Abstract
The α7β1 integrin is a heterodimeric transmembrane receptor that links laminin in the extracellular matrix to the cell cytoskeleton. Loss of the α7 integrin chain results in partial embryonic lethality. We have previously shown that α7 integrin null embryos exhibit vascular smooth muscle cell defects that result in cerebral vascular hemorrhaging. Since the placenta is highly vascularized, we hypothesized that placental vascular defects in α7 integrin null embryos may contribute to the partial embryonic lethality. Placentae from embryonic day (ED) 9.5 and 13.5 α7 integrin knockout embryos showed structural defects including infiltration of the spongiotrophoblast layer into the placental labyrinth, a reduction in the placental labyrinth and loss of distinct placental layers. Embryos and placentae that lacked the α7 integrin weighed less compared to wild-type controls. Blood vessels within the placental labyrinth of α7 integrin null embryos exhibited fewer differentiated vascular smooth muscle cells compared to wild-type. Loss of the α7 integrin resulted in altered extracellular matrix deposition and reduced expression of α5 integrin. Together our results confirm a role for the α7β1 integrin in placental vascular development and demonstrate for the first time that loss of the α7 integrin results in placental defects.
INTRODUCTION
The placenta is a multifunctional organ essential for maintaining pregnancy, providing nutrients and growth factors to the fetus, removing waste and protecting the fetus from potentially harmful substances [1]. Placental dysfunction can result in insufficient fetal nutrition, which can lead to intrauterine growth restriction (IUGR) [2-6]. At least 8% of human pregnancies end in infant death due to complications that arise from IUGR [1;7;8]. Epidemiological studies indicate IUGR can have lifelong consequences by increasing the risk for heart disease, type-2 diabetes, hypertension, and stroke [9-12]. Abnormalities in placental structure and function have been associated with the majority of reported cases of fetal IUGR [1].
Integrins are a diverse family of cell surface receptors that mediate the interactions between cells and the extracellular matrix [13;14]. Previous studies have implicated integrins as important adhesion molecules in placental development [15-18]. In mice, targeted mutations in α4, α5, α6, αv, β3, and β8 integrin genes result in placental defects and embryonic lethality [18-22].
The α7β1 integrin is a laminin receptor that is highly expressed in vascular smooth muscle [23]. Loss of the α7 integrin causes partial embryonic lethality in which 44% of the α7 integrin null embryos die before birth [24;25]. We have recently demonstrated that loss of the α7 integrin chain leads to cerebral vascular defects which may contribute to the partial embryonic lethality observed in α7 integrin null mice [25]. Studies have shown α7 integrin expression increases in vascular smooth muscle cells (VSMCs) isolated from rats treated with allylamine to induce vascular damage [26]. In addition, treatment of rat VSMCs with platelet derived growth factor (PDGF) increased α7 integrin expression and promoted α7 integrin-mediated adhesion to laminin-1 [27]. Together these results suggest an important role for the α7β1 integrin in vascular development and the progression of vasculoproliferative diseases.
The α7β1 integrin is expressed in the embryonic yolk sac, trophoectoderm cells of the blastocyst and later in the trophoblast layer of the placenta [15]. Although the interaction of the α7β1 integrin with laminin is necessary for trophoblast adhesion during implantation [15], little is known about how loss of the α7 integrin chain affects placental development. Since the placenta is well-vascularized, we hypothesized that loss of the α7 integrin might lead to structural defects that contribute to reduced placental vascular function and partial embryonic lethality.
To investigate if loss of the α7 integrin causes vascular defects in the placenta, we analyzed VSMCs in blood vessels of the placentae from α7 integrin null embryos. Biochemical and histological measurements were used to determine if loss of the α7 integrin in the placenta resulted in vascular defects. The observed vascular smooth muscle abnormalities in placental blood vessels support the hypothesis that the α7β1 integrin has an important role in placental development and suggest loss of this integrin results in placental defects that contribute to the partial embryonic lethality observed in α7 integrin null mice.
MATERIALS AND METHODS
Isolation of placentae
Timed homozygous matings were set up to produce wild-type (C57BL6 strain) and α7 integrin null (C57BL6-α7βgal strain) embryos and placentae. Pregnant female mice were euthanized in accordance with a protocol approved by the University of Nevada, Reno Animal Care and Use Committee. ED9.5 and ED13.5 placentae were dissected out of the uterus, rinsed in PBS and frozen in liquid nitrogen cooled isopentane.
Histological Staining
ED9.5 placentae were cryoprotected by fixing with 4% paraformaldehyde for 2 hours, then washed with phosphate buffered saline (PBS) and placed in a series of 10%, 15%, and 20% sucrose solutions. Placentae were cut cross-sectionally at 10 microns with a Leica CM1850 series cryostat. Hematoxylin and eosin staining was performed following a previously published protocol [28].
Wild-type and α7-/- integrin placentae at ED13.5 were fixed in formalin and embedded in paraffin. Samples were processed and stained with hematoxylin and eosin. Images were obtained using a Nikon SMZ800 light microscope (Nikon, Tokyo, Japan), a Spot Slider RT digital camera (Diagnostic Instruments, Sterling Heights, MI) and Spot software (Diagnostic Instruments, Sterling Heights, MI).
For alkaline phosphatase staining, 20 μm sections were stained using an alkaline phosphatase kit according to the manufacturer’s instructions (Sigma Aldrich, St. Louis, MO). Images were acquired using a Zeiss Axioskop 2 Plus fluorescence microscope, Zeiss Axiocam HRc digital camera and Axiovision 4.1 software. Samples were processed and stained with Mason’s Trichrome by the Department of Pathology at the University of Nevada, Reno.
β -galactosidase staining
α7 integrin null mice were produced by replacing exon 1 of the α7 integrin gene with a LacZ reporter gene. Expression of the α7 integrin promoter was detected by β-galactosidase staining. β-galactosidase staining of wild-type and α7 integrin null placentae was performed following a previously published protocol [25].
Western blot analysis
Placentae were powdered in liquid nitrogen. Protein was extracted in 2% Triton X-100, 100mM Tris-HCL, 50mM NaCl, 10mM MgCl2, 10mM CaCl2, 1:200 Protease Inhibitor Cocktail Set III (Calbiochem, EMD Biosciences, San Diego, CA) and 1mM PMSF. Protein samples were quantified by a Bradford assay. To detect the α7 integrin, 20 μg of protein was loaded on a 7.5% polyacrylamide gel (BioRad Laboratories Inc. Hercules, CA) under non-reducing conditions and transferred to nitrocellulose membranes. To detect tropomyosin, 50 μg of protein was loaded on a 12% polyacrylamide gel under reducing conditions (addition of 5% β-mercaptoethanol and boiling the sample for 5 minutes) and transferred to nitrocellulose membranes. Smooth muscle myosin heavy chain, the α3 integrin, the α5 integrin, and the α6 integrin were detected by loading 50 μg of protein on a 7.5% polyacrylamide gel under reducing conditions and transferring to nitrocellulose membranes.
The α7 integrin was identified with a rabbit polyclonal antibody anti-α7B (B2 347) (Dr. Stephen Kaufman, University of Illinois, Urbana, IL) at a dilution of 1:2000. Tropomyosin was detected with a mouse monoclonal anti-tropomyosin antibody (Sigma Aldrich, St. Louis, MO) at a dilution of 1:2000. Smooth muscle myosin heavy chain was identified with an anti-smooth muscle myosin heavy chain rabbit polyclonal antibody (Biomedical Technologies, Stoughton, MA) at a dilution of 1:1000. The α3 integrin was detected with an anti-α3 integrin rabbit polyclonal antibody (Chemicon International, Temecula, CA) at a dilution of 1:1000. The α5 integrin was detected with an anti-α5 integrin rabbit polyclonal antibody (Dr. Maria Valencik, University of Nevada, Reno, NV). The α6 integrin was detected with an anti-α6 integrin rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:200. Alexa Fluor 680 goat anti-rabbit IgG (Molecular Probes, Eugene, OR) or Alexa Fluor 800 donkey anti-mouse IgG (Molecular Probes, Eugene, OR) was used to detect primary antibodies. Blots were normalized for protein loading by reprobing with sheep anti-gamma actin (Chemicon International, Temecula, CA) or goat anti-Cox-1 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Bands were detected with an Odyssey Imaging System (LiCor Biosciences, Lincoln, NE) and quantified with Odyssey Imaging software.
Immunofluorescence
Placentae were embedded in Tissue-TEK OCT compound (Sakura Finetek USA Inc., Torrance, CA) and 10 micron sections cut with a Leica CM1850 cryostat. Sections were fixed in either cold methanol or 4% paraformalydehyde for two minutes, washed in PBS and then blocked in PBS containing 5% Bovine Serum Albuminin (BSA) for 20 minutes. The α7 integrin was detected with the CA5.5 rat monoclonal antibody (Sierra Biosource, Morgan Hill, CA) at a 1:500 dilution followed by a 1:1000 dilution of FITC-conjugated anti-rat secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Fibronectin was detected with an anti-fibronectin rabbit polyclonal antibody (Sigma Aldrich, St. Louis, MO) at a 1:400 dilution followed by a 1:500 dilution of FITC-conjugated anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). A Cy3-labeled anti-smooth muscle actin monoclonal antibody (Sigma Aldrich, St. Louis, MO) was used at a dilution of 1:500 to identify blood vessels located in the placental labyrinth. Sections were mounted in Vectashield+DAPI (Vector Laboratories, Burlingame, CA). A Zeiss Axioskop 2 Plus fluorescence microscope was used to visualize sections and images were captured with a Zeiss Axiocam HRc digital camera and Axiovision 4.1 software. Nuclei were counted in placentae from ED9.5 wild-type and α7 integrin null embryos over 10 random fields at 1000X magnification.
Statistical Analysis
All averaged data are reported as the mean ± standard deviation unless otherwise stated. Student’s t-tests were performed using SigmaStat 1.0 software (Jandel Corporation, San Rafael, CA). A p-value of P<0.05 was considered statistically significant.
RESULTS
α7 integrin is expressed in placental VSMCs
Loss of α7 integrin results in partial embryonic lethality that begins at ED10.5 [24;25]. To determine which cells within the placenta express the α7 integrin during this critical period, placentae from embryos at ED9.5 and ED13.5 were analyzed by immunofluorescence using anti-α7 integrin antibodies. The α7 integrin was detected in VSMCs within the fetal blood vessels and placental labyrinth from wild-type ED9.5 and ED13.5 embryos and was absent in the placentae from α7 integrin null mice (Fig. 1A). Western analysis confirmed expression of the α7 integrin in wild-type placentae and loss of the α7 integrin in α7 integrin null placentae from ED9.5 and ED13.5 embryos (Fig. 1B). To identify cells expressing the α7 integrin in the placenta, wild-type and α7 integrin null placentae were stained for β-galactosidase activity. β-galactosidase was detected in the labyrinth of α7 integrin null placentae (Fig. 1C). These results demonstrate that the α7β1 integrin promoter is highly active in the placental labyrinth from ED9.5 and 13.5 embryos.
Figure 1. α7 integrin is expressed in placental VSMCs.
A. Immunofluorescence revealed the α7 integrin (green) localized in placental cells that were positive for smooth muscle actin (red). Immunofluorescence confirmed the loss of the α7 integrin from the placentae of ED9.5 and ED13.5 α7 integrin null embryos. Scale bar = 10 μm. B. Western analysis confirmed the absence of the α7 integrin protein in placentae of α7 integrin null embryos at ED9.5 and 13.5. C. β-galactosidase staining confirmed expression from the α7 integrin promoter in the placental labyrinth (PL). Scale bar = 50 μm.
α7 integrin expression increases during placental development
As the embryo develops there is an increase in size and vascularization of the placenta. Quantitative western analysis was performed to determine if there were corresponding changes in the expression of the α7 integrin during placental development. After normalization to gamma actin, a 2.1 fold increase in α7 integrin protein was observed in the placentae from ED9.5 to ED13.5 mice (Fig. 2A). These results indicate increased α7 integrin expression coincides with an increase in the size and vascularization of the placenta.
Figure 2. α7 integrin null mice exhibit reduced fetal and placental weight.
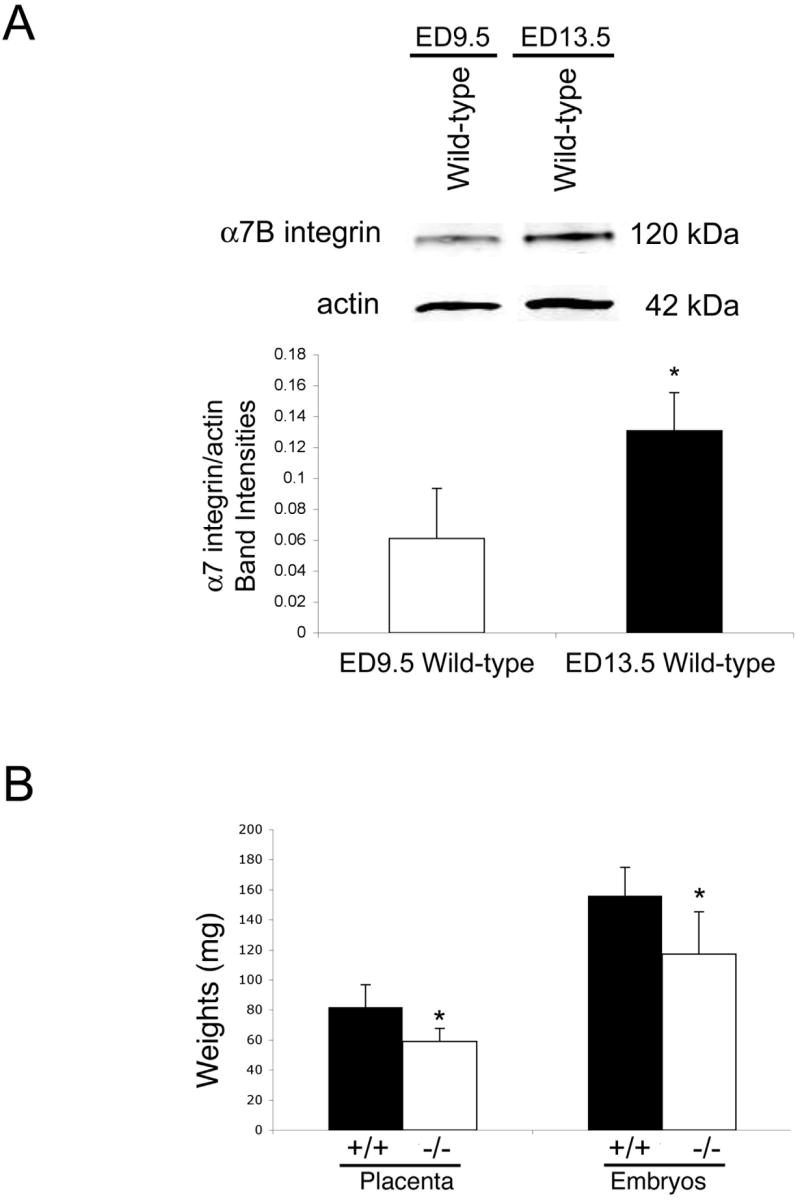
A. α7 integrin expression in the placentae from wild-type embryos was quantitated by western analysis. α7 integrin protein was more abundant in placentae from ED13.5 embryos compared to placentae from ED9.5 embryos. B. The average weight of ED13.5 α7 integrin null placentae and embryos (n=30) was significantly reduced compared to wild-type controls (n=30) (*P<0.05).
Loss of α7 integrin results in reduced placental and fetal weight
Previous studies have shown that placental abnormalities result in placental insufficiency which is characterized by decreased placental and fetal weight [29]. To investigate if loss of the α7 integrin contributes to placental insufficiency, placental and embryonic weights were recorded. Placental weights of α7 integrin null embryos were 28% lower compared to wild-type embryos (Fig. 2B). α7 integrin null embryos were 25% lighter than their wild-type littermates (Fig. 2B). Our results suggest that the α7 integrin is required for normal placental and embryonic growth and that the absence of this integrin chain results in placental insufficiency.
α7-/- placentae exhibit structural defects
To examine if structural defects in the placentae of α7 integrin null fetuses could account for the placental insufficiency, cryosections of placentae from ED9.5 and ED13.5 embryos were stained with hematoxylin and eosin. As expected wild-type placentae showed three distinct layers: the decidua, the spongiotrophoblast, and the fetal blood vessels or the placental labyrinth (Figs 3A, B). In contrast, these layers were not clearly distinguishable in the placentae from ED9.5 α7-/- embryos (Fig. 3A). The placental layers from ED13.5 α7 integrin null embryos appeared disrupted with the spongiotrophoblast layer infiltrating into the placental labyrinth (Fig. 3B). No significant increase in the area occupied by the spongiotrophoblast layer was observed in placentae from α7 integrin null embryos (data not shown). Mason’s Trichrome stain revealed no significant change in the basement membrane or the expression of collagen in the α7 integrin null placentae (data not shown). To examine placental blood vessels, we performed alkaline phosphatase staining on ED13.5 wild-type and α7 integrin null placentae. The labyrinth in α7 integrin null placentae was disorganized and contained irregularly-shaped cells compared to wild-type controls (Figure 3C). These results demonstrate that loss of the α7 integrin results in histological defects that may affect placental function.
Figure 3. Placentae of α7 integrin null embryos exhibit histological defects.
A. Hematoxylin and eosin staining showed extensive fetal blood vessels in placentae from ED9.5 wild-type embryos (FBV). In contrast, placentae from ED9.5 α7 integrin null embryos showed fewer FBV. Scale bar = 0.5 mm. B. Hematoxylin and eosin staining of placentae from wild-type embryos at ED13.5 showed two distinct tissue layers: Spongiotrophoblast (ST) and the Placental Labyrinth (PL). In contrast, the spongiotrophoblast was invading into the labyrinth of α7 integrin null placentae (arrow). Scale bar = 20 μm and 100 μm. C. Alkaline phosphatase staining (blue color) was used to identify structural defects in the placental labyrinth. ED13.5 α7 integrin null placentae exhibited disorganized blood vessels and gaps in the labyrinth. Scale bar = 20 μm.
Placentae from α7-/- embryos contain fewer differentiated VSMCs
To determine if loss of the α7 integrin affected the number of VSMCs in α7 integrin null placentae, placental cryosections were subjected to immunofluorescence with an anti-smooth muscle actin antibody. Compared to wild-type, fewer VSMCs expressing smooth muscle actin were observed in the placentae from ED9.5 α7 integrin null embryos (Fig. 4A). To determine if loss of the α7 integrin resulted in increased cellular proliferation, nuclei were counted in placentae from ED9.5 wild-type and α7 integrin null embryos. Placenta from α7 integrin null embryos contained 54% more cells than wild-type controls (Figure 4B). Quantitative immunoblotting of tropomyosin and smooth muscle myosin heavy chain, two markers of smooth muscle cell differentiation, was performed to confirm these observations (Figure 4C). Tropomyosin expression was 67% lower in ED9.5 α7 integrin null placentae compared to wild-type (Fig. 4C), while expression of smooth muscle myosin heavy chain was 39% lower in ED9.5 α7 integrin null placentae compared to wild-type (Fig. 4C). These results show α7 integrin null placentae exhibit reduced expression of markers associated with the differentiated, contractile smooth muscle phenotype.
Figure 4. Placentae of ED9.5 α7 integrin null embryos have fewer VSMCs.
A. Immunofluorescence showed fewer cells expressing smooth muscle actin (red) in the vascular beds of placentae from ED9.5 α7 integrin null embryos compared to wild-type embryos. Scale bar = 20 μm. B. Nuclei counts revealed a 54% increase in the number of nuclei per field in placentae from ED9.5 α7 integrin null embryos (*P<0.05). C. Quantitative western analysis confirmed lower levels of tropomyosin and smooth muscle myosin heavy chain in the placentae of α7 integrin null embryos at ED9.5 (*P<0.05).
Placentae of ED13.5 α7 integrin null embryos contained fewer smooth muscle actin positive VSMCs compared to wild-type controls (Fig. 5A). These observations were confirmed by quantitative immunoblotting with smooth muscle myosin heavy chain bands normalized to cyclooxygenase-1 (Cox-1). Smooth muscle myosin heavy chain expression was 29% lower in placentae from ED13.5 α7 integrin null embryos (Fig. 5B). These results show that fewer cells expressing the contractile marker smooth muscle myosin heavy chain are present in placentae from ED13.5 α7 integrin null embryos, which could affect placental blood vessel integrity and function.
Figure 5. Placentae of ED13.5 α7 integrin null embryos exhibit vascular defects.
A. Fewer smooth muscle actin positive VSMCs were observed in placentae of α7 integrin null embryos compared to wild-type controls. Scale bar = 10 μm. B. Quantitative western analysis showed a significant reduction in smooth muscle myosin heavy chain in placentae from ED13.5 α7 integrin null embryos compared to wild-type controls (*P<0.05).
Loss of the α7 integrin affects the deposition of extracellular matrix
The α7β1 integrin is a laminin receptor that has been shown to regulate expression of extracellular matrix proteins [28;30]. In addition, the α7β1 integrin can regulate the expression of other integrin chains [25]. To determine if loss of the α7 integrin altered the deposition of extracellular matrix proteins, immunofluorescence was used to detect fibronectin and laminin-1 in wild-type and α7 integrin null placentae. We observed a reduction in fibronectin in α7 integrin null placentae compared to wild-type controls (Fig. 6A). Immunofluorescence analysis revealed no change in laminin-1 expression (data not shown) as a result of the loss of the α7 integrin within the placenta. The observed changes in matrix deposition could result in altered expression of other integrin chains. To determine if the expression of other laminin and fibronectin receptors were altered, expression of α3, α5, and α6 integrin were examined by western analysis. No significant changes in α3 or α6 integrin were observed in α7 integrin null placentae (data not shown). In contrast, ED13.5 α7 integrin null placentae had approximately a 33% decrease in α5 integrin compared to wild-type placentae (Figure 6B). Together, these results provide evidence that loss of the α7 integrin affects matrix deposition and expression of other integrin chains in the placenta.
Figure 6. Loss of the α7 integrin results in altered extracellular matrix deposition and expression of α5 integrin.
A. Wild-type and α7 integrin null placentae were subjected to immunofluorescence using anti-fibronectin to determine if fibronectin deposition was altered. A decrease in fibronectin was observed in α7 integrin null placentae compared to wild-type controls. Scale bar = 10μm. B. Western blot analysis was used to determine changes in the expression of the α5 integrin in ED13.5 wild-type and α7 integrin null placentae. A 33% decrease in α5 integrin was observed in α7 integrin null placentae (*P<0.05, N=4).
DISCUSSION
This study demonstrates that the α7β1 integrin plays an important role in the vascularization of the placenta. Placental vascularization is a critical event during embryonic development allowing the proper exchange of nutrients, oxygen and removal of wastes between maternal and fetal blood supplies [31;31;32;32;33;33;34;34].
Previous studies have shown that the α7 integrin is expressed in cells derived from the trophoectoderm of the developing placenta [15]. Our results showed strong immunolocalization of the α7 integrin to placental blood vessels and increased α7 integrin expression from ED9.5 to ED13.5. α7 integrin expression correlates strongly with placental vascularization during this period. In addition, we confirmed expression from the α7 integrin promoter in the placental labyrinth by β-galactosidase activity.
To confirm the role of the α7 integrin in placental vascularization, we next analyzed the consequences of loss of the α7 integrin on placental development. We speculated that loss of the α7 integrin might lead to growth and structural defects in the placenta. We found that α7 integrin deficient fetuses have reduced placental and fetal weight at ED13.5, consistent with a role for the α7 integrin in placental development.
The wild-type placenta consists of three layers: the decidua, the spongiotrophoblast layer, and placental labyrinth [32;35-37]. Previous studies have shown that a reduction or deletion of one or all of these layers can contribute to fetal death [29;38-40]. To investigate the reason for reduced fetal and placental weight in the α7 integrin null embryos, we examined if histological changes occurred in the placentae of α7 integrin deficient embryos. Our results showed that the spongiotrophoblast layer infiltrated into the placental labyrinth of α7 integrin null placentae. Histologically, the blood vessels in the labyrinth appeared to be disorganized in α7 integrin null placentae, suggesting the α7 integrin plays an important role in placental vascularization.
Previous studies have shown that the α7 integrin is expressed in the smooth muscle cells of the vasculature [23]. VSMC differentiation is associated with the expression of specific smooth muscle cell markers including tropomyosin and smooth muscle myosin heavy chain [41]. VSMCs are known to switch to a less differentiated state after vascular damage [42]. Loss of α7 integrin results in fewer differentiated and contractile VSMCs leading to cerebral vascular hemorrhaging [25]. To explore if a similar mechanism occurred within the placentae of α7 integrin null mice, we examined smooth muscle cell differentiation by examining expression of smooth muscle cell markers within the placenta. We observed reduced protein level expression of smooth muscle myosin heavy chain and tropomyosin in placentae from ED9.5 α7 integrin deficient embryos. In addition, lower levels of smooth muscle myosin heavy chain were observed in placentae from α7 integrin deficient embryos at ED13.5. Changes in these markers could indicate that loss of the α7 integrin results in defects in VSMC differentiation. Significant loss of differentiated VSMCs or inability of the VSMCs to differentiate in the vasculature of α7 integrin null placentae may affect vascular function and be a contributing factor to the observed partial embryonic lethality. The results from ED13.5 α7 integrin null placentae suggest that both placenta defects and cerebral vascular hemorrhaging in embryos are indicative of global vascular defects, which together contribute to the lethality.
Loss of the α7 integrin results in increased cell proliferation indicating the integrin may regulate signaling pathways that maintain the differentiated phenotype of VSMCs. In support of this idea we recently demonstrated that the α7β1 integrin negatively regulates the ERK/MAP kinase signaling pathway in VSMCs [43].
The loss of one integrin in vascular smooth muscle has been shown to cause altered expression of other integrins [25]. In addition, extracellular matrix deposition can be altered by loss of the α7 integrin [28;30]. Surprisingly, the expression of fibronectin was lower but laminin expression was not altered in α7 integrin null placentae. Loss of the α7 integrin appears to affect expression of the α5 integrin which could explain the changes in deposition of fibronectin. Interestingly, targeted deletion of the α5 integrin has been shown to contribute to vascular defects leading to embryonic lethality [21]. The observed decrease in fibronectin and α5 integrin in α7 integrin null placentae could be contributing to the placental defects in α7 integrin null mice.
This study shows for the first time a role for the α7β1 integrin in placental vascular development and that loss of the α7 integrin can result in placental defects. Placental vascular defects may be a contributing factor to the partial embryonic lethality observed in α7 integrin null mice. Altered expression of the α7β1 integrin may play an undefined role in placental defects leading to fetal growth defects and miscarriage.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Regnault TRH, Galan HL, Parker TA, Anthony RV. Placental Development in Normal and Compromised Pregnancies-- A Review. Placenta. 2002;23:S119–S129. doi: 10.1053/plac.2002.0792. [DOI] [PubMed] [Google Scholar]
- 2.Giles W, Trudinger B, Baird P. Fetal umbilical artery flow velocity waveforms and placental resistance, pathological correlation. Br J Obstet Gynaecol. 1985;92:31–38. doi: 10.1111/j.1471-0528.1985.tb01045.x. [DOI] [PubMed] [Google Scholar]
- 3.Krebs C, Longo L, Leiser R. Term ovine placental vasculature: comparison of sea level and high altitude condition by corrosion cast and histomorphometry. Placenta. 1996;18:51. doi: 10.1016/s0143-4004(97)90070-9. [DOI] [PubMed] [Google Scholar]
- 4.Lee M, Yeh M. Fetal microcirculation of abnormal human placen. 1. Scanning electromicroscopy of placental vascular casts from small for gestational age fetus. Am J Obstet Gynecol. 1986;154:1133–1139. doi: 10.1016/0002-9378(86)90774-x. [DOI] [PubMed] [Google Scholar]
- 5.Macara L, Kingdom JC, Kaufmann P. Structural analysis of placental terminal villi from growth-restricted pregnancies with abnormal umbilical artery Doppler waveforms. Placenta. 1996;17:37–48. doi: 10.1016/s0143-4004(05)80642-3. [DOI] [PubMed] [Google Scholar]
- 6.Salafia CM, Pezzullo JC, Minior VK, Divon MY. Placental pathology of absent and reversed end-diastolic flow in growth-restricted fetuses. Obstet Gynecol. 1997;90:830–836. doi: 10.1016/S0029-7844(97)00473-0. [DOI] [PubMed] [Google Scholar]
- 7.Anthony R, Limesand S, Jeckel K. Transcriptional regulation in the placenta during normal and compromised fetal growth. Biochem Soc Trans. 2001;29:42–48. doi: 10.1042/0300-5127:0290042. [DOI] [PubMed] [Google Scholar]
- 8.Pollack R, Divon M. Intrauterine growth retardation: Definition, classification, and etiology. Clin Obstet Gynecol. 1992;35:99–107. doi: 10.1097/00003081-199203000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Barker D, Osmond C, Golding J, Kuth D, Wadsworth M. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. Brit Med J. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker D, Bull A, Osmond C, Simmonds S. Fetal and placental size and risk of hypertension in adult life. Brit Med J. 1990;301:259–262. doi: 10.1136/bmj.301.6746.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker D, Gluckman P, Godfrey K, Harding J, Owens J, Robinson J. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 12.Barker D, Hales C, Fall C, Osmond C, Phipps K, Clark P. Type 2 diabetes mellitus, hypertension, and hyperlipidaemia: relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 13.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 14.van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 15.Klaffky E, Williams R, Yao CC, Ziober B, Kramer R, Sutherland A. Trophoblast-specific expression and function of the integrin alpha 7 subunit in the peri-implantation mouse embryo. Dev Biol. 2001;239:161–175. doi: 10.1006/dbio.2001.0404. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblast. J Clin Invest. 1993;91:950–960. doi: 10.1172/JCI116316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophobalst to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zygmunt M, Boving B, Wienhard J, Munstedt K, Braems G, Bohle RM, Lang U. Expression of cell adhesion molecules in the extravillous trophoblast is altered in IUGR. Am J Reprod Immunol. 1997;38:295–301. doi: 10.1111/j.1600-0897.1997.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 19.Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- 20.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- 22.Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao CC, Breuss J, Pytela R, Kramer RH. Functional expression of the alpha 7 integrin receptor in differentiated smooth muscle cells. J Cell Sci. 1997;110(Pt 13):1477–1487. doi: 10.1242/jcs.110.13.1477. [DOI] [PubMed] [Google Scholar]
- 24.Mayer U, Saher G, Fassler R, Bornemann A, Echtermeyer F, von der MH, Miosge N, Poschl E, von der MK. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet. 1997;17:318–323. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- 25.Flintoff-Dye NL, Welser J, Rooney J, Scowen P, Tamowski S, Hatton W, Burkin DJ. Role for the alpha7beta1 integrin in vascular development and integrity. Dev Dyn. 2005;234:11–21. doi: 10.1002/dvdy.20462. [DOI] [PubMed] [Google Scholar]
- 26.Chao JT, Meininger GA, Patterson JL, Jones SA, Partridge CR, Neiger JD, Williams ES, Kaufman SJ, Ramos KS, Wilson E. Regulation of alpha7-integrin expression in vascular smooth muscle by injury-induced atherosclerosis. Am J Physiol Heart Circ Physiol. 2004;287:H381–H389. doi: 10.1152/ajpheart.00939.2003. [DOI] [PubMed] [Google Scholar]
- 27.Chao JT, Martinez-Lemus LA, Kaufman SJ, Meininger GA, Ramos KS, Wilson E. Modulation of alpha7-integrin-mediated adhesion and expression by platelet-derived growth factor in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2006;290:C972–C980. doi: 10.1152/ajpcell.00136.2005. [DOI] [PubMed] [Google Scholar]
- 28.Rooney JE, Welser JV, Dechert MA, Flintoff-Dye NL, Kaufman SJ, Burkin DJ. Severe muscular dystrophy in mice that lack dystrophin and alpha7 integrin. J Cell Sci. 2006;119:2185–2195. doi: 10.1242/jcs.02952. [DOI] [PubMed] [Google Scholar]
- 29.Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings BA. Protein Kinase Bα/Akt1 Regulates Placental Development and Fetal Growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Rao H, Burkin D, Kaufman SJ, Wu C. The muscle integrin binding protein (MIBP) interacts with alpha7beta1 integrin and regulates cell adhesion and laminin matrix deposition. Dev Biol. 2003;261:209–219. doi: 10.1016/s0012-1606(03)00304-x. [DOI] [PubMed] [Google Scholar]
- 31.Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between Trophoblast Cells and the Maternal and Fetal Circulation in the Mouse Placenta. Developmental Biology. 2002;250:358–373. doi: 10.1016/s0012-1606(02)90773-6. [DOI] [PubMed] [Google Scholar]
- 32.Cross JC, Hemberger M, Lu Y, Nozaki T, Whiteley K, Masutani M, Adamson SL. Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Molecular and Cellular Endocrinology. 2002;187:207–212. doi: 10.1016/s0303-7207(01)00703-1. [DOI] [PubMed] [Google Scholar]
- 33.Pardi G, Marconi AM, Cetin I. Placental-fetal Interrelationship in IUGR Fetuses--A Review. Placenta. 2002;23:S136–S141. doi: 10.1053/plac.2002.0802. [DOI] [PubMed] [Google Scholar]
- 34.Rossant J, Cross JC. Placental development: Lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 35.Cross JC. Genetic insights into trophoblast differentiation and placental morphogenesis. Seminars in Cell & Developmental Biology. 2000;11:105–113. doi: 10.1006/scdb.2000.0156. [DOI] [PubMed] [Google Scholar]
- 36.Hemberger M, Cross JC. Genes governing placental development. Trends in Endocrinology and Metabolism. 2001;12:162–168. doi: 10.1016/s1043-2760(01)00375-7. [DOI] [PubMed] [Google Scholar]
- 37.Wooding FBP, Flint APF. Placentation. In: Lamming GE, editor. Marshall’s Physiology of Reproduction. 4. New York: Chapman and Hall; 1994. pp. 233–460. [Google Scholar]
- 38.Anson-Cartwright L, Dawson K, Holmyard D, Fisher SJ, Lazzarini RA, Cross JC. The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat Genet. 2000;25:311–314. doi: 10.1038/77076. [DOI] [PubMed] [Google Scholar]
- 39.Guillemot F, Nagy A, Auerbach A, Rossant J, Joyner AL. Essential role of Mash-2 in extraembryonic development. Nature. 1994;371:333–336. doi: 10.1038/371333a0. [DOI] [PubMed] [Google Scholar]
- 40.Schreiber M, Wang ZQ, Jochum W, Fetka I, Elliott C, Wagner EF. Placental vascularisation requires the AP-1 component fra1. Development. 2000;127:4937–4948. doi: 10.1242/dev.127.22.4937. [DOI] [PubMed] [Google Scholar]
- 41.Spencer JA, Hacker SL, Davis EC, Mecham RP, Knutsen RH, Li DY, Gerard RD, Richardson JA, Olson EN, Yanagisawa H. Altered vascular remodeling in fibulin-5-deficient mice reveals a role of fibulin-5 in smooth muscle cell proliferation and migration. Proc Natl Acad Sci U S A. 2005;102:2946–2951. doi: 10.1073/pnas.0500058102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thyberg J. Phenotypic modulation of smooth muscle cells during formation of neointimal thickenings following vascular injury. Histol Histopathol. 1998;13:871–891. doi: 10.14670/HH-13.871. [DOI] [PubMed] [Google Scholar]
- 43.Welser J, Lange N, Singer C, Elorza M, Scowen P, Keef KD, Gerthoffer WT, Burkin D. Loss of the α7 integrin promotes ERK activation and altered vascular remodeling. Circ Res. 2007 doi: 10.1161/CIRCRESAHA.107.151415. In Press. [DOI] [PubMed] [Google Scholar]







