Abstract
Background
Sudden cardiac death remains the leading cause of death claiming more than 1000 lives per day in the US alone. Noninvasive means to diagnose rhythm disorders of the heart have relied heavily on the 12-lead ECG and to a lesser extent on higher resolution body surface mapping. These suffer from lack of sensitivity and specificity due to the smoothing effect of the torso volume conductor. In contrast, noninvasive Electrocardiographic Imaging (ECGI) reconstructs potentials, electrograms and activation sequences directly on the heart surface from body surface ECGs and has been applied in animal as well as clinical studies. This presentation summarizes the application of ECGI for imaging epicardial arrhythmogenic substrates and associated properties; in particular dispersion of myocardial repolarization, fractionated electrograms and heterogeneous multipolar potential distributions.
Methods
ECGI was evaluated in a canine model of temperature induced dispersion of myocardial repolarization through localized warming and cooling and in three patients with preserved left ventricular ejection fraction (≥50%) undergoing open heart surgery. Noninvasively reconstructed epicardial potentials, electrograms (and derived measures) as well as activation sequences were compared to their measured counterparts.
Results
Epicardial measures of dispersion of repolarization (activation recovery intervals [ARIs] and QRST integrals) accurately reflected the underlying repolarization properties; prolonged ARIs and increased QRST (warming), shortened ARIs and decreased QRST (cooling) and gradients of adjacent prolonged and shortened ARIs (increased and decreased QRST) during simultaneous warming and cooling. In open heart surgery patients, ECGI reflected the underlying arrhythmogenic substrate by noninvasively reconstructing fractionated electrograms (cross correlation with measured electrograms = 0.72 ± 0.25), regions of heterogeneous multipolar potential distributions and areas of slow conduction.
Conclusion
These studies demonstrate that ECGI can capture and localize noninvasively important electrophysiological properties of the heart. Its clinical significance lies in mapping arrhythmogenic substrates, evaluation and guidance of therapy, and risk stratification.
Introduction
Cardiac arrhythmias remain a leading cause of death and disability with over 300,000 annual deaths in the US alone. Cardiac electrical activity is generally assessed using electrocardiography (ECG) or vectorcardiography (VCG)1, both of which are noninvasive yet lack sensitivity and specificity. This is due to the fact that each electrode on the body surface reflects at each time instant the distance weighted integration of the entire cardiac sources. Consequently, one to one relationships between body surface ECGs and a specific location on the heart do not exist. Additionally, the torso volume conductor consisting of fat, skeletal muscle, sternum, lungs and spine acts as a spatial low pass filter resulting in a smoothed distribution of body surface potentials for a given distribution of cardiac sources. Therefore, geometrical relationships between cardiac sources are not preserved on the body surface1.
Electrocardiographic Imaging (ECGI) is a noninvasive imaging modality that reconstructs noninvasively potentials, electrograms and isochrones on the epicardial surface of the heart from body surface ECGs. Clinical application of ECGI requires the acquisition of body surface ECGs as well as the geometrical relationships between the epicardial surface and the location of recording ECG electrodes2. To date, ECGI has been validated extensively in controlled torso-tank and canine experiments in normal3,4 and abnormal hearts5–7 and during ventricular arrhythmias5,8. More recently, ECGI has been applied in humans9,10 to reconstruct epicardial activation and repolarization during normal sinus rhythm, right bundle branch block, ventricular pacing and atrial flutter and open heart surgery patients11 and in patients receiving devices for cardiac resynchronizations therapy12. Here, we present observations on the ability of ECGI to reconstruct arrhythmogenic properties and substrates in canine and human hearts. Specifically, examples of imaging dispersion of myocardial repolarization as well as fractionated electrograms, and potential gradients will be presented.
Electrocardiographic Imaging (ECGI)
Noninvasive ECGI methodology in humans has been described previously10. Briefly, ECGs are acquired from 224 electrodes on the body surface. The geometrical relationship relating the epicardial surface to the location of the recording ECG electrodes is determined using high resolution computed tomography (CT). Following segmentation of the epicardial surface and digitization of the body surface ECG electrodes, a model of the human torso is constructed using boundary element methods13. The transfer matrix relating the triangulated epicardial and body surfaces is computed. Using the transfer matrix and the body surface ECG recordings as inputs, epicardial potentials are reconstructed using regularized inverse solutions such Tikhonov zero-order14 or the Generalized Minimal Residual methods15. Regularization is necessary because of the ill-posed nature of the inverse problem (that is, large noise fluctuations in the input data [noise on the ECGs or inaccurate electrode locations] may precipitate large errors in the solution).
Imaging Dispersion of Myocardial Repolarization
Dispersion of myocardial repolarization is highly arrhythmogenic. Adjacent myocardial regions with repolarization heterogeneity create substrates for unidirectional block leading to reentry16,17. Mechanistic optical mapping studies in isolated ventricular wedge preparations have confirmed the role of spatial gradients of transmural repolarization in the genesis and maintenance of polymorphic VT in heart failure18 and long QT syndrome19 models. In the clinical setting, noninvasive body surface measures of dispersion of repolarization such as QT dispersion have met with limited success20–22. Additionally, we have shown in a torso-tank model with realistic geometries, and using temperature induced dispersion of repolarization, that body surface measures of dispersion, specifically QT dispersion and QRST integrals lack sensitivity23. Paradoxically, body surface QT dispersion is decreased during left ventricular warming compared to control, whereas corresponding epicardial measures of ARIs dispersion is markedly increased. It is therefore concluded that epicardial measures of dispersion of repolarization reflect the underlying myocardial properties more accurately than body surface measures.
We subsequently applied ECGI to noninvasively reconstruct these measures from the body surface. The noninvasively reconstructed epicardial measures of repolarization dispersion (ARIs and QRST integral maps) were compared to their measured counterpart. Figure 1 shows measured, noninvasively reconstructed and body surface QRST integral maps during control, LV warming, LV cooling and simultaneous LV warming and cooling. While the measured epicardial QRST integral maps (Figure 1, top row) captured accurately the changes in intrinsic repolarization properties (increased QRST integrals during warming, decreased QRST integrals during cooling and both increased and decreased QRST integrals during adjacent warming and cooling of the LV), the body surface QRST integrals (Figure 1, bottom row) showed a similar distribution with a local maximum on the anterior chest throughout all the interventions. The power of ECGI, therefore, was in the ability to reconstruct and localize noninvasively the corresponding spatial dispersion of QRST integrals (Figure 1, middle row) albeit with some smoothing (Figure 1, middle row, third and last columns) starting with these seemingly identical body surface distributions.
Figure 1.
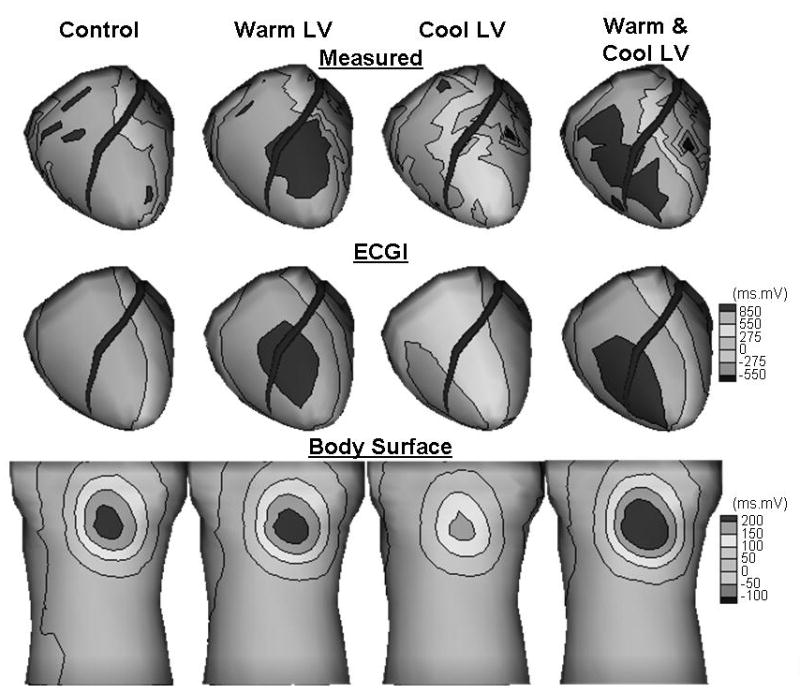
Measured (top), noninvasively reconstructed (middle) and body surface (bottom) QRST integral maps during control, LV warming, LV cooling and simultaneous LV warming and cooling.
Imaging Arrhythmogenic Substrates in Humans
ECGI was more recently applied in three patients undergoing open heart surgery11. Two patients (OR1 and OR2) had ischemic cardiomyopathy and underwent bypass surgery to repair coronary circulation with one patient having undergone prior stent placement. The third patient (OR3) had an ascending aortic arch aneurysm for which he underwent surgery. All patients had preserved LV ejection fraction (LVEF>50%). During surgery, epicardial potentials were recorded intraoperatively from the exposed heart using a 200 electrode sock. Patients underwent the ECGI procedure (CT scan and ECG recording) pre- and post- surgery. Note that CT was performed only during the pre-operative mapping procedure. Noninvasively reconstructed potentials, electrograms and isochrones using ECGI were compared to their measured counterparts during sinus rhythm, endocardial and epicardial pacing. Noninvasively reconstructed electrograms were well correlated to those measured intraoperatively. In addition, ECGI successfully localized the pacing site to within 5–20mm error. The ability of ECGI to image pacing sites provided implications for its potential application for the localization of arrhythmogenic foci.
It is worth noting that patient OR1 has multiple occluded arteries including a severe within-stent restenosis in the proximal right coronary artery (RCA) and patient OR2, while having normal LVEF, suffers from mildly impaired systolic RV function with moderate enlargement of the RV. Indeed, examination of the noninvasively reconstructed electrograms using ECGI reveals negative S-wave electrograms with superimposed small deflections in the mid to basal region and the right margin of the right ventricle for OR1 (Figure 2A). These deflections have been shown to reflect slow conduction in a thin layer of surviving myocardium in an otherwise infarcted tissue. In contrast, electrograms recorded from more remote sites exhibit a normal RS morphology (Figure 2B). These are invasively measured and confirmed with intraoperative mapping. Additionally, invasively measured potentials for patient OR2, show a bipolar potential distribution on the left ventricle (Figure 2C, right column) during repolarization (T-peak), while those measured over the right ventricle show multiple dispersed islands of maxima and minima (Figure 2C, first column). These potential patterns are captured noninvasively, albeit with smoother potential gradients due to the smoothing effect of the mathematical methodology of ECGI. The potential gradient measured and noninvasively reconstructed on the LV is consistent with those observed in healthy volunteers9. This is also consistent with the fact that patient OR2 had preserved LVEF. It can be speculated, on the other hand, that the multipolar potential distribution during T-peak on the right ventricle is indicative of substrate remodeling due to RV cardiomyopathy.
Figure 2.
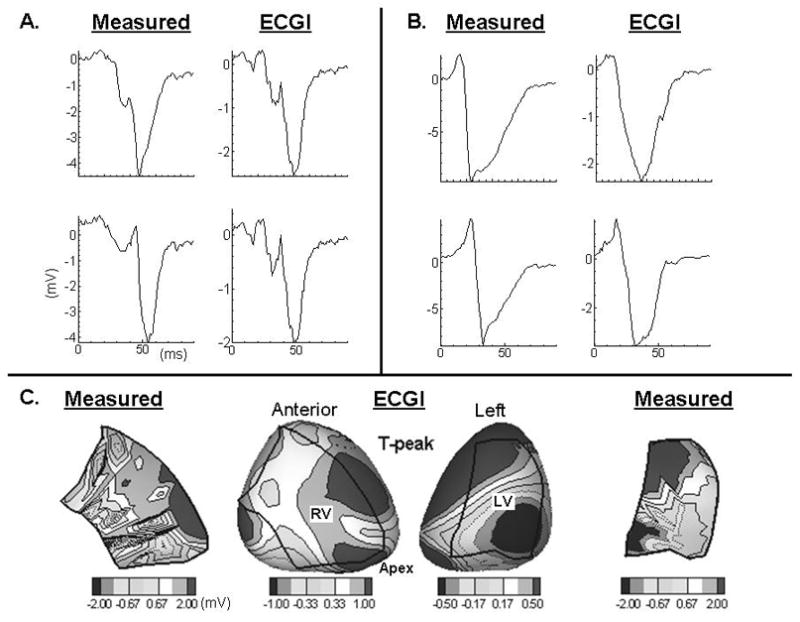
Panel A, invasively measured and noninvasively reconstructed electrograms from the mid to basal region and the right margin of the RV. Panel B, invasively and noninvasively reconstructed electrograms from remote sites closer to the interventricular septum. Panel C, invasively measured (first and last column) and noninvasively reconstructed epicardial potentials during repolarization. Boundaries of the intraoperative 200 electrode sock are overlaid over the RV and LV.
Clinical Implications
Imaging focal activity is critical for characterizing arrhythmia mechanism and guiding ablation therapies. Additionally, imaging epicardial activity during a single beat can provide insight into the mechanisms of ventricular tachycardia with epicardial components. With close to 30% of ventricular tachycardia having epicardial circuits24, and with the advent of transthoracic catheter ablation of epicardial targets25,26, the role for ECGI in mapping noninvasively cardiac electrical activity and guiding ablation therapy will be emphasized further. In fact, noninvasively reconstructed ECGI maps of activation during a single premature ventricular complex with QRS morphology similar to that measured during VT, uncovered and localized the site of earliest activation in an athlete with focal tachycardia and guided ablation therapy27. Because CT imaging is unavailable in the electrophysiology (EP) laboratory where mapping and ablation procedures are performed and in order to render the ECGI procedure more practical for mainstream adoption, new methods for obtaining patient specific geometry using biplane fluoroscopy28 or pseudo-3D ultrasound29 have been developed and successfully tested in the context of ECGI in the EP laboratory. The ability to image noninvasively regions of dispersion of repolarization in the form of QRST integral maps (or other metrics of repolarization dispersion) during a single beat provides a feasible and computationally efficient method for evaluating the severity of the substrate in patients at risk of developing arrhythmias. Noninvasive reconstruction of epicardial measures of repolarization dispersion can therefore provide a tool for rapid screening of patients at risk of sudden death. The significance of applying ECGI for risk stratification is amplified by the lack of sensitivity of body surface measures (such as QT dispersion) at reflecting underlying dispersion of repolarization.
Acknowledgments
I thank Drs. Yoram Rudy, Alan Markowitz, Albert Waldo for their mentorship, data analysis and clinical collaborations; Drs. Charulatha Ramanathan, Ping Jia and Kyungmoo Ryu for their assistance in data collection and analysis; Leslie Ciancibello for technical assistance with CT and Dr. John Haaga for access to CT facilities at University Hopsitals of Cleveland; Dr. Jayakumar Sahadevan for advice and assistance in intraoperative mapping; Celeen Khresitian and James Golebiewski for their assistance in the repolarization experiments. This work was supported by NIH-NHLBI grants R37-HL-33343 and R01-HL-49054 (Dr. Rudy) and R01-HL-38408 (Dr. Waldo).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rudy Y. The electrocardiogram and cardiac excitation. In: Sperelakis N, Kurachi Y, Terzic A, Cohen MV, editors. Heart Physiology and Pathophysiology. San Diego, CA: Academic Press; 2000. pp. 133–48. [Google Scholar]
- 2.Rudy Y, Burnes JE. Noninvasive electrocardiographic imaging. Annals of Noninvasive Electrocardiology. 1999;4:340–59. [Google Scholar]
- 3.Messinger Rapport B, Rudy Y. Noninvasive recovery of epicardial potentials in a realistic heart-torso geometry. Normal sinus rhythm. Circ Res. 1990;66:1023–1039. doi: 10.1161/01.res.66.4.1023. [DOI] [PubMed] [Google Scholar]
- 4.Oster HS, Taccardi B, Lux RL, Ershler PR, Rudy Y. Noninvasive electrocardiographic imaging: reconstruction of epicardial potentials, electrograms, and isochrones and localization of single and multiple electrocardiac events. Circulation. 1997;96:1012–1024. doi: 10.1161/01.cir.96.3.1012. [DOI] [PubMed] [Google Scholar]
- 5.Burnes JE, Taccardi B, Ershler PR, Rudy Y. Noninvasive electrocardiogram imaging of substrate and intramural ventricular tachycardia in infarcted hearts. Journal of the American College of Cardiology. 2001;38:2071–8. doi: 10.1016/s0735-1097(01)01653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnes JE, Taccardi B, MacLeod RS, Rudy Y. Noninvasive ECG imaging of electrophysiologically abnormal substrates in infarcted hearts : A model study. Circulation. 2000;101:533–40. doi: 10.1161/01.cir.101.5.533. [DOI] [PubMed] [Google Scholar]
- 7.Ghanem RN, Burnes JE, Waldo AL, Rudy Y. Imaging dispersion of myocardial repolarization, II: noninvasive reconstruction of epicardial measures. Circulation. 2001;104:1306–12. doi: 10.1161/hc3601.094277. [DOI] [PubMed] [Google Scholar]
- 8.Burnes JE, Taccardi B, Rudy Y. A noninvasive imaging modality for cardiac arrhythmias. Circulation. 2000;102:2152–58. doi: 10.1161/01.cir.102.17.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramanathan C, Jia P, Ghanem R, Ryu K, Rudy Y. Activation and repolarization of the normal human heart under complete physiological conditions. PNAS. 2006;103:6309–6314. doi: 10.1073/pnas.0601533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramanathan C, Ghanem RN, Jia P, Ryu K, Rudy Y. Electrocardiographic Imaging (ECGI): A Noninvasive Imaging Modality for Cardiac Electrophysiology and Arrhythmia. Nature Medicine. 2004;10:422–428. doi: 10.1038/nm1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghanem RN, Jia P, Ramanathan C, Ryu K, Markowitz A, Rudy Y. Noninvasive Electrocardiographic Imaging (ECGI): Comparison to intraoperative mapping in patients. Heart Rhythm. 2005;2:339–354. doi: 10.1016/j.hrthm.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia P, Ramanathan C, Ghanem RN, Ryu K, Varma N, Rudy Y. Electrocardiographic imaging of cardiac resynchronization therapy in heart failure: Observation of variable electrophysiologic responses. Heart Rhythm. 2006;3:296–310. doi: 10.1016/j.hrthm.2005.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brebbia CA, Telles JCF, Wrobel LC. Boundary Element Techniques: Theory and Applications in Engineering. Berlin, Germany: Springer Verlag; 1984. [Google Scholar]
- 14.Tikhonov AN, Arsenin VY. Solutions of Ill-Posed Problems. New York, NY: John Wiley & Sons; 1977. [Google Scholar]
- 15.Ramanathan C, Jia P, Ghanem R, Calvetti D, Rudy Y. Noninvasive electrocardiographic imaging (ECGI): application of the generalized minimal residual (GMRes) method. Ann Biomed Eng. 2003;31:981–94. doi: 10.1114/1.1588655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiological Reviews. 1989;69:1049–169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- 17.El-Sherif N, Caref EB, Yin H, Restivo M. The electrophysiological mechanism of ventricular arrhythmias in the long QT syndrome. Tridimensional mapping of activation and recovery patterns. Circulation Research. 1996;79:474–92. doi: 10.1161/01.res.79.3.474. [DOI] [PubMed] [Google Scholar]
- 18.Akar FG, Rosenbaum DS. Transmural Electrophysiological Heterogeneities Underlying Arrhythmogenesis in Heart Failure. Circ Res. 2003;93:638–645. doi: 10.1161/01.RES.0000092248.59479.AE. [DOI] [PubMed] [Google Scholar]
- 19.Akar FG, Yan G-X, Antzelevitch C, Rosenbaum DS. Unique Topographical Distribution of M Cells Underlies Reentrant Mechanism of Torsade de Pointes in the Long-QT Syndrome. Circulation. 2002;105:1247–1253. doi: 10.1161/hc1002.105231. [DOI] [PubMed] [Google Scholar]
- 20.Malik M, Batchvarov VN. Measurement, interpretation and clinical potential of QT dispersion. Journal of the American College of Cardiology. 2000;36:1749–66. doi: 10.1016/s0735-1097(00)00962-1. [DOI] [PubMed] [Google Scholar]
- 21.Statters DJ, Malik M, Ward DE, Camm AJ. QT dispersion: problems of methodology and clinical significance. Journal of Cardiovascular Electrophysiology. 1994;5:672–85. doi: 10.1111/j.1540-8167.1994.tb01190.x. [DOI] [PubMed] [Google Scholar]
- 22.Surawicz B. Will QT dispersion play a role in clinical decision-making? Journal of Cardiovascular Electrophysiology. 1996;7:777–84. doi: 10.1111/j.1540-8167.1996.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 23.Burnes JE, Ghanem RN, Waldo AL, Rudy Y. Imaging dispersion of myocardial repolarization, I - Comparison of body-surface and epicardial measures. Circulation. 2001;104:1299–1305. doi: 10.1161/hc3601.094276. [DOI] [PubMed] [Google Scholar]
- 24.Sosa E, Scanavacca M, D’Avila A, Piccioni J, Sanchez O, Velarde JL, Silva M, Reolao B. Endocardial and epicardial ablation guided by nonsurgical transthoracic epicardial mapping to treat recurrent ventricular tachycardia. Journal of Cardiovascular Electrophysiology. 1998;9:229–39. doi: 10.1111/j.1540-8167.1998.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 25.Sapp J, Soejima K, Couper GS, Stevenson WG. Electrophysiology and anatomic characterization of an epicardial accessory pathway. J Cardiovasc Electrophysiol. 2001;12:1411–4. doi: 10.1046/j.1540-8167.2001.01411.x. [DOI] [PubMed] [Google Scholar]
- 26.Swarup V, Morton JB, Arruda M, Wilber DJ. Ablation of epicardial macroreentrant ventricular tachycardia associated with idiopathic nonischemic dilated cardiomyopathy by a percutaneous transthoracic approach. J Cardiovasc Electrophysiol. 2002;13:1164–8. doi: 10.1046/j.1540-8167.2002.01164.x. [DOI] [PubMed] [Google Scholar]
- 27.Intini A, Goldstein RN, Jia P, Ramanathan C, Ryu K, Giannattasio B, Gilkeson R, Stambler BS, Brugada P, Stevenson WG, Rudy Y, Waldo AL. Electrocardiographic imaging (ECGI), a novel diagnostic modality used for mapping of focal left ventricular tachycardia in a young athlete. Heart Rhythm. 2005;2:1250–1252. doi: 10.1016/j.hrthm.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghanem RN, Ramanathan C, Jia P, Rudy Y. Heart-surface reconstruction and ECG electrodes localization using fluoroscopy, epipolar geometry and stereovision: application to noninvasive imaging of cardiac electrical activity. IEEE Trans Med Imaging. 2003;22:1307–18. doi: 10.1109/TMI.2003.818263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng LK, Sands GB, French RL, Withy SJ, Wong SP, Legget ME, Smith WM, Pullan AJ. Rapid construction of a patient-specific torso model from 3D ultrasound for non-invasive imaging of cardiac electrophysiology. Medical and Biological Engineering and Computing. 2005;43:325–330. doi: 10.1007/BF02345808. [DOI] [PubMed] [Google Scholar]


