Abstract
Recent studies suggest that in female monkeys and rats, estrogens elicit dendritic spine synapse formation in the prefrontal cortex, an area that similar to the hippocampus, plays a critical role in cognition. However, whether gonadal hormones induce synaptic remodeling in the male prefrontal cortex remains unknown. Here we report that gonadectomy reduced, while administration of 5α-dihydrotestosterone or estradiol-benzoate to castrated male rats increased the number of medial prefrontal cortical (mPFC) spine synapses, with estradiol-benzoate being less effective than 5α-dihydrotestosterone. To investigate whether the androgen receptor contributes to the mediation of these changes, we compared the response of testicular feminization mutant (Tfm) male rats to that of wild-type animals. The number of mPFC spine synapses in gonadally intact Tfm rats and 5α-dihydrotestosterone-treated castrated Tfm males was considerably reduced compared to intact wild-type animals, while the synaptogenic effect of estradiol-benzoate was surprisingly enhanced in Tfm rats. These data are consistent with the hypothesis that remodeling of spine synapses in the prefrontal cortex may contribute to the cognitive effect of gonadal steroids. Our findings in Tfm animals indicate that androgen receptors may mediate a large part of the synaptogenic action of androgens in the mPFC of adult males. However, because this effect of 5α-dihydrotestosterone is not completely lost in Tfm rats, additional mechanisms may also be involved.
Keywords: gonadal hormones, androgen receptor, synaptic plasticity, prefrontal cortex, Tfm rat, stereology
Introduction
It is well documented that gonadal steroid hormones significantly influence cognitive performance in both humans and laboratory animals (1). Recent studies also suggest that remodeling and subsequent stabilization of dendritic spine synapses may represent a mechanism by which new memories are made and stored (2, 3). Consequently, it is believed that remodeling of spine synapses significantly contributes to the cognitive effect of gonadal hormones. Indeed, pioneering studies from Woolley and McEwen (4) have shown that during the estrous cycle of rats, fluctuating levels of estradiol mediate a change in the density of spine synapses in the hippocampus that is critically involved in memory and cognitive functions. Another brain area that plays a crucial role in cognition, especially in working memory, is the prefrontal cortex (PFC) (5). The function and structure of PFC neurons is also affected by gonadal steroids. Estrogens improve PFC-related cognitive performance of ovariectomized nonhuman primates (6) and rats (7). In line with this, changing levels of circulating estrogens influence the total number of spines in layer I of Walker’s area 46 in young female rhesus monkeys (8). Similarly, Wallace et al. (7) have shown that ovariectomy decreases the dendritic spine density of layer II/III pyramidal cells in the medial PFC (mPFC) of female rats, suggesting that changes in spine synapses may mediate the effect of estrogens on PFC-related cognitive functions. Based on the fact that Walker’s area 46 in the monkey dorsolateral PFC and the mPFC of rats share many functional and anatomical characteristics, the analogy of the two areas is well accepted. For example, both the dorsolateral PFC and mPFC show similar connectivity patterns with other brain regions. Moreover, damage to these two areas elicits analogous changes in behaviors such as response inhibition, temporal ordering, spatial orientation, social or affective behavior, behavioral spontaneity, olfaction, and habituation (5).
Whether gonadal steroids induce spine synapse remodeling in the adult male PFC, however, remains unknown. To address this issue, we investigated the effects of androgens and estradiol on the number of spine synapses in the mPFC of adult male rats. In addition, to examine the role of the androgen receptor in this cellular response, we utilized testicular feminization mutant (Tfm) male rats. Naturally occurring mutations in the androgen receptor gene cause the syndrome of X-linked testicular feminization that has been extensively studied in humans as well as experimental animals (9-12). In the rat, the Tfm mutation results in the transcription of a defective androgen receptor, with a glutamine for arginine substitution at position 734 in the ligand-binding domain (13). This reduces the binding capacity of the receptor to ∼10-15% of normal. The phenotypic expression of this defect includes essentially complete loss of normal developmental responses to androgen in the reproductive tract. Thus, although these animals secrete Mullerian Inhibiting Hormone and therefore lack a female internal reproductive tract, androgen-dependent differentiation of both the external genitalia and Wolffian duct-derived structures, such as the seminal vesicles and prostate, fail to occur (9). Many androgen receptor-mediated responses in the central nervous system of Tfm males are also either completely abolished or severely impaired (10, 11), indicating that the 10-15% residual binding capacity of the androgen receptor in Tfm males is clearly not sufficient to maintain androgen receptor-mediated normal physiological responses.
Materials and Methods
Adult male Tfm Long Evans rats and littermate wild-type males (n=24, 230-250 g) were bred in the animal facilities of Michigan State University and maintained on a 12/12-h light/dark cycle. Rats were group housed and provided with unlimited access to water and rodent chow. Tfm males were distinguished from their wild-type brothers at weaning based on the presence of nipples and testes. Sex was also confirmed at the time of gonadectomy. All animal protocols used in this study were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Michigan State University.
Six groups of rats (n=3 animals per group) were gonadectomized under isoflurane anesthesia and one week later, they were treated with 200 μl/rat/day sesame oil, 500 μg/rat/day 5α-dihydrotestosterone (DHT, a non-aromatizable androgen) or 10 μg/rat/day estradiol-benzoate (EB, a long-acting estradiol ester). Each hormone was dissolved in 200 μl sesame oil and delivered daily by subcutaneous injections for 2 days. This dose of DHT is at the very low end of the dose-response curve established for powerful androgens to maintain peripheral androgen-target tissues such as the ventral prostate in castrated male rats (14). Previous studies have also shown that these doses of DHT and EB are sufficient to reproduce the density of hippocampal spine synapses observed in proestrus females (4) or in intact males (15). Thus, the hippocampi of these animals were also processed and the number of CA1 spine synapses was determined as published previously (16), which served as evidence of appropriate hormonal influence on the brain. Two additional groups of animals were left gonadally intact and untreated to serve as positive controls. Thus, this experiment included the following treatment groups: intact/wild-type, oil/wild-type, DHT/wild-type, EB/wild-type, intact/Tfm, oil/Tfm, DHT/Tfm and EB/Tfm. Two days after the last injections, rats were overdosed with i.p. sodium pentobarbital and perfused transcardially with heparinized saline, followed by a fixative containing 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M phosphate buffer (pH 7.35). The brains were removed and postfixed overnight in the same fixative without glutaraldehyde.
The number of spine synapses in layer II/III of the mPFC was calculated as published previously (17). Due to the labor-intensive nature of electron microscopic stereology, the analysis was focused on these particular layers because a recent study has demonstrated that ovariectomy induces specific changes in dendritic spine density of layer II/III pyramidal neurons in the mPFC of female rats (7). Briefly, vibratome sections throughout the mPFC were cut and embedded in Durcupan (Electron Microscopy Sciences, Fort Washington, PA). Using the embedded sections, the volume of the sampling area was estimated utilizing the Cavalieri Estimator module of the Stereo Investigator® system (MicroBrightField Inc, Villiston, VT) mounted on a Zeiss Axioplan 2 light microscope. Thereafter, sampling regions were localized and approximately four 75 nm thick consecutive ultrasections were cut from each site. Digitized electron micrographs (Fig. 1) were taken for the physical disector at a magnification of ×11,000 in a Tecnai 12 transmission electron microscope (FEI Company, Hillsboro, OR) furnished with an Hamamatsu HR/HR-B CCD camera system (Hamamatsu Photonics, Hamamatsu, Japan). The micrographs were taken by a person, who was blind to the treatment and genotype of individual animals. Spine synapses were counted according to the rules of the disector technique (18), and the volumetric density of spine synapses (synapse/μm3) was determined. Thereafter, this volumetric density was multiplied by the volume of the sampling area to arrive at the total number of spine synapses (reported in billions, i.e., 109). The number of spine synapses was determined independently by two different investigators, and the results were cross-checked to preclude systematic analytical errors.
Figure 1.
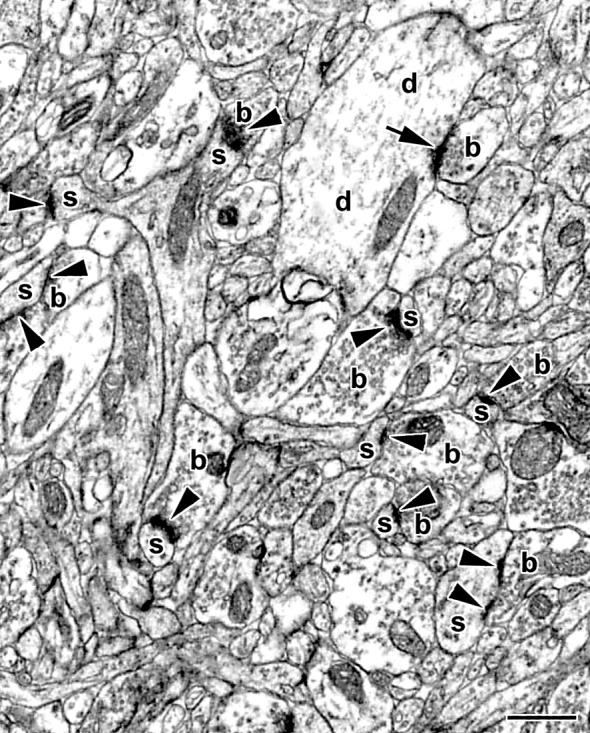
A representative electron micrograph demonstrating the ultrastructure of spine synapses (arrowheads) in the medial prefrontal cortex of a 5α-dihydrotestosterone-treated, castrated, wild-type male rat. The arrow points to a synapse on a dendritic shaft. b, boutons; d, dendritic shafts; s, spines. Scale bar: 500 nm.
Estimates of the total number of spine synapses and the volume of the sampling area obtained from individual animals were used to calculate group means (±S.E.M.) for each treatment group. Results were analyzed with Bartlett’s test for homogeneity of variance and with two-way (treatment × genotype) analysis of variance (ANOVA), followed by the Newman-Keuls multiple comparison test. A criterion for statistical confidence of p<0.05 was adopted. Typically, with these methods, the standard deviation for spine synapse counts was ∼5% of the mean. With a standard deviation of 5% and sample sizes of 3 animals per group, a 15% change in the mean number of spine synapses can be detected with α=0.05 and 80% power. Hence, for purposes of the present study, the minimum treatment group size was set at 3 animals.
Results
Spine synapse counts in the mPFC of adult male rats are summarized in Figure 2. Treatment groups differed only in group mean, with no significant inhomogeneity of variance across treatment groups. Numbers of spine synapses differed markedly depending on both steroid treatment (F3,16=94.38, p<0.0001) and genotype (F1,16=11.27, p=0.004). Moreover, there was a significant interaction between steroid treatment and genotype (F3,16=52.91, p<0.0001), reflecting marked differences in the effect of castration and steroid replacement between wild-type and Tfm males.
Figure 2.
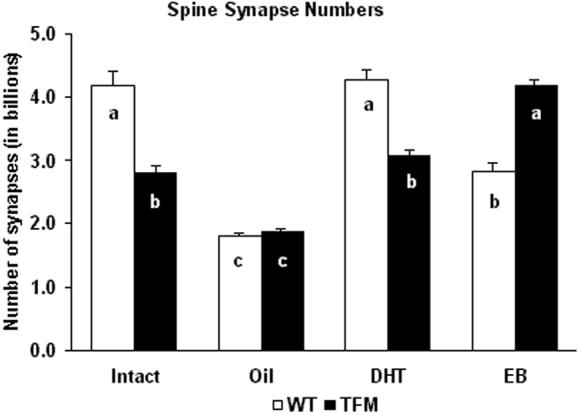
Effects of administration of sesame oil (Oil), 5α-dihydrotestosterone (DHT) and estradiol-benzoate (EB) to wild-type (WT, open bars) and testicular feminization mutant (TFM, solid bars), adult, castrated male rats (n=3 animals per group) on the number of spine synapses in the medial prefrontal cortex. Intact male rats were untreated and gonadally intact. Columns labeled with the same letter are statistically indistinguishable (Newman-Keuls multiple comparison test).
For wild-type male rats, gonadectomy reduced the number of spine synapses by 56.9% (from 4.194 ± 0.213 billion in the intact/wild-type group to 1.808 ± 0.052 billion in the oil/wild-type group, p<0.001), while DHT administration to castrated wild-type males increased this number by 136% (from 1.808 ± 0.052 billion in the oil/wild-type group to 4.268 ± 0.157 billion in the DHT/wild-type group, p<0.001), restoring the number of spine synapses to intact wild-type levels (DHT/wild-type group vs. intact/wild-type group, p>0.05). EB administration to castrated wild-type males elicited a 56.4% increase in the number of spine synapses (from 1.808 ± 0.052 billion in the oil/wild-type group to 2.828 ± 0.118 billion in the EB/wild-type group, p<0.001). The effect of EB, however, was significantly smaller than that of DHT (EB/wild-type group vs. DHT/wild-type group, p<0.001, Fig. 2).
For Tfm male rats, gonadectomy decreased the number of spine synapses by 32.6% (from 2.788 ± 0.12 billion in the intact/Tfm group to 1.879 ± 0.048 billion in the oil/Tfm group, p<0.001), while DHT administration to castrated Tfm males restored spine synapse numbers to intact Tfm levels (from 1.879 ± 0.048 billion in the oil/Tfm group to 3.054 ± 0.119 billion in the DHT/Tfm group, a 62.6% increase, p<0.001; DHT/Tfm group vs. intact/Tfm group, p>0.05). EB administration to castrated Tfm males led to a 123.1% increase in the number of spine synapses (from 1.879 ± 0.048 billion in the oil/Tfm group to 4.191 ± 0.087 billion in the EB/Tfm group, p<0.001), significantly larger than that caused by DHT (EB/Tfm group vs. DHT/Tfm group, p<0.001, Fig. 2).
DHT had a significantly smaller effect on the number of spine synapses in castrated Tfm males than in castrated wild-type rats (DHT/Tfm group vs. DHT/wild-type group, p<0.001), reproducing spine synapse levels found in gonadally intact animals. EB elicited the opposite pattern, inducing the formation of more spine synapses in castrated Tfm males than in castrated wild-type rats (EB/Tfm group vs. EB/wild-type group, p<0.001). There was no significant difference between the number of spine synapses of oil-injected castrated wild-type and castrated Tfm animals (oil/wild-type group vs. oil/Tfm group, p>0.05, Fig. 2).
Two-way ANOVA revealed no significant effect of either genotype (F1,16=0.362, p=0.556) or treatment (F3,16=0.005, p=0.999) on the measured tissue volumes (Fig. 3).
Figure 3.
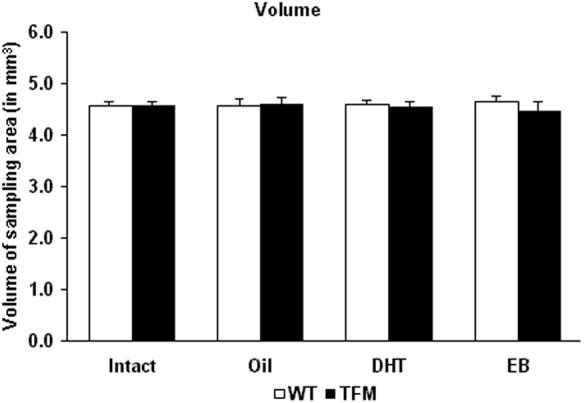
Effects of administration of sesame oil (Oil), 5α-dihydrotestosterone (DHT) and estradiol-benzoate (EB) to wild-type (WT, open bars) and testicular feminization mutant (TFM, solid bars), adult, castrated male rats (n=3 animals per group) on the volume of the sampling area. Intact male rats were untreated and gonadally intact.
Discussion
These results indicate that both androgens and estradiol are effective in inducing spine synapse formation in the mPFC of castrated male rats, although estradiol is less potent. To our knowledge, this is the first direct demonstration at the electron microscopic level that gonadal steroids are able to regulate the number of spine synaptic contacts in the mPFC of adult male rats. We observed a considerable decrease in the number of prefrontal spine synapses both in gonadally intact and in DHT-treated castrated Tfm males, although this synaptogenic effect of androgens is not completely lost in Tfm rats. Surprisingly, prefrontal spine synapse growth in response to estradiol is considerably enhanced in the Tfm males.
Androgens enhance spine synapse formation in the mPFC of adult males
Several studies have investigated the effect of gonadal steroids on spine synapse remodeling in the hippocampus (4, 15). In contrast, only a few studies have examined such gonadal steroid-induced changes in the PFC, despite the fact that this cortical area plays a critical role in working memory (5). It has been shown that changing levels of circulating estrogens influence the total number of dendritic spines in layer I of Walker’s area 46 of young female rhesus monkeys (8), as well as dendritic spine density in layer II/III of the female rat mPFC (7). The results reported in this paper provide direct evidence that spine synapse remodeling in the male rat mPFC is also regulated by androgens, just as estrogens appear to stimulate dendritic spine growth in the female PFC (7, 8). It is hypothesized that the positive effect of gonadal steroids on PFC-dependent cognitive performance (6, 19, 20) is mediated, at least in part, via remodeling of synaptic contacts made by cortical pyramidal neurons (7, 8). Consistent with this idea, Tfm male rats have been found to show impaired performance in the Morris water maze memory task (21), which correlates with the decreased prefrontal spine synapse counts reported in this paper.
Estradiol induces prefrontal synaptogenesis in adult males
A few studies indicate that, similar to androgens (19), estrogen is also effective in enhancing cognitive performance in males (22-24), as well as in females. In contrast to the beneficial cognitive effect of estrogens in males, our previous study indicates that estradiol does not increase the density of spine synapses in the CA1 hippocampal area of castrated male rats (15). This finding seems to contradict the hypothesis that spine synapse remodeling may mediate changes in cognitive performance. However, earlier observations in primates (6, 8), as well as a recent study on female rats (7) suggest that estrogens may exert their effect on synaptogenesis not only in the hippocampus, but also in the PFC. Although EB was not as potent as DHT, our findings show that estradiol enhances spine synapse formation in the mPFC of castrated wild-type male rats. Thus, spine synapse remodeling in the PFC, rather than in the hippocampus, may contribute to the cognitive benefits of estrogen treatment in males.
Gonadal steroid-induced prefrontal synaptogenesis is altered in Tfm animals
We have previously reported that administration of flutamide, an androgen receptor antagonist, fails to block the synaptogenic effect of androgens in the hippocampal CA1 area of both male and female rats (25). This finding raised the question of whether the influence of androgens on the number of hippocampal spine synapses might be mediated via mechanisms different from those involved in the flutamide-sensitive anabolic responses of non-neural target tissues. To examine this issue further, we utilized Tfm male rats. We have found that the effect of DHT on CA1 spine synapse remodeling is virtually identical in Tfm and wild-type castrated male rats, indicating that the androgen receptor is not involved in this response (16). In contrast to the hippocampus, the potency of DHT to enhance spine synapse formation in the mPFC of castrated Tfm males is markedly reduced, when compared to wild-type rats, but not completely lost. In addition, the present results indicate that certain changes occur in the mPFC of Tfm males, which make it more sensitive to estrogens. The mechanisms underlying this alteration in prefrontal estrogen sensitivity, however, remain unknown. An early study has reported that the brain of Tfm mice has the same estrogen binding capacity as wild-type males (26), suggesting that the Tfm mutation causes no change in the expression of estrogen receptors in general, at least in mice. Another level where alterations may occur is at the intracellular signaling pathways downstream of steroid receptors. Unfortunately, data specific to prefrontal estrogen receptor expression or to estrogen-related signaling mechanisms are currently not available in Tfm animals. More importantly, because estrogen-treated Tfm animals generate prefrontal spine synapse levels comparable to those of gonadally intact, wild-type males, indicating that the synaptogenic mechanisms are fully functional in Tfm rats, the most logical conclusion is that androgen-induced spine synapse remodeling in the mPFC of male rats is dependent, at least to some extent, on functional androgen receptors.
The observed residual synaptogenic activity in the mPFC of gonadally intact and DHT-treated castrated Tfm males may be explained by the fact that in Tfm rats, the androgen receptor remains partially functional (13). However, it cannot be excluded that alternative mechanisms are also involved, especially in light of our previous observations in the Tfm male hippocampus (16). Testosterone, the primary androgen secreted by the testis can be aromatized to estradiol, while DHT is metabolized in the brain to 5α-androstane-3β,17β-diol that is a potent agonist at estrogen receptor-β (27). Because prefrontal spine synapse remodeling in the male is sensitive to estrogens, conversion of androgens to estrogenic metabolites may be one of the alternative mechanisms. However, it appears unlikely that the estrogenic metabolites play a significant role based on the fact that prefrontal spine synapse formation in response to androgens remains reduced in Tfm males despite the increased sensitivity of these animals to estrogens. Another metabolite of DHT, 5α-androstane-3α,17β-diol potentiates the effect of GABA on the GABA-A receptor as an allosteric modulator (28), offering another alternative mechanism to explain the residual synaptogenic action of androgens in Tfm animals. Based on in vivo studies, Rudick and Woolley (29) have concluded that the effect of estradiol on dendritic spine density involves disinhibition of pyramidal neurons. Similarly, the effect of androgens on spine synapse remodeling in both the mPFC and hippocampus could involve GABA receptor signaling in the male, leading to the disinhibition of pyramidal cells and proliferation of spine synapses.
Summary
Our findings support the conclusion that testosterone derived from the testis plays a critical role in sustaining the normal rate of synaptogenesis in the mPFC of adult male rats. This androgen-dependent spine synapse remodeling in the mPFC may contribute, at least partially, to the cognitive effects of this steroid hormone. It is well known that circulating testosterone is converted to DHT in the rat brain, where both testosterone and DHT binds to nuclear androgen receptors (30). Although additional studies are required to better define the mechanisms underlying the synaptogenic effect of androgens in the rat mPFC, the data reported here suggest a role for the androgen receptor in this effect. In this respect, the mPFC is considerably different from the hippocampus, where the effect of androgens on spine synapse remodeling seems to be entirely independent of the androgen receptor (16).
Acknowledgments
This work was supported by NIH grants MH074021 (TH), NS045195 (CLJ), MH060858 (CL), NS042644 (CL), as well as by a grant from the Hungarian National Office for Research and Technology RET-08/04.
The authors are indebted to Klara Szigeti-Buck and Jeremy Bober for excellent technical assistance.
Footnotes
Disclosure summary
Disclosure statement: The authors have nothing to disclose.
NIH statement
This is an un-copyedited author manuscript copyrighted by The Endocrine Society. This may not be duplicated or reproduced, other than for personal use or within the rule of “Fair Use of Copyrighted Materials” (section 107, Title 17, U.S. Code) without permission of the copyright owner, The Endocrine Society. From the time of acceptance following peer review, the full text of this manuscript is made freely available by The Endocrine Society at http://www.endojournals.org/. The final copy edited article can be found at http://www.endojournals.org/. The Endocrine Society disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The citation of this article must include the following information: author(s), article title, journal title, year of publication and DOI.
References
- 1.Dohanich GP. Gonadal steroids, learning and memory. In: Rubin RI, editor. Hormones, Brain and Behavior. Academic Press; San Diego: 2002. pp. 265–327. [Google Scholar]
- 2.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 3.Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 4.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolb B. Functions of the frontal cortex of the rat: a comparative review. Brain Res. 1984;320:65–98. doi: 10.1016/0165-0173(84)90018-3. [DOI] [PubMed] [Google Scholar]
- 6.Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006 doi: 10.1016/j.brainres.2006.07.064. In Press. [DOI] [PubMed] [Google Scholar]
- 8.Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- 9.Bardin CW, Bullock L, Blackburn WR, Sherins RJ, Vanha-Perttula T. Testosterone metabolism in the androgen-insensitive rat: a model for testicular feminization. Birth Defects Orig Artic Ser. 1971;7:185–192. [PubMed] [Google Scholar]
- 10.Beach FA, Buehler MG. Male rats with inherited insensitivity to androgen show reduced sexual behavior. Endocrinology. 1977;100:197–200. doi: 10.1210/endo-100-1-197. [DOI] [PubMed] [Google Scholar]
- 11.Krey LC, Lieberburg I, MacLusky NJ, Davis PG, Robbins R. Testosterone increases cell nuclear estrogen receptor levels in the brain of the Stanley-Gumbreck pseudohermaphrodite male rat: Implications for testosterone modulation of neuroendocrine activity. Endocrinology. 1982;110:2168–2176. doi: 10.1210/endo-110-6-2168. [DOI] [PubMed] [Google Scholar]
- 12.Griffin JE, Leshin M, Wilson JD. Androgen resistance syndromes. Am J Physiol. 1982;243:E81–87. doi: 10.1152/ajpendo.1982.243.2.E81. [DOI] [PubMed] [Google Scholar]
- 13.Yarbrough WG, Quarmby VE, Simental JA, Joseph DR, Sar M, Lubahn DB, Olsen KL, French FS, Wilson EM. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J Biol Chem. 1990;265:8893–8900. [PubMed] [Google Scholar]
- 14.Dorfman RI. Androgens and anabolic agents. In: Dorfman RI, editor. Methods in Hormone Research. Academic Press; New York: 1962. pp. 275–313. [Google Scholar]
- 15.Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacLusky NJ, Hajszan T, Johansen JA, Jordan CL, Leranth C. Androgen effects on hippocampal CA1 spine synapse numbers are retained in Tfm male rats with defective androgen receptors. Endocrinology. 2006;147:2392–2398. doi: 10.1210/en.2005-0673. [DOI] [PubMed] [Google Scholar]
- 17.Hajszan T, Leranth C, Roth RH. Subchronic phencyclidine treatment decreases the number of dendritic spine synapses in the rat prefrontal cortex. Biol Psychiatry. 2006;60:639–644. doi: 10.1016/j.biopsych.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- 19.Janowsky JS, Chavez B, Orwoll E. Sex steroids modify working memory. J Cogn Neurosci. 2000;12:407–414. doi: 10.1162/089892900562228. [DOI] [PubMed] [Google Scholar]
- 20.Bimonte-Nelson HA, Singleton RS, Nelson ME, Eckman CB, Barber J, Scott TY, Granholm AC. Testosterone, but not nonaromatizable dihydrotestosterone, improves working memory and alters nerve growth factor levels in aged male rats. Exp Neurol. 2003;181:301–312. doi: 10.1016/s0014-4886(03)00061-x. [DOI] [PubMed] [Google Scholar]
- 21.Jones BA, Watson NV. Spatial memory performance in androgen insensitive male rats. Physiol Behav. 2005;85:135–141. doi: 10.1016/j.physbeh.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 22.Kampen DL, Sherwin BB. Estradiol is related to visual memory in healthy young men. Behav Neurosci. 1996;110:613–617. doi: 10.1037//0735-7044.110.3.613. [DOI] [PubMed] [Google Scholar]
- 23.Friedman G. The effects of estrogen on short-term memory in genetic men. J Am Med Dir Assoc. 2000;1:4–7. [PubMed] [Google Scholar]
- 24.Cordova M, Jacome L, Lachhman V, Luine VN. Castration impairs and gonadal hormones restore recognition memory in rats. ENDO Meeting. 2004:P2–214. [Google Scholar]
- 25.MacLusky NJ, Hajszan T, Leranth C. Effects of dehydroepiandrosterone and flutamide on hippocampal CA1 spine synapse density in male and female rats: implications for the role of androgens in maintenance of hippocampal structure. Endocrinology. 2004;145:4154–4161. doi: 10.1210/en.2004-0477. [DOI] [PubMed] [Google Scholar]
- 26.Attardi B, Geller LN, Ohno S. Androgen and estrogen receptors in brain cytosol from male, female, and testicular feminized (tfm/y hermaphrodite) mice. Endocrinology. 1976;98:864–874. doi: 10.1210/endo-98-4-864. [DOI] [PubMed] [Google Scholar]
- 27.Pak TR, Chung WC, Lund TD, Hinds LR, Clay CM, Handa RJ. The androgen metabolite, 5alpha-androstane-3beta, 17beta-diol, is a potent modulator of estrogen receptor-beta1-mediated gene transcription in neuronal cells. Endocrinology. 2005;146:147–155. doi: 10.1210/en.2004-0871. [DOI] [PubMed] [Google Scholar]
- 28.Reddy DS. Anticonvulsant activity of the testosterone-derived neurosteroid 3alpha-androstanediol. Neuroreport. 2004;15:515–518. doi: 10.1097/00001756-200403010-00026. [DOI] [PubMed] [Google Scholar]
- 29.Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21:6532–6543. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lieberburg I, McEwen BS. Brain cell nuclear retention of testosterone metabolites, 5alpha-dihydrotestosterone and estradiol-17beta, in adult rats. Endocrinology. 1977;100:588–597. doi: 10.1210/endo-100-2-588. [DOI] [PubMed] [Google Scholar]


