Abstract
Transferrin receptor (TfR)-mediated endocytosis and transcytosis in enterocyte-like Caco-2 cells was investigated in order to elucidate the transport mechanism of orally administered Tf-fusion proteins. Cellular uptake and pulse chase studies were performed in Caco-2, MCF-7 and bladder carcinoma (5637) cells using 125I-labeled Tf (125I-Tf). Co-localization studies of Rab 11 and FITC-Tf endocytosed at either the apical or basolateral membrane were performed in polarized Caco-2 cells grown on Transwells, using confocal laser scanning microscopy (LSM510, Zeiss). Unlike in MCF-7 or 5637 cells, where rapid recycling of Tf was observed, a significant amount of endocytosed 125I-Tf accumulated in Caco-2 cells. This accumulation was especially noticeable with the internalization of 125I-Tf from the apical membrane of polarized Caco-2 cells. Confocal microscopy studies showed that apically, but not basolaterally, endocytosed FITC-Tf was delivered to a Rab11-positive compartment. Our results suggest that a significant amount of apically endocytosed Tf in intestinal epithelial cells is transported to a Rab11-positive compartment, possibly a late endosomal and slow recycling compartment. The Rab11-positive compartment may control the release of apically internalized Tf for either slow recycling to apical membrane or processing to transcytotic compartments.
Keywords: Transferrin, Transferrin Receptor, Caco-2 cells, accumulation, oral absorption
1. INTRODUCTION
During recent years, transferrin (Tf) and its receptor (TfR) has been developed as a potential ligand-receptor binding system to enable drug targeting and delivery of therapeutic agents that would normally suffer from poor pharmacokinetic characteristics [1]. TfR-directed targeting has enabled the efficient delivery of therapeutic agents to sites of interest, including the central nervous system [2] and malignant tissues [3, 4]. In addition, by utilizing knowledge of the intracellular sorting and recycling pathways of TfR, including Rab and PI(3)K mediated processes [5, 6], one can maximize the transepithelial delivery of peptide-based therapeutics. Depending upon the desired result, apparently paradoxical effects can be achieved. For example, TfR-based strategies can selectively achieve either an accumulation of the carried drug within targeted tissues, or the delivery of the therapeutic entity across tissues of interest [7].
Our previous studies demonstrated that Tf-based chemical conjugation could be applied for non-invasive delivery of therapeutic proteins across the absorptive barriers, such as the small intestinal [8] and alveolar epithelial [9] cells, which express TfR on the surface. More importantly, a hypoglycemic effect was observed from using orally administered insulin-Tf conjugate in streptozotocin-induced diabetic rats [10, 11]. Similarly, an increase of neutrophil number was observed when a Tf conjugate of G-CSF was administered orally to BDF1 mice [9, 12]. However, the major obstacle with the chemical conjugation methodology is that the chemically cross-linked products are mostly heterogeneous mixtures of various size and composition [9] and, conceivably, are not suitable as therapeutic drugs. In addition, the high cost of preparing Tf chemical conjugates with a reasonable purity also prohibits developing them into marketable drugs. Consequently, recombinant DNA technology has been used to prepare fusion proteins that consist of both Tf or anti-TfR antibody and therapeutic protein moieties for transport and biological activity [13][14].
To demonstrate the feasibility of using a Tf-fusion protein for oral drug delivery, we recently prepared a recombinant plasmid consisting of cDNA from both human Tf and human G-CSF [14]. This fusion protein showed a marked effect on the increase of absolute neutrophil count (ANC) when orally administered to BDF1 mice [14][15]. In oral administration, the fusion protein, G-CSF-Tf, maintained an increased ANC in mice for 4 to 5 days, while only 2 days effect was observed in subcutaneous administration of either the native G-CSF or the fusion protein [14]. Since the life span for neutrophils is only about 12 h, this finding implies that there is a sustained release mechanism of G-CSF-Tf transport from the intestine to the blood stream. The fact that subcutaneously injected G-CSF and G-CSF-Tf have a similar effect on neutrophil counts suggests that the prolonged effect of orally administered G-CSF-Tf is most likely due to an accumulation in the intestinal epithelium, followed by a slow release into blood circulation.
In this report, we use Caco-2 cells as a model to study the process of TfR in intestinal epithelium. We found that Caco-2 cells indeed are different from other cultured cells in the recycling and accumulation of apically internalized Tf. The accumulation of Tf in Caco-2 cells supports our hypothesis that intestinal epithelial cells may play an important role in the sustained release of orally absorbed Tf from the GI tract into the circulation.
2. MATERIALS AND METHODS
2.1. Materials
Recombinant human transferrin was purchased from Sigma (St. Louis, MO). Cell culture medium and reagents were purchased from Gibco BRL (Rockville, MD). Transwells and other culture dishes were products of Corning (Corning, NY). All other chemicals that are not specified above were purchased from Sigma. Transferrin was loaded with iron [16] and iodinated using the chloramines-T method [17]. The specific activities of 125I-Tf ranged from 400 cpm/ng to 900 cpm/ng. Rabbit polyclonal antibody against Rab11 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The rabbit polyclonal antibody against ZO-1 and Alexa-Fluor labeled secondary antibodies were purchased from Molecular Probes (Eugene, OR).
FITC-Tf was prepared by reacting FITC (Sigma) with human recombinant Tf with a molar ratio of 50 to 1 in 1 ml of 1 M sodium bicarbonate, pH 9.0, under constant stirring for 1 h at 25°C. Subsequently, the reaction mixture was dialyzed 20 h against 2 L PBS at 4°C to remove unreacted FITC. The degree of labeling was estimated as 4 FITC per Tf molecule using the extinction coefficients of FITC and Tf.
2.2. Cell culture
All experiments were performed using human colon carcinoma cells (Caco-2 or its subclone, C2BBe1), human breast adenocarcinoma cells (MCF-7), and human bladder carcinoma cells (5637). These cells were obtained from American Type Culture Collection (Rockville, MD, USA). The procedure used for culturing Caco-2, MCF-7 and 5637 cells was adapted from the report by Grasset E. et al. [18], Strobl J.S. et al. [19] and Grups J.W. et al. [20] respectively. Cells were seeded on 12-well cluster plates. Confluent cell monolayers were obtained within a week after passage and ready for experiments. In some experiments, Caco-2 cell was seeded on 0.4 μm pore size polycarbonate filters in Transwell (Costar, Cambridge, MA) and grew for a week after confluence. The transepithelial electrical resistance (TEER), which was measured by using an epithelial voltohmmeter (EVOM, World Precision Instruments, West Haven, CT), of Caco-2 cell was 400 ohms. cm2.
2.3. Determination of cellular uptake
Confluent (6 day grown) Caco-2, MCF-7 and 5637 cell monolayers and subconfluent (3 day-grown) Caco-2 cell monolayers grown on 12-well cluster plates were washed twice with serum free medium at room temperature and then preincubated with serum free medium with 1 mg/ml bovine serum albumin (BSA) at 37°C for 1 h to deplete serum Tf. Subsequently, 125I-Tf (3 μg/ml) was added to each cell lines in each well in serum free medium with 1 mg/ml BSA. Since the concentration of Tf, i.e., 3 μg/ml or 37 nM, was about 30-fold higher than the reported Kd (1.2 nM) for the binding of Tf to human TfR [21], all studies were under the condition that would saturate all TfR on the surface of Caco-2 cells. After incubation for different time intervals (15 min., 1, 2, 4, 6 and 8 h) at 37°C, cells were then washed with cold PBS (pH 7.4) thrice and solubilized by incubation with 1 N NaOH at 37°C for 10 min. After mixing the content of each well with a Pasteur pipette, the cell lysate was assayed for 125I content by using a Packard gamma counter. Non-specific binding was determined in parallel wells containing 125I-Tf and a 100-fold excess of unlabeled Tf. TfR-mediated cellular uptake was calculated by subtracting non-specific surface binding from the total cellular uptake. Cellular uptakes in subconfluent Caco-2 cells were performed similarly as in the confluent monolayers, except that cells were grown for only 3 days and approximately a 25% confluence was maintained.
Cellular uptake of Caco-2 grown on Transwell was similar to above description. 125I-Tf (3 μg/ml) was added to either the apical or basolateral compartment in individual wells at 37°C in serum free medium with 1mg/ml BSA for various time intervals incubation. The filter grown cells were then washed with cold PBS thrice. The cell monolayer together with the filter membrane were cut and assayed for radioactivity as cellular uptake. The cell integrity during experiment was monitored by TEER.
2.4. Pulse chase studies
After depletion of serum Tf by preincubation (the same procedure as described in cellular uptake study), confluent Caco-2 and MCF-7 cells were incubated with 125I-Tf (3 μg/ml) for 15 min or 4 h in serum free medium (with 1 mg/ml BSA) at 37°C. Non-specific binding was determined in parallel wells containing 125I-Tf and a 100-fold excess of unlabeled Tf. The unbound 125I-Tf was then removed by three washes of serum free medium. The cells were chased at 37°C for 2 h in the presence of excess unlabeled Tf to prevent reinternalization of 125I-Tf. The chase medium from each sample was removed and assayed for radioactivity by gamma counter as total release from the cells. Cell surface associated 125I-Tf was removed by 5-min incubation in mild acid wash buffer. To determine the percentage of intact recycled 125I-Tf, chase medium, which contained 0.3 mg of unlabeled Tf, was precipitated by incubation with 15% of trichloroacetic acid for 15 min at 4°C. After centrifugation, the radioactivity in the precipitate and supernatant was used to measure the intact and degraded 125I-Tf, respectively. Finally, cells were detached by incubation with trypsin 37 °C for 5 min and medium with BSA was added to the cells. Cells were then centrifuged down and assayed as the intracellular retention. The sum of release, cell surface association and intracellular retention is the total initially endocytosed 125I-Tf. Data were presented as percentage of initially endocytosed ligands.
2.5 Confocal fluorescence microscopy
Caco-2 cell monolayers grown on 12 mm Transwells (Costar, Cambridge, MA), with about >90% confluency, were subjected to the pulse-chase assay with FITC-Tf. However, the fields with 100% confluency were selected for confocal analysis. After binding of FITC-Tf (45 ng/ml) to the apical or basolateral surface of the cells for 45 min on ice, the cells were incubated at 37°C for 30 min. The surface-bound FITC-Tf was removed by sequential PBS (containing 0.1 mM Ca2+ and 0.05 mM Mg2+) and mild acid buffer (0.15 M NaCl, 0.5 M Acetate, pH; 2.2) washes and then the cells were fixed using 3.7% para-formaldehyde (Sigma) in PBS. The fixed cell monolayers were permeabilized with 0.2% saponin for 5 min and blocked with 10% FBS in PBS. Permeabilized cells were subsequently incubated with appropriate primary antibodies diluted in 1.5% FBS in PBS. Alexa-Fluor 568 goat anti-rabbit antibody was used for the secondary detection of the protein. The nucleus was labeled by incubation with 4',6-diamidino-2-phenylindole, dilactate (DAPI, dilactate, Invitrogen) dye for 15 minutes on a platform shaker. The prepared slides were subjected to the investigation using a confocal laser scanning microscope (LSM 510 Meta NLO imaging system, Carl Zeiss). Caco-2 cell monolayers were optically sectioned in the z-axis from the apical to the basolateral side of the cell. The step size in the z-axis was 0.4 μm.
3. RESULTS
3.1. A linear increase of cellular uptake of Tf in Caco-2 cells
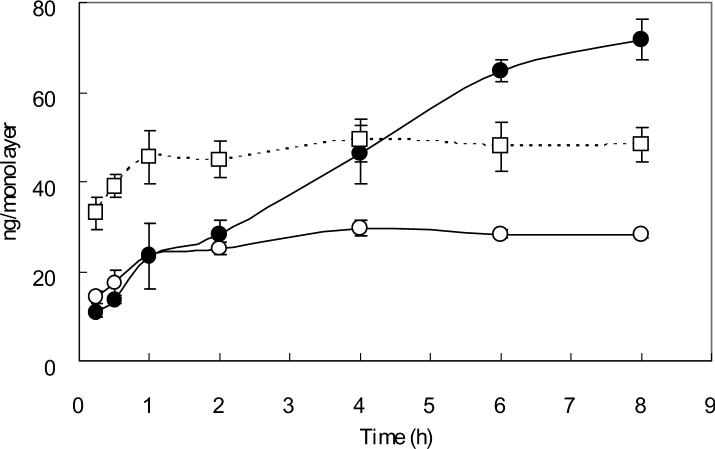
TfR-mediated cellular uptakes of Tf versus time in MCF-7, 5637, and Caco-2 cells are shown in Fig. 1. A biphasic pattern uptake was observed in MCF-7 cells. The initial rapid phase reached a plateau within 1 h. The second phase began after 2 h and reached a plateau within 4 h. The cellular uptake pattern in 5637 cells was similar to that of MCF-7 cells. However, in Caco-2 cells, even though a biphasic pattern uptake was also observed, the second phase began after 2 h increased linearly. The increase was observed up to 8 h.
Fig. 1. Cellular uptake of 125I-Tf in confluent MCF-7, 5637 and Caco-2 cell monolayers.
Confluent MCF-7 (open circles), 5637 (open squares) and Caco-2 (solid circles) cells grown for 6 days on 12-well cluster plates were pre-incubated with serum free medium at 37°C for 1 h to deplete serum Tf. Subsequently, 125I-Tf (3 μg/ml) was added to cells and incubated for different time intervals at 37°C. Cells were then washed with cold PBS and solubilized by incubation with 1 N NaOH. The cell lysate was assayed by using a Packard gamma counter. Non-specific binding was determined in parallel wells. Each point represents the mean of three measurements with error bars representing the standard deviation.
3.2. Long intracellular retention of Tf in Caco-2 cells
Cells were incubated with 125I-Tf for 15 min or 4 h, and chased for 2 h as described in method of pulse chase studies. By interpretation of percentage of initially endocytosed ligands, intracellular retention of Tf in Caco-2 cells was about 20-fold higher than that in MCF-7 cells when cells were incubated with 125I-Tf for 15 min and it was about 14 fold-higher when cells were incubated for 4 h (Table I). More than 90% of the recycled Tf in the medium could be precipitated with TCA in both cell lines, indicating very little degradation of internalized Tf occurred. The intracellular retention increased 3.3-fold when the incubation time was increased from 15 min to 4 h (Table I), indicating a very slow recycling or degradation process occurred in Caco-2 cells.
Table 1.
Comparison of the accumulation of Tf in Caco-2 and MCF-7 cells at different time of incubation
| Type of Cells | Accumulation (% of initial internalized Tf)a | |
|---|---|---|
| 15 min | 4 h | |
| Caco-2 | 9.9 ± 1 | 33.4 ± 1.8 |
| MCF-7 | 0.5 ± 0.03 | 2.4 ± 0.2 |
The percentage represents the mean of three measurements with the standard deviation.
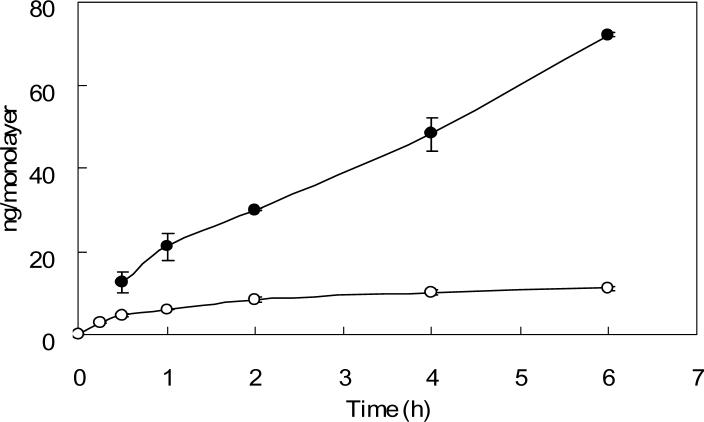
3.3. The retention of Tf is accessible to apical side rather than basolateral side
The profile of cellular uptake of Tf through the apical side and basolateral side of Caco-2 cell is shown in Fig. 2. The cellular uptake through apical dosing increased linearly during the whole experiment until the experiment terminated at 4 h. However, the cellular uptake through basolateral dosing increased linearly up to 1 h and then reached a plateau within 4 h.
Fig. 2. Cellular uptake of 125I-Tf in Caco-2 cells grown on transwells.
125I-Tf was added to either the apical (solid circles) or basolateral (open circles) compartment at 37°C in serum free medium for various time intervals incubation. The filter grown cells were then washed with cold PBS and the cell monolayer together with the filter membrane were cut and assayed for radioactivity as cellular uptake. Non-specific binding was determined in parallel wells. Each point represents the mean of three measurements with error bars representing the standard deviation.
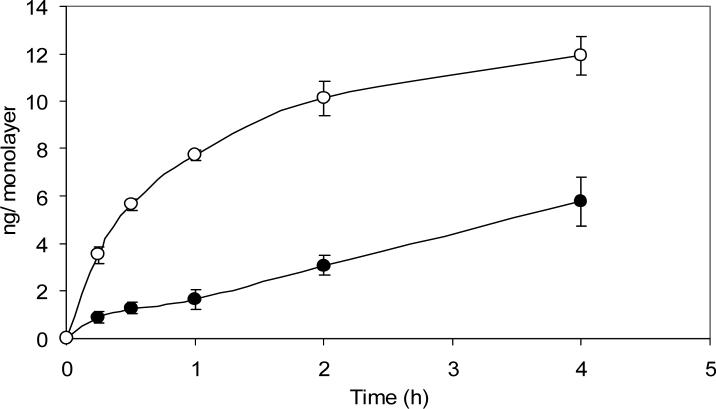
3.4. Long intracellular retention of Tf was not shown in subconfluent Caco-2 cells
The cellular uptake in subconfluent Caco-2 cells (Fig. 3) increased rapidly during the first two hours, followed by a significant slow-down of the uptake and reached a plateau. The cellular uptake in subconfluent Caco-2 cells did not increase linearly during the 6 h experiment as that shown in confluent Caco-2 cells.
Fig. 3. Cellular uptake of 125I-Tf in confluent and subconfluent Caco-2 cell monolayers.
Caco-2 cell monolayers with approximately 25% confluence were used as subconfluent cells. Confluent (solid circles) and subconfluent (open circles) Caco-2 cell monolayers on 12-well cluster plates were pre-incubated with serum free medium at 37°C for 1 h to deplete serum Tf. Subsequently, 125I-Tf was added to cells and incubated for different time intervals at 37°C. Cells were then washed with cold PBS and solubilized by incubation with 1 N NaOH. The cell lysate was assayed by using a Packard gamma counter. Non-specific binding was determined in parallel. Each point represents the mean of three measurements with error bars representing the standard deviation.
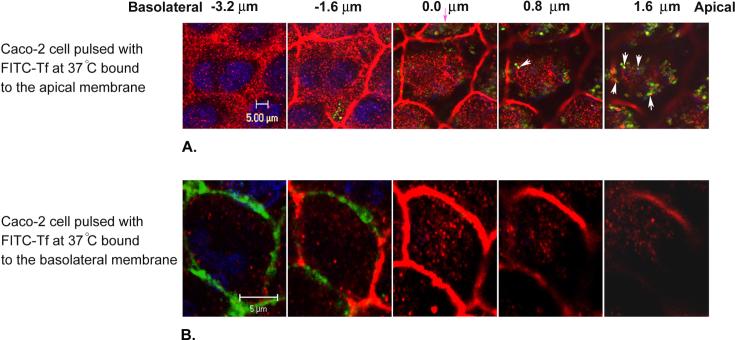
3.5. Co-localization of FITC-Tf and Rab11 was observed only for the apically internalized FITC-Tf
Caco-2 cells were pulsed with FITC-Tf at either the apical or basolateral membrane for 30 minutes at 37°C. Some optical sections, obtained from the galleries of the cells, from the apical towards the basolateral membrane are shown in Fig. 4. In the collected z-series, the first z-section from the apical surface in which the ZO-1 was first observed was set to zero. In the z-sections located above the tight junction, a high degree of co-localization between the apically endocytosed FITC-Tf and Rab11 was observed (Fig. 4A). The co-localization between the apically endocytosed FITC-Tf and Rab11 is shown in the z-section located about 1.6 μm above the tight junction (Fig. 4A, arrows). On the other hand, in the cells that FITC-Tf was endocytosed at the basolateral membrane, the FITC-Tf was mainly found in the z-sections localized basolaterally and under the intracellular level at which the tight junction begins to appear (Fig. 4B). No co-localization between the basolaterally endocytosed FITC-Tf and Rab11 was observed in the z-sections located above the tight junction.
Fig. 4. Co-localization of FITC-Tf and Rab11 was observed only for the apically internalized FITC-Tf.
Caco-2 cells were pulsed with FITC-Tf (green) at either the apical or basolateral membrane for 30 min at 37°C, which was followed by the removal of the unbound and surface-bound FITC-Tf by multiple PBS and acid washes. The cells were immunolabeled with primary and fluorescent secondary antibodies against Rab11 and ZO-1 (both red). The nucleus (blue) was labeled using DAPI. Images selected from a series of optical sections from the apical towards the basolateral membrane of the cells pulsed with FITC-Tf at the apical membrane (A) and the basolateral membrane (B) are shown. The intracellular level at which ZO-1 begins to appear is set to zero (the pink arrow). Some of the co-localizations between the apically endocytosed FITC-Tf and Rab11 are shown as white arrows in (A).
4. DISCUSSION
Results from our recent studies of the in vivo pharmacological effect of Tf conjugates or fusion proteins indicate that there is a sustained release of the protein drugs into the blood stream after oral absorption via TfR-mediated transcytosis. To identify the intestinal epithelial cells as the potential depot for the Tf-conjugates, we used enterocyte-like Caco-2 cells as a model to investigate the intracellular processing of internalized Tf. The cellular uptake of Tf was compared in Caco-2 cells and, as controls, two other human carcinoma cell lines, MCF-7 and 5637 cells. We found that a linear increase in cellular uptake of 125I-Tf was observed only in Caco-2 cells, but not in MCF-7 or 5637 cells. In MCF-7 and 5637 cells, the uptake of 125I-Tf reached a plateau within one hour (Fig. 1) which is consistent with the general belief that a rapid recycling of TfR occurs in most mammalian cells [22]. The linear uptake of 125I-Tf, which is unique in Caco-2 cells, was observed only from the apically, but not the basolaterally, internalized Tf (Fig. 2) and not in subconfluent cells (Fig. 3) In addition, the pulse-chase study also indicated that there was an accumulation of Tf in Caco-2 cells but not in MCF-7 cells (Table I). These findings suggest that apically internalized Tf is retained longer in an intracellular compartment in Caco-2, but not in MCF-7, cells. Furthermore, the accumulation increases as the incubation time is prolonged from 15 min to 4 h (Table I). Since the intracellular retention of Tf has not been reported in other cell culture studies, and has only been mentioned recently as a regulatory mechanism for the intestinal absorption of iron [23], we believe that it supports our hypothesis that the sustained release of orally absorbed Tf is due to the storage of Tf in the intestinal epithelial cells.
To further demonstrate the difference between apically and basolaterally internalized Tf in Caco-2 cells, the co-localization of Tf with Rab11-positive compartments was investigated. Rab11 has been shown to be involved in the slow recycling pathway of internalized Tf in non-polarized cells [24], and both dominant positive (Q70L) and dominant negative (S25N) mutant forms of Rab11 inhibited Tf recycling from perinuclear recycling endosomes in CHO cells [25]. It would be of interesting to find if the targeting of endocytosed Tf to the slow recycling compartments from the apical surface is different from that from the basolateral surface. Fig. 4 shows that a significant amount of apically internalized Tf was co-localized with Rab11, while no such co-localization was detectable when Tf was internalized from the basolateral surface. Conceivably, the delivery of Tf to Rab11-positive compartments can increase the accumulation and, subsequently, the transcytosis of Tf from the apical to the basolateral surface. However, the correlation between the accumulation in Rab11-positive compartments and the regulatory function in iron-absorption of Tf in intestinal epithelial cells needs further investigation.
We believe that intestinal epithelium, rather than the liver, is more likely the depot site of the sustained release of Tf for the oral absorption. The reason is that, once delivered into the portal vein, the Tf from the intestinal absorption will be mixed with a high concentration of endogenous Tf in the blood before reaching the liver [26]. Such a dilution effect will unlikely make exogenous Tf selectively retained in the liver. Therefore, it remains to be demonstrated whether or not the retention of Tf in Caco-2 cells controls the slow transport of Tf from the apical to the basolateral surface. A better understanding of the sustained release mechanism of TfR-mediated transcytosis in intestinal epithelium will help to achieve an optimal efficacy for the oral delivery of Tf-fusion proteins in therapeutics.
5. CONCLUSION
TfR-mediated endocytosis of Tf from the apical surface of polarized Caco-2 cell monolayers is slowly accumulated inside the cell, possibly through the transport into Rab11-positive compartments. Our results support the hypothesis that orally administered Tf can be retained in intestinal epithelium. This retention may explain the prolonged biological effect which has been observed when Tf-fusion proteins were administered orally in animal models.
ACKNOWLEGMENTS
This work was supported in part by NIH Grant GM063647. We thank Dr. Sarah Hamm-Alvarez for providing us with the confocal microscope for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Widera A, Norouziyan F, Shen WC. Mechanisms of TfR-mediated transcytosis and sorting in epithelial cells and applications toward drug delivery. Adv Drug Deliv Rev. 2003;55:1439–1466. doi: 10.1016/j.addr.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Pardridge WM. Molecular Trojan horses for blood-brain barrier drug delivery. Curr Opin Pharmacol. 2006;6:494–500. doi: 10.1016/j.coph.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Weaver M, Laske DW. Transferrin Receptor Ligand-Targeted Toxin Conjugate (Tf-CRM107) for Therapy of Malignant Gliomas. J Neuro-Oncol. 2003;65:3–14. doi: 10.1023/a:1026246500788. [DOI] [PubMed] [Google Scholar]
- 4.Singh M. Transferrin as a targeting ligand for liposomes and anticancer drugs. Curr Pharm Design. 1999;5:443–451. [PubMed] [Google Scholar]
- 5.Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Dam EM, Ten Broeke T, Jansen K, Spijkers P, Stoorvogel W. Endocytosed transferrin receptors recycle via distinct dynamin and phosphatidylinositol 3-kinase-dependent pathways. J Biol Chem. 2002;277:48876–48883. doi: 10.1074/jbc.M206271200. [DOI] [PubMed] [Google Scholar]
- 7.Shah D, Shen WC. The paradox of transferrin receptor-mediated drug delivery--intracellular targeting or transcellular transport? J Drug Target. 1995;3:243–245. doi: 10.3109/10611869509015952. [DOI] [PubMed] [Google Scholar]
- 8.Shah D, Shen WC. The establishment of polarity and enhanced transcytosis of transferrin receptors in enterocyte-like Caco-2 cells. J Drug Target. 1994;2:93–99. doi: 10.3109/10611869409015897. [DOI] [PubMed] [Google Scholar]
- 9.Widera A, Kim KJ, Crandall ED, Shen WC. Transcytosis of GCSF-transferrin across rat alveolar epithelial cell monolayers. Pharm Res. 2003;20:1231–1238. doi: 10.1023/a:1025005232421. [DOI] [PubMed] [Google Scholar]
- 10.Xia CQ, Wang J, Shen WC. Hypoglycemic effect of insulin-transferrin conjugate in streptozotocin-induced diabetic rats. J Pharmacol Exp Ther. 2000;295:594–600. [PubMed] [Google Scholar]
- 11.Xia CQ, Shen WC. Tyrphostin-8 enhances transferrin receptor-mediated transcytosis in Caco-2- cells and increases hypoglycemic effect of orally administered insulin-transferrin conjugate in diabetic rats. Pharm Res. 2001;18:191–5. doi: 10.1023/a:1011032502097. [DOI] [PubMed] [Google Scholar]
- 12.Widera A, Bai Y, Shen WC. The transepithelial transport of a GCSF-transferrin conjugate in Caco-2 cells and its myelopoietic effect in BDF1 mice. Pharm Res. 2004;21:278–284. doi: 10.1023/b:pham.0000016240.81059.ec. [DOI] [PubMed] [Google Scholar]
- 13.Li JY, Sugimura K, Boado RJ, Lee HJ, Zhang C, Pardridge WM. Genetically engineered brain drug delivery vectors: cloning, expression and in vivo application of an anti-transferrin receptor single chain antibody-streptavidin fusion gene and protein. Protein Engineering. 1999;12:787–796. doi: 10.1093/protein/12.9.787. [DOI] [PubMed] [Google Scholar]
- 14.Bai Y, Ann DK, Shen WC. Recombinant granulocyte colony-stimulating factor-transferrin fusion protein as an oral myelopoietic agent. Proc Natl Acad Sci U S A. 2005;102:7292–7296. doi: 10.1073/pnas.0500062102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai Y Y, Shen WC. Improving the oral efficacy of recombinant granulocyte colony-stimulating factor and transferring fusion protein by spacer optimization. Pharm Res. 2006;23:2116–2121. doi: 10.1007/s11095-006-9059-5. [DOI] [PubMed] [Google Scholar]
- 16.Larrick JW, Cresswell P. Transferrin receptors on human B and T lymphoblastoid cell lines. Biochim Biophys Acta. 1979;583:483–590. doi: 10.1016/0304-4165(79)90065-5. [DOI] [PubMed] [Google Scholar]
- 17.Sonoda S, Schlamowitz M. Studies of 125I trace labeling of immunoglobulin G by chloramines-T. Immunochemistry. 1970;7:885–898. doi: 10.1016/0019-2791(70)90051-0. [DOI] [PubMed] [Google Scholar]
- 18.Grasset E, Bernareu J, Pinto M. Epithelial properties of human colonic carcinoma cell line Caco-2 effect of secretagogues. Am. J. Physiol. 1978;173:723–737. doi: 10.1152/ajpcell.1985.248.5.C410. [DOI] [PubMed] [Google Scholar]
- 19.Strobl JS, Kirkwood KL, Lantz TK, Lewine MA, Peterson VA, Worley JF., 3rd Inhibition of human breast cancer cell proliferation in tissue culture by the neuroleptic agents pimozide and thioridazine. Cancer Res. 1990;50(17):5399–5405. [PubMed] [Google Scholar]
- 20.Konstantinov SM, Berger MR. Human urinary bladder carcinoma cell lines respond to treatment with alkylphosphocholines. Cancer Lett. 1999;144(2):153–160. doi: 10.1016/s0304-3835(99)00219-0. [DOI] [PubMed] [Google Scholar]
- 21.Gross CN, Irrinki A, Feder JN, Enns CA. Co-trafficking of HFE, a nonclassical major histocompatibility complex class I protein, with the transferrin receptor implies a role in intracellular iron regulation. J Biol Chem. 1998;273:22068–22074. doi: 10.1074/jbc.273.34.22068. [DOI] [PubMed] [Google Scholar]
- 22.Dautry-Varsat A. Receptor-mediated endocytosis: the intracellular journey of transferrin and its receptor. Biochimie. 1986;68(3):375–381. doi: 10.1016/s0300-9084(86)80004-9. [DOI] [PubMed] [Google Scholar]
- 23.Flemming R. Advances in understanding the molecular basis for the regulation of dietary iron absorption. Curr Opin Gastroenterol. 2005;21:201–206. doi: 10.1097/01.mog.0000153362.98276.db. [DOI] [PubMed] [Google Scholar]
- 24.Urbe S, Huber LA, Zerial M, Tooze SA, Parton RG. Rab11, a small GTPase associated with both constitutive and regulated secretory pathways in PC12 cells. FEBS Lett. 1993;334:175–182. doi: 10.1016/0014-5793(93)81707-7. [DOI] [PubMed] [Google Scholar]
- 25.Sachse M, Ramm G, Strous G, Klumperman J. Endosomes: multipurpose designs for integrating housekeeping and specialized tasks. Histochem Cell Biol. 2002;117:91–104. doi: 10.1007/s00418-001-0348-0. [DOI] [PubMed] [Google Scholar]
- 26.Aisen P. Transferrin metabolism and the liver. Semin Liver Dis. 1984;4:193–206. doi: 10.1055/s-2008-1041770. [DOI] [PubMed] [Google Scholar]