNA increased >2-fold by miR-122 antagomir, compared with no miR-122 antagomir. Data are presented as means ± SE from three samples. * differs from non-treatments, p<0.05.
Effects of Bach1-siRNA on up-regulation of HO-1 and down-regulation of HCV expression
Recently, we successfully up-regulated the HO-1 gene by silencing Bach1 gene expression with Bach1-siRNA (57; 58). To further investigate whether up-regulation of HO-1 gene involved down-regulation HCV expression, we silenced the Bach1 gene with Bach1-siRNA. 50 nM Bach1-siRNA or antagomir of miR-122 were single- or co-transfected into 9–13 or CNS3 replicon cells. The Bach1 gene was down-regulated by antagomir of miR-122 (50%) or Bach1-siRNA (80%), but was not further down-regulated by the combination of antagomir of miR-122 and Bach1-siRNA (Fig. 6A). HO-1 mRNA level was up-regulated ~3-fold by antagomir of miR-122, ~ 8-fold by Bach1-siRNA, and ~11-fold by co-transfection with antagomir of miR-122 and Bach1-siRNA (Fig. 6B). Interestingly, HCV NS5A mRNA level was down-regulated by Bach1-siRNA (60%), which was the same amount of down-regulation caused by antagomir of miR-122 (60%). However, there was no further down-regulation by combined administration of Bach1-siRNA and miR-122 antagomir (Fig. 6C). We also transfected Bach1-siRNA into CNS3 cells. As expected, silencing Bach1 gene expression, with Bach1-siRNA, up-regulated HO-1 gene expression nearly 1.8-fold, and reciprocally down-regulated HCV core mRNA by over 50% (Fig. 7A & 7B).
Figure 6. Silencing Bach1 gene up-regulates HO-1 and down-regulates HCV replication in 9–13 cells.
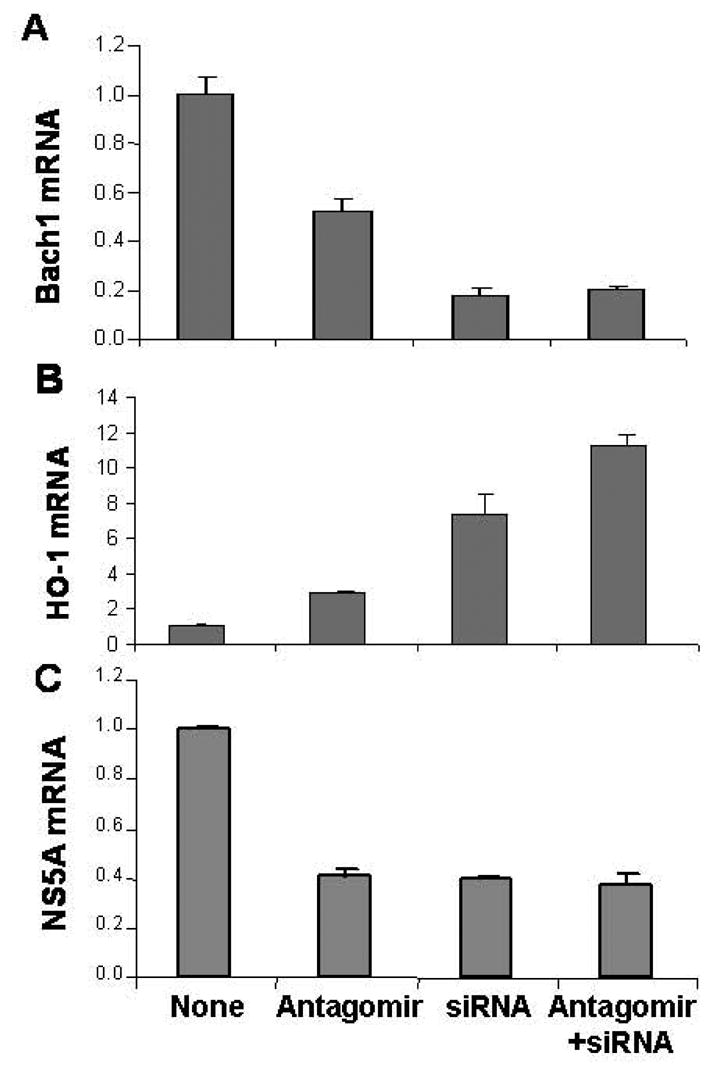
9–13 cells were transfected with 50 nM Bach1-siRNA, antagomir of miR-122, or the combination of Bach1-siRNA and antagomir of miR-122 for 48 h, after which cells were harvested. Bach1 (A), HO-1 (B), and NS5A (C) mRNA levels were measured by quantitative RT-PCR, as described in the Methods. Data are presented as means ± SE from three samples.
Figure 7. Silencing Bach1 gene up-regulates HO-1 and down-regulates HCV replication in CNS3 cells.
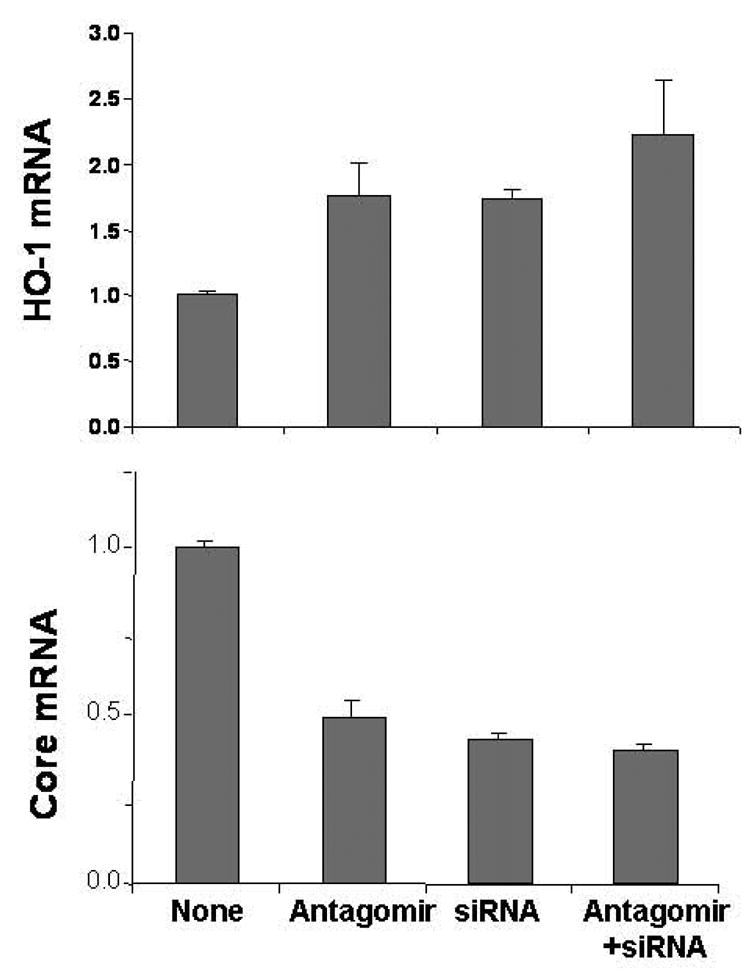
CNS3 cells were transfected with 50 nM Bach1-siRNA, antagomir of miR-122, or the combination of Bach1-siRNA and antagomir of miR-122 for 48 h, after which cells were harvested. HO-1 (A) and HCV Core (B) mRNA levels were measured by quantitative RT-PCR, as described in the Methods. Data are presented as means ± SE from three samples.
Up-regulation of HO-1 gene expression by CoPP down-regulates HCV replication
CoPP, like heme, is known to be a potent and effective inducer of HO-1 activity in many tissues. We up-regulated HO-1 gene expression with CoPP or heme, to further determine whether the HO-1 gene is involved in the regulation of HCV replication in hepatocytes. CNS3 cells were treated with 1–10 μM of CoPP for 16–72 h. As we expected, CoPP markedly up-regulated HO-1 mRNA levels (40-fold, Fig 8A), concomitant with reciprocal reduction of the HCV core mRNA expression (50%) in CNS3 cells in a dose-dependent (Fig 8B). HCV replication was significantly inhibited at 1–10 μM CoPP treatment for 24 h. Exposure of HCV replicon cells to CoPP for 24 hours produced a greater decrease of HCV RNA expression than exposure for other times (data not shown). The expression of HCV core protein was decreased ~50% by 2.5–5 μM CoPP treatments for 24 hours (Fig 8C). HCV core protein was also decreased by 2.5 μM heme (~50%), another putative HO-1 inducer. The results presented here further confirm that HO-1 is involved in the regulation of HCV replication in human hepatocytes.
Figure 8. Up-regulation of HO-1 by CoPP down-regulates HCV replication in CNS3 cells.
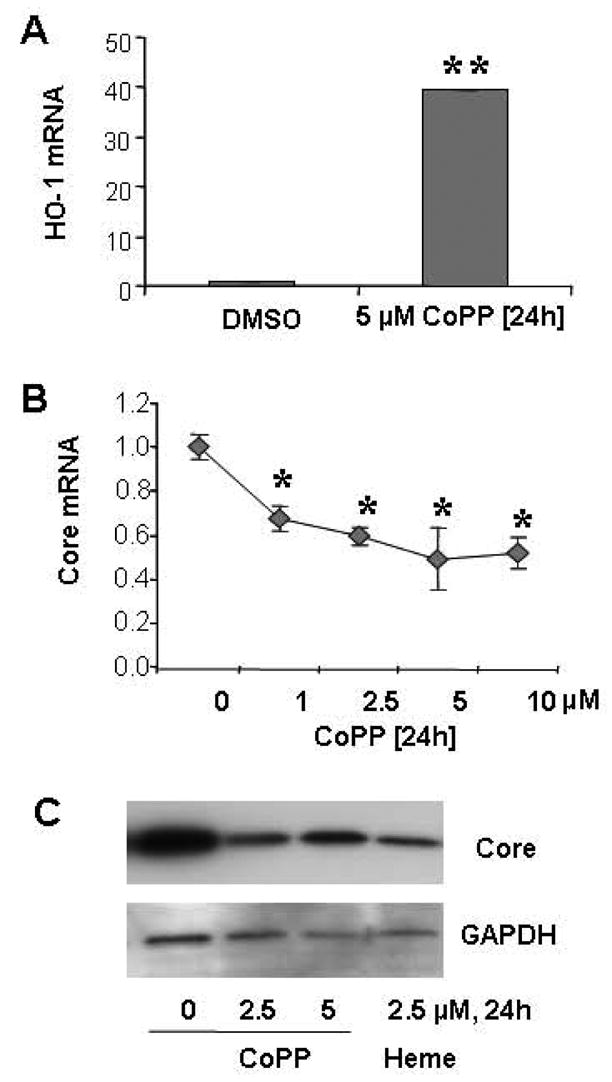
A) HO-1 mRNA was increased by treatment with 5 μM CoPP for 24 h. B) HCV core mRNA was down-regulated by CoPP in a dose-dependent fashion at 24h. C) HCV core protein was down-regulated by CoPP and heme. CNS3 cells were treated with 1–10 μM CoPP, or 2.5 μM heme for 24 h, after which cells were harvested. HO-1, HCV Core, and GAPDH mRNA levels were measured by quantitative RT-PCR. HO-1 and HCV Core were normalized by GAPDH. Data are presented as means ± SE from three samples. HCV Core and GAPDH proteins were measured by Western Blot. 50 μg of protein were separated by 4–15% SDS-polyacrylamide gel, transferred to a PVDF membrane, and probed with anti-HCV Core and GAPDH specific antibodies. Representative results from one of three experiments are shown. * differs from DMSO (0 μM) treatment, p<0.05; ** differs from DMSO treatment, p<0.001.
Discussion
The major findings of this study are 1) an antagomir of miR-122 reduces the abundance of HCV mRNA and protein in CNS3 and 9–13 cells; 2) transfection with mimic-miR-122 increases HCV levels; whereas transfection with NSCA did not change the level of HCV, when compared to the cells that were not transfected; 3) antagomir of miR-122 decreased Bach1 and increased HO-1 mRNA levels in CNS3 and 9–13 replicon cells; 4) silencing Bach1 with Bach1-siRNA increases HO-1 mRNA levels and decreases HCV RNA by 50% in CNS3 and 9–13 replicon cells; and 5) up-regulation of HO-1 by CoPP significantly down-regulates HCV mRNA and protein levels in replicon cells.
Approaches to the study of miRNA function have largely focused on the inhibition of miRNAs with 2′-O-methylated oligonucleotides. Jopling et al employed a 2′-O-methylated miR-122 with exact complementarity to miR-122 to inactivate miR-122. In our previous study, we silenced Bach1 and Nrf2 gene expression in hepatocytes with chemically synthesized siRNA (57; 58). By use of a chemically synthesized antagomir of miR-122, we successfully inhibited HCV replication in two HCV replicon cell lines. Our results are consistent with the previous finding by Jopling et al, and extend the finding to include two different replicon cell lines, CNS3 and 9–13 cells. These results also suggest that chemically synthesized miRNA can cause a functional inactivation of miRNA that is just as effective as the 2′-O-methylated oligonucleotides. Also, silencing of HCV RNA replication using chemically synthesized antagomir targeted to miR-122 was effective, specific, and selective.
Jopling et al reported that the putative miR-122 binding site in 5′ UTR of HCV genome is required for miR-122 to affect HCV RNA abundance, whereas the 3′-UTR miR-122 binding site is not. miR-122 neither affects HCV RNA stability nor RNA translation. It is possible that miR-122 antagomir also acts indirectly, for example, by leading to up-regulation of non-HCV genes, which, in turn, alter HCV gene expression. Using antagomir of miR-122 also resulted in reduced plasma cholesterol levels, increased hepatic fatty-acid oxidation, and a decrease in hepatic fatty-acid and cholesterol synthesis rates (61).
HO-1 is induced as a protective mechanism to guard against heme mediated oxidative damage to cellular lipids, proteins, and nucleoproteins. Increased oxidative stress is an important pathogenetic mechanism in CHC (3–9). Hence, induction of HO-1 might have potential in management of CHC. Our and other previous studies indicated that Bach1 is an HO-1 repressor (43; 57; 58; 62). We further investigated whether HO-1 and/or Bach1 gene expression is regulated by miR-122. Our results showed that miR-122 is involved the regulation of HO-1 and Bach1 gene expression in hepatocytes. These results indicate that miR-122 down-regulates HCV replication through at least two pathways: directly facilitating the viral replication, and indirectly through regulation of HO-1 and Bach1 gene expression.
miRNAs regulate the expression of a large number of protein-coding genes. Their primary transcripts (pri-miRNAs) undergo multiple processing steps before reaching their final functional form. The RNA-binding protein DiGeorge critical region-8 (DGCR8) is essential for this first processing step of miRNA from pri-miRNA to functional form. Faller et al recently reported that DGCR8 is a heme-binding protein (63). Heme promotes dimerization of DGCR8, and the heme-bound DGCR8 dimer seems to trimerize upon binding pri-miRNA. It is this complex that is active in triggering pri-miRNA cleavage, whereas the heme-free monomer is much less active. A heme-binding region of DGCR8 inhibits the pri-miRNA-processing activity of the monomer. This putative autoinhibition is overcome by heme. The involvement of heme in pri-miRNA processing suggests that the gene-regulation network of miRNAs and signal-transduction pathway involving heme might be connected. HO-1 is the rate-controlling enzyme involved in heme degradation. It is still not clear whether heme/CoPP down-regulated HCV replication through up-regulation of HO-1 gene expression or through regulation of miR-122 processing.
HCV is a major cause of cirrhosis and hepatocellular carcinoma. Interferon alone or together with ribavirin, is the cornerstone of therapy for HCV infection; however, a sizeable number of HCV-infected individuals do not respond to this treatment and it has numerous unpleasant side effects. Therefore, the development of new therapeutic options against HCV is a matter of urgency. Our studies indicate that down-regulation of HCV replication using an antagomir targeted to miR-122 is effective, specific, and selective. Increasing HO-1, by silencing the expression of the Bach1 gene or by induction of HO-1 with CoPP/heme, also decreases HCV replication. Thus, miR-122 plays an important role in the regulation of HCV replication and HO-1/Bach1 expression in hepatocytes. Down-regulation of miR-122 and up-regulation of HO-1 may be new strategies for anti-HCV intervention and cytoprotection.
Acknowledgments
This work was supported by NIH RO1-DK38825 and contracts NO-1 DK92326 and UO-1 DK065193. The opinions expressed herein are those of the authors. They do not necessarily reflect the official views of the U.S.P.H.S. or The University of Connecticut Health Center.
Abbreviations used
- CO
carbon monoxide
- CHC
chronic hepatitis C
- CoPP
cobalt protoporphyrin
- DGCR8
the DiGeorge syndrome critical region gene 8
- DMSO
dimethyl sulfoxide
- GAPDH
glyceraldehyde phosphate dehydrogenase
- HCV
hepatitis C virus
- HO
heme oxygenase
- miRNA
micro RNA
- NCR
non-coding region
- Nrf2
NF-E2-related factor 2
- PAGE
polyacrylamide gel electrophoresis
- NS3
non-structural protein 3 of HCV
- NS5B
non-structural protein 5b of HCV
- NSCA
non-specific control antagomir
- RT-PCR
reverse transcription-polymerase chain reaction
- SDS
sodium dodecyl sulfate
- ROS
reactive oxygen species
- WT
wild type
- UTR
untranslated region
- PVDF
polyvinylidene fluoride
Footnotes
No conflict of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shepard CW, Finelli L, Alter M. Global epidemiology of hepatitis C virus infection. Lancet Infectious Diseases. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 2.Shiffman ML, Saab S, Feng S, Abecassis MI, Tzakis AG, Goodrich NP, Schaubel DE. Liver and intestine transplantation in the United States, 1995–2004. Am J Transplant. 2006;6:1170–1187. doi: 10.1111/j.1600-6143.2006.01273.x. [DOI] [PubMed] [Google Scholar]
- 3.Lieber CS. Role of oxidative stress and antioxidant therapy in alcoholic and nonalcoholic liver diseases. Adv Pharmacol. 1997;38:601–628. doi: 10.1016/s1054-3589(08)61001-7. [DOI] [PubMed] [Google Scholar]
- 4.Tsutsumi T, Suzuki T, Moriya K, Shintani Y, Fujie H, Miyoshi H, Matsuura Y, Koike K, Miyamura T. Hepatitis C virus core protein activates ERK and p38 MAPK in cooperation with ethanol in transgenic mice. Hepatology. 2003;38:820–828. doi: 10.1053/jhep.2003.50399. [DOI] [PubMed] [Google Scholar]
- 5.Wen F, Abdalla MY, Aloman C, Xiang J, Ahmad IM, Walewski J, McCormick ML, Brown KE, Branch AD, Spitz DR, Britigan BE, Schmidt WN. Increased prooxidant production and enhanced susceptibility to glutathione depletion in HepG2 cells co-expressing HCV core protein and CYP2E1. J Med Virol. 2004;72:230–240. doi: 10.1002/jmv.10567. [DOI] [PubMed] [Google Scholar]
- 6.Mahmood S, Kawanaka M, Kamei A, Izumi A, Nakata K, Niiyama G, Ikeda H, Hanano S, Suehiro M, Togawa K, Yamada G. Immunohistochemical evaluation of oxidative stress markers in chronic hepatitis C. Antioxid Redox Signal. 2004;6:19–24. doi: 10.1089/152308604771978318. [DOI] [PubMed] [Google Scholar]
- 7.Barbaro G, Di Lorenzo G, Asti A, Ribersani M, Belloni G, Grisorio B, Filice G, Barbarini G. Hepatocellular mitochondrial alterations in patients with chronic hepatitis C: ultrastructural and biochemical findings. Am J Gastroenterol. 1999;94:2198–2205. doi: 10.1111/j.1572-0241.1999.01294.x. [DOI] [PubMed] [Google Scholar]
- 8.Kageyama F, Kobayashi Y, Kawasaki T, Toyokuni S, Uchida K, Nakamura H. Successful interferon therapy reverses enhanced hepatic iron accumulation and lipid peroxidation in chronic hepatitis C. Am J Gastroenterol. 2000;95:1041–1050. doi: 10.1111/j.1572-0241.2000.01979.x. [DOI] [PubMed] [Google Scholar]
- 9.Abdalla MY, Ahmad IM, Spitz DR, Schmidt WN, Britigan BE. Hepatitis C virus-core and non structural proteins lead to different effects on cellular antioxidant defenses. J Med Virol. 2005;76:489–497. doi: 10.1002/jmv.20388. [DOI] [PubMed] [Google Scholar]
- 10.Qadri I, Iwahashi M, Capasso JM, Hopken MW, Flores S, Schaack J, Simon FR. Induced oxidative stress and activated expression of manganese superoxide dismutase during hepatitis C virus replication: role of JNK, p38 MAPK and AP-1. Biochem J. 2004;378:919–928. doi: 10.1042/BJ20031587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray RB, Steele R, Meyer K, Ray R. Transcriptional repression of p53 promoter by hepatitis C virus core protein. J Biol Chem. 1997;272:10983–10986. doi: 10.1074/jbc.272.17.10983. [DOI] [PubMed] [Google Scholar]
- 12.Marusawa H, Hijikata M, Chiba T, Shimotohno K. Hepatitis C virus core protein inhibits Fas- and tumor necrosis factor alpha-mediated apoptosis via NF-kappaB activation. J Virol. 1999;73:4713–4720. doi: 10.1128/jvi.73.6.4713-4720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 14.Korenaga M, Wang T, Li Y, Showalter LA, Chan T, Sun J, Weinman SA. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases ROS production. J Biol Chem. 2005 doi: 10.1074/jbc.M506412200. [DOI] [PubMed] [Google Scholar]
- 15.Korenaga M, Okuda M, Otani K, Wang T, Li Y, Weinman SA. Mitochondrial dysfunction in hepatitis C. J Clin Gastroenterol. 2005;39:S162–S166. doi: 10.1097/01.mcg.0000155517.02468.46. [DOI] [PubMed] [Google Scholar]
- 16.Otani K, Korenaga M, Beard MR, Li K, Qian T, Showalter LA, Singh AK, Wang T, Weinman SA. Hepatitis C virus core protein, cytochrome P450 2E1, and alcohol produce combined mitochondrial injury and cytotoxicity in hepatoma cells. Gastroenterology. 2005;128:96–107. doi: 10.1053/j.gastro.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 17.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 18.Elbirt KK, Bonkovsky HL. Heme oxygenase: recent advances in understanding its regulation and role. Proc Assoc Am Physicians. 1999;111:438–447. [PubMed] [Google Scholar]
- 19.Bonkovsky HL, Elbirt KK. Heme Oxygenase: Its Regulation and Role. In: Cutler RG, Rodriguez H, editors. Oxidative Stress and Aging. River Edge, NJ: World Scientific; 2002. pp. 690–706. [Google Scholar]
- 20.Lambrecht RW, Fernandez M, Shan Y, Bonkovsky H. Heme oxygenase and carbon monoxide in cirrhosis and portal hypertension. In: Gine P, Arroyo V, Rodes J, Schrier RW, editors. Ascites and Renal Dysfunction in Liver Disease, Pathogenesis, Diagnosis & Treatment. 2. London: Blackwell Science; 2005. pp. 125–136. [Google Scholar]
- 21.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi T, Shioji I, Sugimoto A, Komoda Y, Nakajima H. Chemical structure of a new family of bile pigments from human urine. J Biochem (Tokyo) 1994;116:298–303. doi: 10.1093/oxfordjournals.jbchem.a124523. [DOI] [PubMed] [Google Scholar]
- 23.Abraham NG, Lavrovsky Y, Schwartzman ML, Stoltz RA, Levere RD, Gerritsen ME, Shibahara S, Kappas A. Transfection of the human heme oxygenase gene into rabbit coronary microvessel endothelial cells: protective effect against heme and hemoglobin toxicity. Proc Natl Acad Sci U S A. 1995;92:6798–6802. doi: 10.1073/pnas.92.15.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee PJ, Alam J, Wiegand GW, Choi AM. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc Natl Acad Sci U S A. 1996;93:10393–10398. doi: 10.1073/pnas.93.19.10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, Vercellotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J Clin Invest. 2006;116:808–816. doi: 10.1172/JCI26857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res. 2002;90:E17–E24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- 27.Yachie A, Toma T, Mizuno K, Okamoto H, Shimura S, Ohta K, Kasahara Y, Koizumi S. Heme oxygenase-1 production by peripheral blood monocytes during acute inflammatory illnesses of children. Exp Biol Med. 2003;228:550–556. doi: 10.1177/15353702-0322805-26. [DOI] [PubMed] [Google Scholar]
- 28.Alam J, Shibahara S, Smith A. Transcriptional activation of the heme oxygenase gene by heme and cadmium in mouse hepatoma cells. J Biol Chem. 1989;264:6371–6375. [PubMed] [Google Scholar]
- 29.Shan Y, Pepe J, Lu TH, Elbirt KK, Lambrecht RW, Bonkovsky HL. Induction of the heme oxygenase-1 gene by metalloporphyrins. Arch Biochem Biophys. 2000;380:219–227. doi: 10.1006/abbi.2000.1921. [DOI] [PubMed] [Google Scholar]
- 30.Shan Y, Pepe J, Lambrecht RW, Bonkovsky HL. Mapping of the chick heme oxygenase-1 proximal promoter for responsiveness to metalloporphyrins. Arch Biochem Biophys. 2002;399:159–166. doi: 10.1006/abbi.2001.2742. [DOI] [PubMed] [Google Scholar]
- 31.Mitani K, Fujita H, Sassa S, Kappas A. A heat-inducible nuclear factor that binds to the heat-shock element of the human haem oxygenase gene. Biochem J. 1991;277 (Pt 3):895–897. doi: 10.1042/bj2770895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AM. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem. 1997;272:5375–5381. [PubMed] [Google Scholar]
- 33.Wood SM, Wiesener MS, Yeates KM, Okada N, Pugh CW, Maxwell PH, Ratcliffe PJ. Selection and analysis of a mutant cell line defective in the hypoxia-inducible factor-1 alpha-subunit (HIF-1alpha). Characterization of HIF-1 alpha-dependent and -independent hypoxia-inducible gene expression. J Biol Chem. 1998;273:8360–8368. doi: 10.1074/jbc.273.14.8360. [DOI] [PubMed] [Google Scholar]
- 34.Numazawa S, Yamada H, Furusho A, Nakahara T, Oguro T, Yoshida T. Cooperative induction of c-fos and heme oxygenase gene products under oxidative stress in human fibroblastic cells. Exp Cell Res. 1997;237:434–444. doi: 10.1006/excr.1997.3825. [DOI] [PubMed] [Google Scholar]
- 35.Taketani S, Kohno H, Yoshinaga T, Tokunaga R. The human 32-kDa stress protein induced by exposure to arsenite and cadmium ions is heme oxygenase. FEBS Lett. 1989;245:173–176. doi: 10.1016/0014-5793(89)80215-7. [DOI] [PubMed] [Google Scholar]
- 36.Tyrrell RM, Basu-Modak S. Transient enhancement of heme oxygenase 1 mRNA accumulation: a marker of oxidative stress to eukaryotic cells. Methods Enzymol. 1994;234:224–235. doi: 10.1016/0076-6879(94)34089-7. [DOI] [PubMed] [Google Scholar]
- 37.Vile GF, Basu-Modak S, Waltner C, Tyrrell RM. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci U S A. 1994;91:2607–2610. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci U S A. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi BM, Pae HO, Kim YM, Chung HT. Nitric oxide-mediated cytoprotection of hepatocytes from glucose deprivation-induced cytotoxicity: involvement of heme oxygenase-1. Hepatology. 2003;37:810–823. doi: 10.1053/jhep.2003.50114. [DOI] [PubMed] [Google Scholar]
- 42.Oyake T, Itoh K, Motohashi H, Hayashi N, Hoshino H, Nishizawa M, Yamamoto M, Igarashi K. Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Mol Cell Biol. 1996;16:6083–6095. doi: 10.1128/mcb.16.11.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Igarashi K, Hoshino H, Muto A, Suwabe N, Nishikawa S, Nakauchi H, Yamamoto M. Multivalent DNA binding complex generated by small Maf and Bach1 as a possible biochemical basis for beta-globin locus control region complex. J Biol Chem. 1998;273:11783–11790. doi: 10.1074/jbc.273.19.11783. [DOI] [PubMed] [Google Scholar]
- 44.Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, Taketo MM, Yamamoto M, Igarashi K. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shan Y, Lambrecht RW, Ghaziani T, Donohue SE, Bonkovsky HL. Role of Bach-1 in regulation of heme oxygenase-1 in human liver cells: Insights from studies with small interfering RNAs. J Biol Chem. 2004;279:51769–51774. doi: 10.1074/jbc.M409463200. [DOI] [PubMed] [Google Scholar]
- 46.Tomari Y, Zamore PD. Perspective: machines for RNAi. Genes Dev. 2005;19:517–529. doi: 10.1101/gad.1284105. [DOI] [PubMed] [Google Scholar]
- 47.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 48.Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang J, Provost P, Taylor JM. Resistance of human hepatitis delta virus RNAs to dicer activity. J Virol. 2003;77:11910–11917. doi: 10.1128/JVI.77.22.11910-11917.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia M. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1:106–113. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- 51.Chang JH, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, Xu C, Mason WS, Moloshok T, Bort R. miR-122, a mammalian liver-specific microRNA, is processed from hcv mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1:106–113. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- 52.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 53.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 54.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 55.Appel N, Bartenschlager R. A novel function for a micro RNA: Negative regulators can do positive for the hepatitis C virus. Hepatology. 2006;43:612–615. doi: 10.1002/hep.21092. [DOI] [PubMed] [Google Scholar]
- 56.Ghaziani T, Shan Y, Lambrecht RW, Donohue SE, Pietschmann T, Bartenschlager R, Bonkovsky HL. HCV proteins increase expression of heme oxygenase-1 (HO-1) and decrease expression of Bach1 in human hepatoma cells. J Hepatol. 2006:1–8. doi: 10.1016/j.jhep.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 57.Shan Y, Lambrecht RW, Bonkovsky HL. Identification of key elements that are responsible for heme-mediated induction of the avian heme oxygenase-1 gene. Biochimica et Biophysica Acta-Gene Structure and Expression. 2004;1679:87–94. doi: 10.1016/j.bbaexp.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Shan Y, Lambrecht RW, Donohue SE, Bonkovsky HL. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J. 2006;20:2651–2653. doi: 10.1096/fj.06-6346fje. [DOI] [PubMed] [Google Scholar]
- 59.Dinev D, Jordan BW, Neufeld B, Lee JD, Lindemann D, Rapp UR, Ludwig S. Extracellular signal regulated kinase 5 (ERK5) is required for the differentiation of muscle cells. EMBO Rep. 2001;2:829–834. doi: 10.1093/embo-reports/kve177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 61.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, Subramaniam A, Propp S, Lollo BA, Freier S, Bennett CF, Bhanot S, Monia BP. miR–122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 62.Kanezaki R, Toki T, Yokoyama M, Yomogida K, Sugiyama K, Yamamoto M, Igarashi K, Ito E. Transcription factor BACH1 is recruited to the nucleus by its novel alternative spliced isoform. J Biol Chem. 2001;276:7278–7284. doi: 10.1074/jbc.M004227200. [DOI] [PubMed] [Google Scholar]
- 63.Faller M, Matsunaga M, Yin S, Loo JA, Guo F. Heme is involved in microRNA processing. Nat Struct Mol Biol. 2007;14:23–29. doi: 10.1038/nsmb1182. [DOI] [PubMed] [Google Scholar]


