Abstract
Objective
Osteoprotegerin (OPG) and osteopontin (OPN) have been identified within unstable atherosclerosis and circulating concentrates have been linked to cardiovascular events. We studied the influence of OPG and OPN on endothelial adhesion molecule expression and monocyte binding.
Methods
Resting or tumor necrosis factor (TNF-α) activated human endothelial cells were incubated with OPG (0, 0.5, 5, and 10ng/mL) or OPN (0, 2.5, 10 and 50nmol/L). The expression of endothelial genes and proteins were investigated with the Olig GEArray microarray series, multiplexed gene expression analysis, flow cytometry, ELISA and immunohistochemistry. Monocyte binding studies were carried out using fluorescently labeled THP-1 cells and analysed by flow cytometry.
Results
OPG but not OPN stimulated a dose dependent increase in the expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and E-selectin by endothelial cells in the presence of TNF-α (p≤0.05) which was reflected by enhanced binding of THP-1 monocytes. In the absence of TNF-α, OPG had no significant effect on adhesion molecule expression but upregulated angiopoietin-2. When the induction of angiopoietin-2 was inhibited using interfering RNA the ability of OPG to upregulate adhesion molecules in the presence of TNF-α was abolished. OPN did not effect adhesion molecule expression by resting or activated endothelial cells.
Conclusion
OPG upregulates angiopoietin-2 in human endothelial cells sensitizing them to the effects of TNF-α. These findings suggest a mechanism by which OPG may stimulate inflammation in atheroma and thereby promote the progression and complications of atherosclerosis.
1. Introduction
Calcification is a frequent finding in advanced atherosclerosis with the process having similarities to bone remodeling [1–3]. A number of bone remodeling cytokines, in particular osteoprotegerin (OPG) and osteopontin (OPN), have been identified adjacent to areas of atheroma calcification [4–6]. The presence of OPG and OPN within atheroma suggests that these cytokines may actively modulate the progression and complications of atherosclerosis. In support of a role for OPG and OPN in atherosclerosis, local atheroma and circulating concentrations of these cytokines have been associated with cardiovascular events in a variety of different patient populations, such as those with hypertension, diabetes and recent cardiac events [6–12].
Evidence supporting a role for OPN in atherosclerosis is also available from experimental studies. In animal models deficiency of OPN inhibits atherosclerosis development while upregulation of OPN promotes atheroma progression [13,14]. In addition, in vitro studies have demonstrated an important role for OPN in inflammatory cell chemotaxis [15,16]. Data from experimental studies examining the importance of OPG in atherosclerosis is more controversial than that for OPN [17,18]. Studies in vitro suggest that OPG stimulates changes in vascular smooth muscle cells typically demonstrated in atherosclerosis by promoting apoptosis and matrix metalloproteinase release [17]. In a pro-atherosclerotic mouse model however deficiency of OPG was associated with enhanced development of atherosclerosis [18].
Accumulation of monocyte-macrophages has been linked to both atherosclerosis progression and plaque rupture and therefore is believed to be an important target for the medical treatment of cardiovascular disease [19–21]. Control of inflammatory cell accumulation is primarily determined by endothelial expression of adhesion molecules [22,23]. At the time of this study the role of OPG in endothelial-monocyte interactions had not been previously examined. We hypothesized that OPG and/or OPN accumulated locally within atheroma or the circulation favoured monocyte-macrophage entry by altering endothelial expression of adhesion molecules. In this study we investigated this hypothesis in vitro.
2. Methods
2.1. Patients
Umbilical cords were collected from consenting mothers (n=30). The study was approved by the ethics committees of the Townsville Hospital and James Cook University and protocol conformed to ethical guidelines of Declaration of Helsinki.
2.2. Materials
Recombinant human OPG was purchased from ImmunoKontact. Endotoxin concentrates were demonstrated to be below detectable limits by the LAL method (<0.1 ng/μg) [24]. We did not exclude LPS contamination by heat-inactivation experiments in addition since mild heat treatment has recently been demonstrated to inactivate 90% of the TNFα inducing ability of LPS and therefore for our experiment would be a poor guide to LPS contamination [25]. OPN, tumour necrosis factor (TNF)- α and interleukin (IL)-1β were purchased from R&D Systems. Specific monoclonal antibodies to vascular cell adhesion molecule (VCAM)-1 (1.4C3), E-selectin (1.2B6), CD31 (JC70A), von willebrand factor (F8/86) and smooth muscle α-actin (1A4), as well as a goat anti-mouse FITC (F0479) and HRP (P0447) and rabbit anti-goat-HRP (P0160)-conjugated secondary antibody were from DakoCytomation. The monoclonal antibody against intercellular adhesion molecule (ICAM)-1 (R6.5) was kindly donated by Boehringer-Ingelheim and a polyclonal antibody against Angiopoietin-2 (N-18) was from SantaCruz Biotechnology.
2.3. Cell Culture
We elected to utilize human umbilical vein endothelial cells (HUVECs) rather than commercial cells. This allowed experiments to be carried out on a range of different endothelial cells rather than limiting studies to those of a single donor. HUVECS were isolated from freshly collected human umbilical cords by collagenase (Worthington Biochemical Corporation) digestion and cultured according to the method of Jaffe et al. [26]. Briefly umbilical cords were rinsed in DMEM (TropBio) and incubated at 4°C overnight in fungizone (Invitrogen) (10μg/mL). Umbilical veins were rinsed with hanks balanced salt solution (JRH Biosciences) and perfused with 0.1% pre-warmed collagenase (type I) in PBS and incubated for 16 minutes at 37°C. Endothelial cells were collected and cultured at 37°C/5% CO2 in EGM-2 culture media (Cambrex Bio Science Australia). Media was changed within 24 hours of plating then every three days. For experiments, cells were passaged with trypsin-versene (Cambrex BioScience Australia) to 24-well plates. HUVEC purity was ascertained by the typical ‘cobblestone’ morphology of endothelial cells and by immunohistochemistry showing positive staining for Von Willebrand Factor and CD31 and negative staining for smooth-muscle α-actin. Cells were used between passages 3 to 5. The monocyte THP-1 cell line was kindly provided by Dr. Rajiv Khanna (Tumor Immunology Laboratory, Queensland Institute of Medical Research) and maintained at 0.5 × 106 cells/mL in RPMI, supplemented with 25mM HEPES (JRH Biosciences), antibiotics and 10% fetal bovine serum (FBS) (Invitrogen).
2.4. Cell Based ELISA
Preliminary studies to investigate the pattern of TNF-α-induced adhesion molecule expression by HUVECs were carried out by a cell based ELISA as previously described [27]. Briefly, after cytokine treatment HUVEC monolayers were washed with PBS and fixed with freshly prepared 0.5% periodate-lysine-paraformaldehye buffer for 10 min at room temperature. For antigen detection, 40μL of monoclonal antibody in PBS/0.05% tween/1% BSA was added to each monolayer and incubated with shaking at room temperature for 1 hr, followed by 3 washes with PBS. Monolayers were then incubated with a peroxidase conjugated goat-anti-mouse antibody and washed. The peroxidase substrate 3,3′,5,5′-Tetramethylbenzidine solution (Sigma Australia) was added (100μL/well) and incubated with shaking in the dark at room temperature for 15min. The colour reaction was stopped by the addition of 100μL 2N HCL and the optical density at 450nm was read in a Sunrise TECAN microplate reader (Q-Lab). To adjust for cell number, wells were washed with tap water and stained with 50μL 0.08% crystal violet in PBS for 5 min. Cells were washed and the nuclear stain was solubilised with 100uL 33% acetic acid and the optical density was measured at 595nm. Time course studies where monolayers were stimulated with TNF-α (300U/mL) for 0, 6, 8, 12 and 24hrs showed that maximal induction of ICAM-1, VCAM-1 and E-selectin expression were 12, 8 and 6hrs respectively (results not shown). Dose-response curves for TNF-α and IL-1β (0–1000U/mL) were also carried out. Based on these curves, a concentration of TNF-α or IL-1β of 50U/mL was chosen to activate the monolayers for latter experiments. This concentration was sub-maximal and allowed for the detection of both an up or down regulation of expression due to other cytokine treatments.
2.5. Flow Cytometry
Analysis of adhesion molecule expression was also performed by flow cytometry. After incubation with various cytokines cell monolayers were rinsed with cold wash buffer (0.1% BSA/0.1% NaN3/PBS). Due to reported trypsin sensitivity cells were labeled directly before trypsinisation for the detection of E-selectin (4μg/mL) and VCAM-1 (4μg/mL) [28]. For ICAM-1 (10μg/mL) cells were stained following trypsinisation. Cells were washed twice with wash buffer and stained with a FITC-conjugated secondary antibody. Labeled cells were re-suspended in PBS and stained with propidium iodide (0.5μg/mL) to detect dead cells. The cell-associated fluorescence of 10 000 events per sample was analysed in a FACScan flow cytometer (Becton Dickinson) using the Cell Quest software and measured as mean fluorescence intensity (MFLI) in the FL-1 channel. Propidium iodide positive cells were excluded from analysis.
2.6. Monocyte Binding Study
a) Monocyte Labelling: THP-1 cells were collected and washed three times in serum-free RPMI. Cells (1–5 × 106) were re-suspended in serum-free RPMI and stained with calcein-AM (Molecular probes) (10μM) for 30 min at 37°C/5% CO2. The staining process was stopped by 3 washes with cold RPMI/10% FBS. b) Monocyte binding: THP-1 cells were re-suspended in RPMI/10% FBS and 0.5ml of cells were added to rinsed cytokine treated HUVEC monolayers in fresh EGM-2 (106 cells per monolayer). Plates were incubated for 30 min at 37°C with shaking (75rpm). Plates were rotated 90° 3 times during the binding step. Wells were washed 4 times with RPMI to remove unbound THP-1s. The remaining cells were removed with 1mM EDTA/PBS, collected and re-suspended in wash buffer. c) Flow cytometry: Re-suspended cells were analysed as described above with 20 000 events collected. To determine the ratio of THP-1 to endothelial cells, calcein-positive and -negative cells were gated and expressed as a ratio. Controls included labeled and unlabeled THP-1 cells, unlabelled HUVECS and HUVECS incubated with the supernatant of stained THP-1 cells.
2.7. Immunohistochemistry and Haematoxylin and Eosin Staining
For haematoxylin and eosin staining, coverslips with HUVECs/THP-1 cells attached were fixed in 75% methanol/PBS for 10 min at 4°C and dried overnight. The coverslips were incubated with haematoxylin (4 min) followed by eosin (2 min) and dehydrated in graded alcohol and mounted with DEPEX (ProScitech). For angiopoietin-2 staining, cells fixed to coverslips were stained with a polyclonal antibody (1/50) followed by a secondary rabbit anti-goat-HRP (1/100). Staining was developed using DAB (DakoCytomation).
2.8. Preparation of total RNA
Total RNA from endothelial cells stored in RNALater (Ambion) was extracted using the RNeasy Mini Kit (Qiagen) using QiaShredder (Qiagen) for homogenisation, according to the manufacturer’s instruction. The RNA was eluted in RNAse-free water and the integrity was verified by electrophoresis on a 1.2% agarose gel and was visualized with ethidium bromide staining. The concentration was quantified by UV absorption with a nanodrop spectrophotometer (BioLab Ltd).
2.9. cDNA SuperArray Analysis
The Oligo GEArray microarray series (SuperArray Inc.) were used to quantify the expression of 113 genes associated with endothelial cell biology. This technique is highly specific (90% homology) and has an inter-assay coefficient of variation <10%. Briefly 3μg total RNA was reverse transcribed into Biotin-16-dUTP-labelled cDNA probes with the TrueLabeling-AMP method according to the manufacturer’s instructions. The SuperArray membranes (OHS-015) were pre-hybridized at 60°C for at least 2 hours. Hybridization of the Biotin-labeled cDNA probes to the membranes was carried out at 60°C overnight with slow agitation in a hybridization oven. The hybridized membranes were washed in saline sodium citrate buffer once in solution I (one in 2x SSC, 1% SCS) and once in solution II (0.1 x SSC, 0.5% SDS). For detection, membranes were incubated with alkaline phosphatase-conjugated streptavidin, washed and incubated with the chemiluminescent substrate CDP-Star. Images of the membranes were acquired using the Chemidoc XRS system (Biorad Laboratories) and analysed with the web-based GEArray Expression Analysis Suite software. The relative expression level of each gene was determined by comparing the signal intensity of each gene in the array after correction for background and normalization.
2.10. Quantitative Gene expression (QGE)
A novel gene expression analysis method that combined competitive PCR and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) was used to confirm the results of the membrane arrays. This technique has excellent reproducibility (inter-assay coefficient of variation <3%) and high sensitivity of 5 copies of a gene in a sample prior to PCR [29]. Total RNA was sent to the Australian Genome Research Facility (AGRF) and the assay was carried out as per the developers protocol which has been previously validated with comparison to other quantitative gene expression analysis methods [30].
2.11. Small interfering RNA (siRNA) studies
Human angiopoietin-2 (RefSeq NM_001147) sense (CCAGACGGCUGUGAUGAUAtt), antisense (UAUCAUCACAGCCGUCUGGtt) and control (Silencer™ control #1) siRNA oligonucleotides were obtained from Ambion. HUVECs were prepared and cultured as described above and transfected in 6 well plates using siPORT Amine transfection reagent (Ambion) according to the manufacturer’s reverse transfection protocol. Trypsinised HUVECs were added at a concentration of 3×105 cells in 2.3 ml EGM-2 medium (without antibiotics) and transfected using 20nM siRNA and 10μl siPORT Amine per well. Growth medium was replaced 8 hrs after transfection to minimise cytotoxicity. Angiopoietin-2 knockdown was assessed by real-time RT-PCR using total RNA extracted from cells 48 hrs post transfection. Cells were used 48 hrs post transfection with siRNAs in experiments to examine the role of angiopoietin-2 in changes induced by OPG in TNF-α activated HUVECs.
2.12: Real-time RT-PCR
Real-time PCR primers for human angiopoietin-2 and GAPDH were designed using PCR Express® primer design software and were as follows: angiopoietin-2 sense (TTCCTCCTGCCAGAGATGGA), angiopoietin-2 antisense (TGCACAGCATTGGACACGTA); GAPDH sense (GACCACTTTGTCAAGCTCATTTCC); GAPDH antisense (GTGAGGGTCTCTCTCTTCCTCTTGT). One-step SYBR green I-based RT-PCR was performed on the Rotor-Gene 3000 (Corbett Research) using the QuantiTect SYBR Green RT-PCR Kit (Qiagen) according to the manufacturer’s instructions. Briefly, 25μl reactions contained total RNA (40–400ng), 500μM each primer, 0.25μl QuantiTect RT mix and 1x QuantiTect SYBR Green RT-PCR Master Mix. Cycling conditions were: reverse transcription for 30 min. at 50ºC; RT inactivation and polymerase activation step 15min. at 95ºC; quantitative real-time PCR for 45 Cycles: 94ºC for 15s (denaturation), 57ºC for 30s (annealing), 72ºC for 30s (elongation). Fluorescence was measured during the elongation phase. Negative controls were included in which template was absent and RNA was tested for DNA-contamination in reactions in which QuantiTect RT mix was omitted. The comparative Ct method was used to detect relative gene expression ratios normalised to the housekeeping gene, GAPDH. A template dilution series using angiopoietin-2 and GAPDH PCR primers, proved that the rate of Ct change versus the rate of target copy change (i.e. reaction efficiency) was identical for both angiopoietin-2 and GAPDH, hence the ΔΔCt method was used to calculate relative gene expression [31]. Amplicons were run as duplicates in separate tubes to allow for quantification of angiopoietin-2 cDNA normalised to GAPDH cDNA. Each reaction contained 40ng total RNA. The relative angiopoietin-2 expression in angiopoietin-2 siRNA-transfected versus negative control siRNA-transfected HUVECS was estimated using the following formula: 2 –(ΔΔCt), where ΔΔCt = [Ct angiopoietin-2 (angiopoietin-2 siRNA) − Ct GAPDH (angiopoietin-2 siRNA)] − [Ct angiopoietin-2 (control siRNA) − Ct GAPDH (control siRNA)]. Each Ct represents the mean Ct value of sample duplicates, and relative expression was calculated using samples from 2 independent experiments. The percent knockdown by angiopoietin-2 siRNA was calculated as (1–2−ΔΔCt) × 100.
2.13. Statistical analysis
Each experiment was carried out on HUVECS isolated from at least 3 different cords. Results were expressed as a mean ± standard error of percentage of the control for flow cytometry. For dose response experiments a one-way ANOVA was used to test for statistical significance. For the membrane arrays and QGE, results were expressed as mean and standard error and statistical significance was tested using the Mann-Whitney U test. P ≤ 0.05 was considered statistically significant. Analyses were performed using SPSS software 13.0 for Windows.
3. Results
3.1. The effect of OPG and OPN on resting endothelial adhesion molecule expression
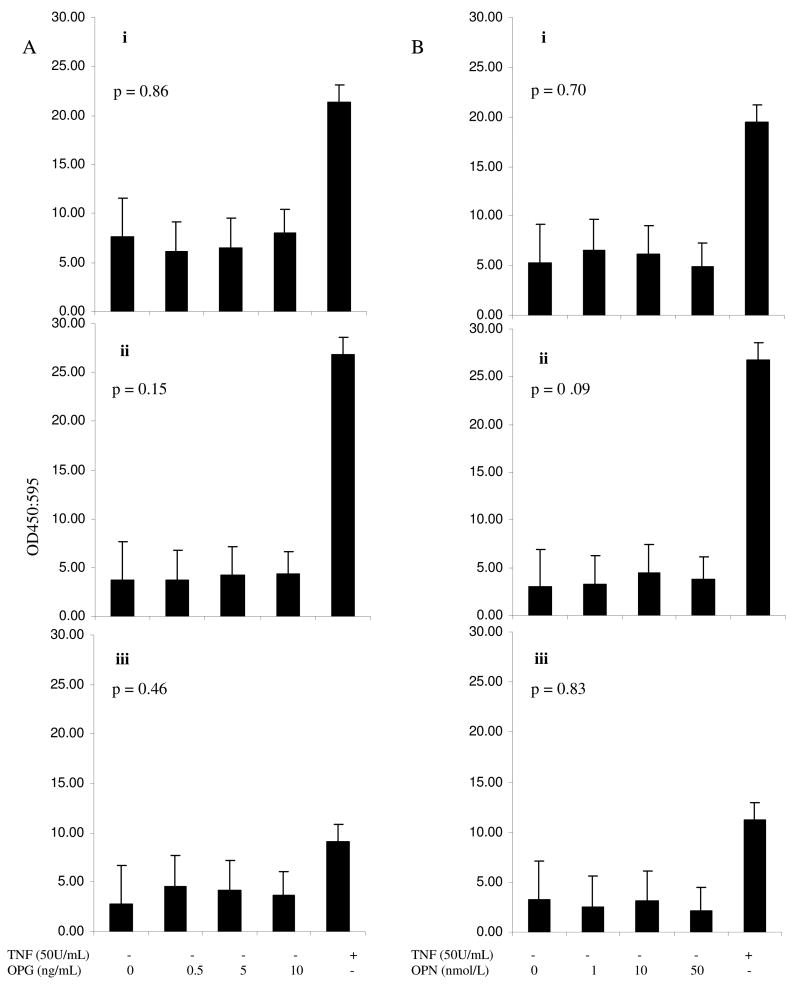
Incubation of OPN or OPG with non-activated HUVEC monolayers did not influence adhesion molecule expression (Figure 1).
Figure 1.
The effect of incubation of OPG (A) and OPN (B) on unstimulated HUVEC ICAM-1 (i), VCAM-1 (ii) and E-selectin (iii) surface expression. Adhesion molecule levels were measured using cell based ELISA at 6, 8 and 12 hours for VCAM-1, E-selectin and ICAM-1, respectively. These incubation periods were based on preliminary time response curves (see methods). Results are expressed as OD 450/595 (n=3). Adhesion molecule expression was not significantly altered. Statistical analysis was by ANOVA.
3.2. Effect of OPG and OPN on endothelial adhesion molecule expression in the presence of TNF-α
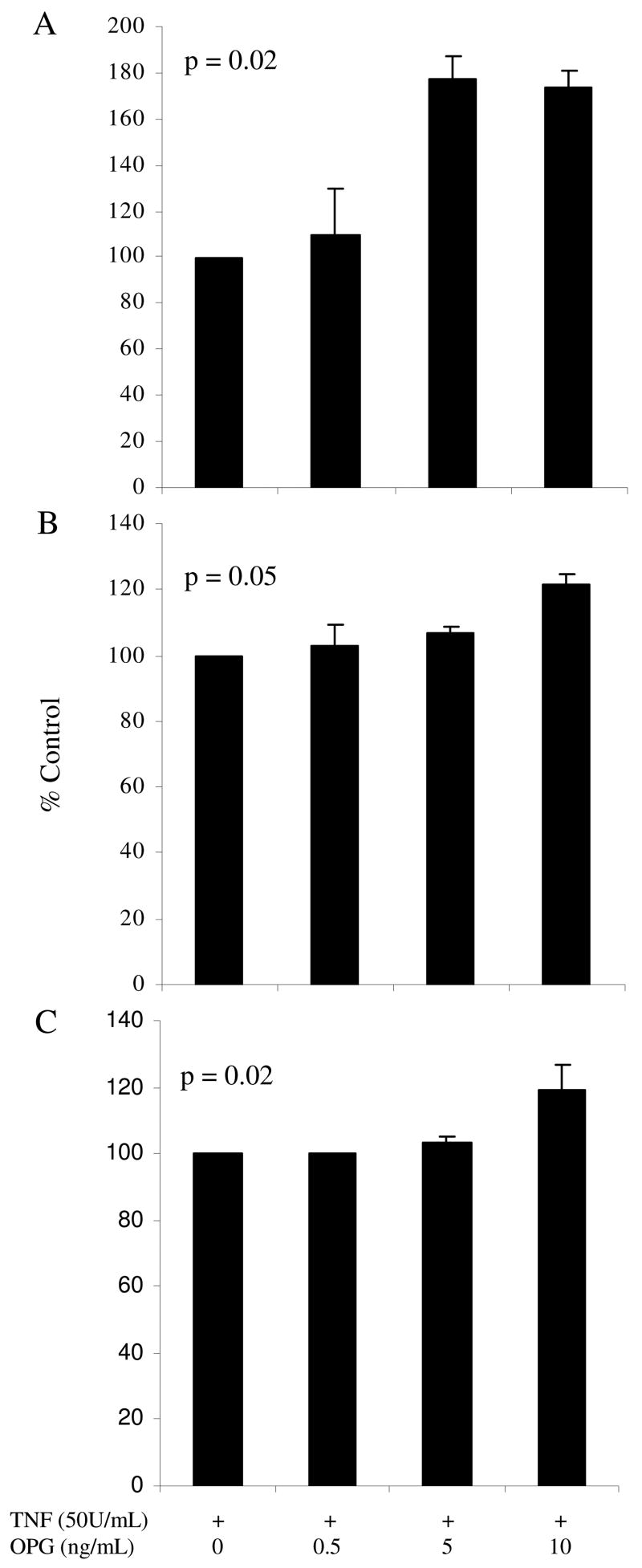
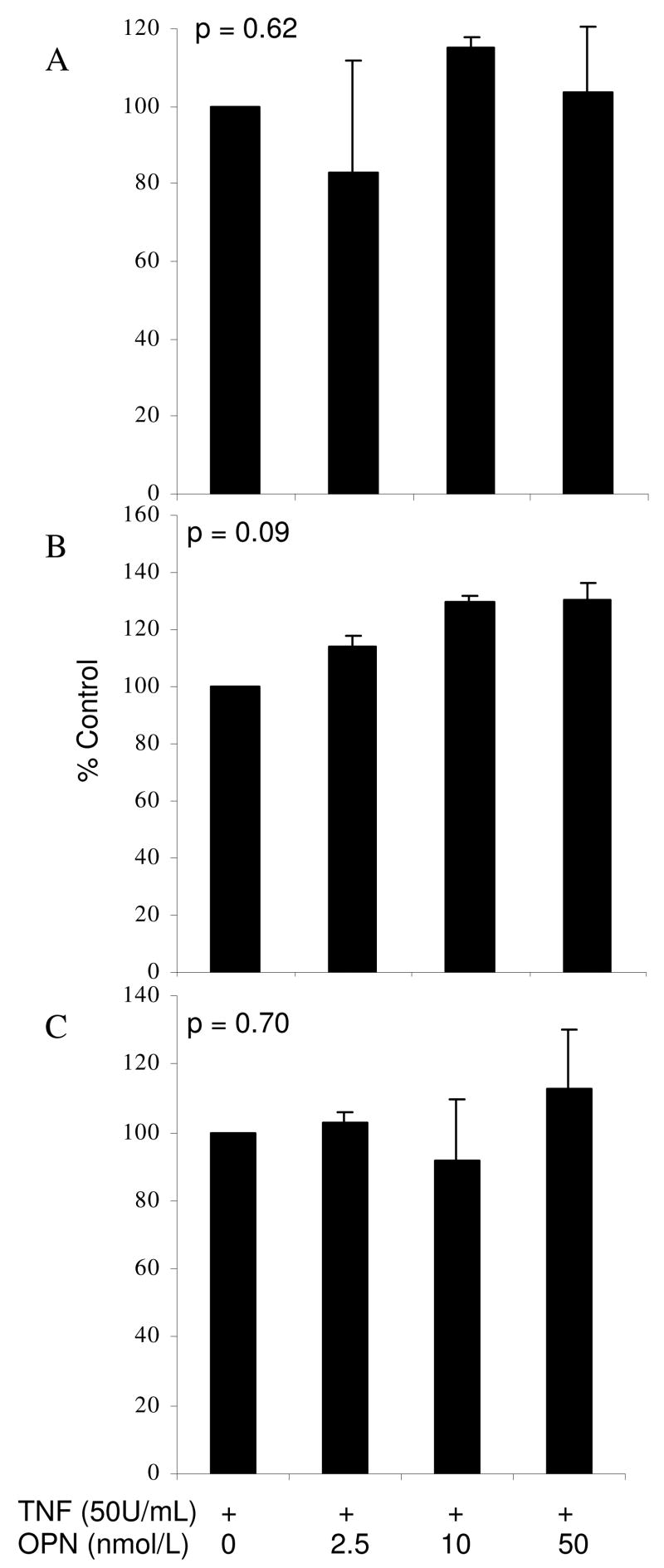
HUVEC monolayers were incubated with both TNF-α (50U/mL) and increasing concentrations of OPG (0.5, 5, 10ng/mL) or OPN (2.5, 10, 50nmoles/L). Based on preliminary time-response experiments, VCAM-1, E-selectin and ICAM-1 surface expression were measured at 6, 8 and 12 hours, respectively. Incubation with OPG stimulated a dose-dependent up regulation of all three adhesion molecules on TNF- α activated HUVECs (Figure 2). OPN had no significant effect on adhesion molecule expression (Figure 3).
Figure 2.
The effect of co-incubation of OPG and TNF-α on HUVEC ICAM-1 (A), VCAM-1 (B) and E-selectin (C) surface expression. Adhesion molecule levels were measured using flow cytometry at 6, 8 and 12 hours for VCAM-1, E-selectin and ICAM-1, respectively. Results are expressed as % of control (± standard error) of 3 independent experiments. Statistical analysis was by ANOVA.
Figure 3.
The effect of co-incubation of OPN and TNF-α on HUVEC ICAM-1 (A), VCAM-1 (B) and E-selectin (C) surface expression. Adhesion molecule levels were measured using flow cytometry at 6, 8 and 12 hours for VCAM-1, E-selectin and ICAM-1, respectively. Results are expressed as % of control (± standard error) of 3 independent experiments. Adhesion molecule expression was not significantly altered. Statistical analysis was by ANOVA.
3.3. The effect of OPN and OPG on monocyte binding
To further investigate the functional and pathological significance of OPG, monocyte-binding studies were carried out using the THP-1 cell line. Confluent activated HUVEC (TNF-α 50U/mL) monolayers in 24-welled plates were treated with increasing concentrations of OPG (0. 0.5 5, 10ng/mL) for 12 hours, prior to incubation with THP-1 monocytic cells for 30 minutes. The number of THP-1 cells firmly adhered per 1000 endothelial cells was measured by flow cytometry. OPG stimulated a dose-dependent up regulation of THP-1 binding (Figure 4A) with a 47% increase in bound THP-1 cells in the presence of 10ng/mL OPG. Parallel experiments of HUVECS grown on cover slips and stained with haematoxylin and eosin supported this finding (Figure 4B). OPN had no effect on monocyte binding (data not shown).
Figure 4.
The effect of incubation of TNF-α activated HUVEC monolayers with OPG on the binding of THP-1 monocytic cells. HUVEC monolayers were pre-incubated with TNF-α (50 U/ml) and OPG (0–10 ng/ml) prior to exposure to monocytes for 30 minutes. The ratio of firmly adherent THP-1 cells to HUVECs was measured by flow cytometry. Results are representative of 1 of 3 independent experiments carried out in triplicate. Monocyte binding is expressed as the ratio of THP-1 cells bound per 1000 HUVECs (± standard error) (A). Parallel wells were fixed and a haematoxylin and eosin stain performed (B) 0 treatment (i), TNF-α (50U/mL) (ii), OPG (5ng/mL)/TNF-α (50U/mL) (iii), OPG (10ng/mL)/TNF-α (50U/mL) (iv). Statistical analysis was by ANOVA.
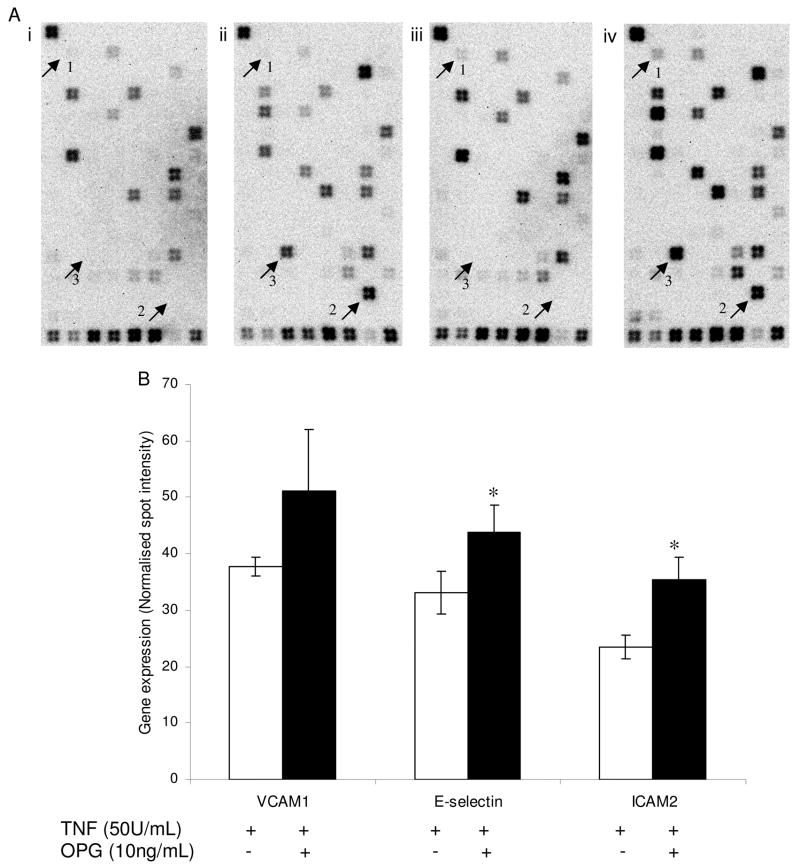
3.4. The effect of OPG on resting and activated endothelial gene expression
Resting and activated (TNF-α 50U/mL) HUVEC monolayers were incubated with OPG (10ng/mL) for 4 hours to investigate the effect of OPG on the expression of genes associated with endothelial cell biology. The original images captured for one complete set of membrane arrays are shown in Figure 5A. This experiment was carried out on 3 separate occasions and the genes that had significant differences between treatments were selected after they were corrected for background and normalized using the interquartile range for the entire set of genes. OPG stimulated significant up-regulation of endothelial E-selectin and ICAM-2 expression in the presence of TNF-α but not alone (Figure 5B). Further gene expression analysis using MALDI-TOF MS supported a role for OPG in upregulating endothelial cell adhesion molecule expression. OPG significantly upregulated the expression of ICAM-1, VCAM-1, E-selectin and ICAM-2 in TNF-α activated HUVECs (Figure 6). OPG did not influence the expression of adhesion molecules in IL-1β activated HUVECs (Table I).
Figure 5.
(A) The effect of OPG on HUVEC gene expression detected using SuperArray membranes. Results are representative of 1 of 3 independent experiments carried out. Endothelial cells were incubated with 0 treatment (i), TNF-α (50U/mL) (ii), OPG (10ng/mL) (iii), OPG (10ng/mL)/TNF-α (50U/mL) (iv). Differences in the expression of genes such as 1, Angiopoietin-2; 2, VCAM-1; 3 E-selectin are noted by an arrowhead. (B) Bars and error bars represent mean ± standard error of intensities for specific genes from three independent membranes. OPG significantly increased expression of E-selectin and ICAM-2 on activated HUVECs. *P≤0.05.
Figure 6.
The effect of OPG on activated HUVEC gene expression detected using quantitative gene expression analysis. TNF-α activated endothelial cells were incubated without or with OPG (10ng/mL) and gene expression for adhesion molecules were measured. The genes that were quantified were ICAM-1 (A), VCAM-1 (B), E-selectin (C) and ICAM-2 (D). Results are representative of 4 independent experiments carried out in duplicate. Bars and error bars represent mean ± standard error. * P≤0.05.
Table I.
Effect of OPG on adhesion molecule expression in IL-1β activated HUVECs.
| Adhesion molecule (pM) | IL-1β (50U/ml) | OPG (10ng/ml) & IL-1β (50U/ml) | P value |
|---|---|---|---|
| ICAM-1 | 3.38 ± 0.43 | 3.53 ± 0.19 | 0.77 |
| VCAM-1 | 0.13 ± 0.03 | 0.19 ± 0.03 | 0.19 |
| E-selectin | 16.57 ± 2.08 | 15.90 ± 0.55 | 1.00 |
| ICAM-2 | 1.19 ± 0.54 | 0.94 ± 0.03 | 0.08 |
HUVECs were incubated with IL-1β (50U/ml) alone or in addition to 10 ng/ml of OPG. Gene expression was assessed by MALDI-TOF MS. Data is presented as mean ± standard error and compared by Mann Whitney U test.
3.5. OPG upregulates endothelial Angiopoietin-2
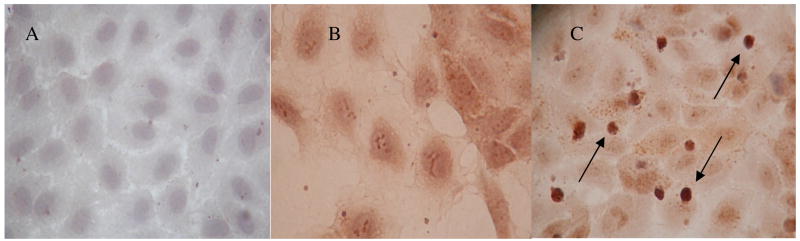
We carried out a number of further experiments in order to investigate the mechanisms that might be responsible for the OPG induced upregulation of adhesion molecules in TNF-α activated HUVECs. Firstly we assessed whether OPG influenced the expression of TNF-α receptors in HUVECS. Incubation of HUVECS with 10ng/ml of OPG for 4 hours had no effect on the expression of TNF receptor subfamily, member 1a (mean expression 35.6±1.9 in OPG treated compared to 41.4±4.1 FM in control cells, n=4, p=0.49) or TNF receptor subfamily, member 1b (mean expression 21.4±1.7 in OPG treated compared to 27.8±3.8 FM in control cells, n=4, p=0.34). Analysis of the SuperArray membranes showed that OPG induced the expression of angiopoietin-2 by resting HUVECs (Figure 5A iii). The adjusted angiopoietin-2 spot intensity for resting HUVECS was 2.8 ± 0.752 compared to 5.6 ± 0.756 for OPG treated cells, p=0.04. These results were supported by analysis using the MALDI-TOF MS, and similarly indicated a significant 2-fold increase in angiopoietin-2 levels (mean expression 162.3±26.9 in OPG treated HUVECS compared to 85.2±8.9 FM in control cells, n=4, p=0.03). HUVEC angiopoietin-2 protein expression was assessed by immunohistochemistry analysis of HUVECs grown on coverslips and treated with OPG (10ng/mL). Initial time course studies (3, 6, 8, 10, 12, 24 and 36 hrs) of OPG treatment were carried out to identify if and when OPG had an effect on angiopoietin-2 protein expression. OPG induced expression at 8 hrs. Representative images (Figure 7) show the effect of OPG on HUVEC angiopoietin-2 expression. Positive staining is seen in a granular pattern in cells exposed to OPG for 10 hrs (Figure 7c). This granular pattern has been previously identified to be angiopoietin-2 expressed within Weibel-Palade bodies [32].
Figure 7.
The effect of OPG on HUVEC angiopoietin-2 expression assessed by immunohistochemistry. Images are representative of 4 independent experiments. Endothelial cells were incubated with isotype control antibody (negative control) (A), 0 Treatment (B) or 10ng/mL OPG for 10 hrs (C). Images were taken at x10 magnification (B and C). Treatment with OPG is associated with increased immunostaining for angiopoietin-2 demonstrated in a granular pattern (positive granules identified by arrows), as previously described [30].
3.6 Interfering with angiopoietin-2 upregulation by OPG inhibits increased expression of adhesion molecules
We used targeted siRNA to investigate whether angiopoietin-2 was important in the effect of OPG on TNF-α activated HUVECs. Angiopoietin-2 expression was measured by real-time PCR in angiopoietin-2 siRNA- and control siRNA-treated cells. Expression was normalised to GAPDH and reported relative to control siRNA. The relative expression of angiopoietin-2 was 0.3±0.05 and 1.0±0 in angiopoietin-2 siRNA- and control siRNA-treated cells, respectively, n=3, p<0.05, which represents a 70% inhibition in expression. Having established that angiopoietin-2 siRNA treatment knocked down angiopoietin-2 expression in HUVECs, we then compared the effect of OPG in angiopoietin-2 siRNA- and control siRNA-treated cells exposed to TNF using quantitative gene expression (Figure 8A–D). OPG had no influence on the expression of angiopoietin-2 in cells transfected with siRNA targeted at angiopoietin-2 (Figure 8). Similarly, OPG did not effect ICAM-1, VCAM-1, E-selectin or ICAM-2 expression in TNF-α activated HUVECs transfected with angiopoietin-2 siRNA (Figure 8). The ability of OPG to upregulate adhesion molecules and angiopoietin-2 in the presence of TNF-α was preserved in HUVECs transfected with control siRNA (Figure 8).
Figure 8.
Effect of OPG on adhesion molecule expression in TNF-α activated HUVECs transfected with control (i) or angiopoietin-2 targeted (ii) siRNA. HUVECs were transfected with siRNA 48 hours prior to incubation with TNF-α (50U/ml) and OPG (10ng/ml) for 4 hours. Gene expression of ICAM-1 (A), VCAM-1 (B), E-selectin (C), ICAM-2 (D) and angiopoietin-2 (E) was measured by MALDI-TOF MS. In control siRNA transfected HUVECs OPG induced an upregulation in ICAM-1, VCAM-1, E-selectin, ICAM-2 and angiopoietin-2 expression (*p≤05). However, in endothelial cells transfected with angiopoietin-2 targeted siRNA OPG had no effect on adhesion molecule or angiopoietin-2 expression (p=0.4–0.9).
4. Discussion
Both OPG and OPN were originally identified in bone [33,34]. OPN is component of the non-collagenous matrix of bone and has been shown to have important effects on cell migration through its ability to bind a variety of integrins [35]. OPG is critical in the control of bone remodeling through inhibition of osteoclast function [33]. Both proteins have been associated with vascular calcification, atherosclerosis and cardiovascular events [4–12]. Since inflammation plays an important role in the progression and complications of atherosclerosis the effect of OPG and OPN on endothelial adhesion molecules is of particular interest.
In this study we demonstrated that OPG upregulates the expression of a number of adhesion molecules in TNF-α activated HUVECs (Figure 2). In contrast OPN failed to stimulate such changes. The functional importance of this effect is supported by the ability of OPG to promote monocyte adhesion to TNF-α activated endothelial cells. The effects stimulated by OPG were dose-dependent being evident at 5 ng/ml (Figures 2 and 4). We have previously demonstrated important effects of OPG at these concentrations on vascular smooth muscle cells, with estimates of tissue concentrations within atherosclerosis and aortic aneurysm being within this range [17]. The circulating concentrations of OPG measured in patients with atherosclerosis have varied between 1 and 6 ng/ml, suggesting the concentrations we used in vitro are relevant to the in vivo situation [36–38]. Of note the actual concentrations measured are dependent on the assay utilized which makes it difficult to relate in vivo concentrations accurately to the in vitro situation [38]. Interestingly OPG has previously been demonstrated to promote endothelial survival in vitro, however, this effect was demonstrated at 500–2000 ng/ml, a concentration unlikely to be relevant to the in vivo situation [39].
The effects of OPG on adhesion molecule expression were only evident when endothelial cells were co-activated with the cytokine TNF-α. OPG had no influence on endothelial adhesion molecule expression alone or in the presence of the cytokine IL-1β (Figure 1 and Table I). Initially we postulated that OPG might act to upregulate TNF receptors in endothelial cells thereby explaining this co-stimulation effect. We demonstrated that OPG had no effect on the expression of TNF receptor subfamily members 1a and 1b. Instead it appears that OPG acts to sensitize endothelial cells to TNF-α by upregulating angiopoietin-2. Angiopoietin-2 has been previously demonstrated to markedly enhance the endothelial adhesion of monocytes in response to TNF-α [40]. These effects were demonstrated both in vitro using HUVECs and in vivo within a mice model [40]. In the present study we demonstrate that OPG induces a 2-fold upregulation of angiopoietin-2 expression using gene expression analysis. Furthermore transfection of HUVECs with siRNA targeted at angiopoietin-2 abolished the ability of OPG to upregulate adhesion molecules in the presence of TNF-α (Figure 8). Our findings therefore suggest that OPG facilitates monocyte adhesion by indirectly sensitizing endothelial cells to the influence of TNF-α which then promotes increased transcription of relevant adhesion molecule genes.
Thus our study supports a role of OPG in promoting inflammation, recognized as an important mechanism in the progression and complications of atherosclerosis [19–21]. Both OPG and TNF-α are present within unstable atherosclerosis and therefore would be able to act together on the endothelial cells of vasa vasorum or the arterial lumen [4,6,41]. It should be noted however that the exact role of OPG in atherosclerosis remains controversial [18]. A large body of epidemiological evidence supports an association between OPG and cardiovascular disease and events [4,5,7–10]. In one study carried out in the pro-atherosclerotic apolipoprotein knock-out mouse, deficiency of OPG was associated with increased development of atherosclerosis [18]. It is unclear how this finding in an animal model relates to atheroma progression and complications in humans. The evidence supporting a role for OPN in atherosclerosis is even stronger than that for OPG. In addition to numerous studies demonstrating an association between circulating concentrations of OPN and atherosclerosis presence and subsequent events, animal studies support the ability of OPN to promote atheroma development [6,11–14]. Our study demonstrates that OPN has no influence on the expression of endothelial adhesion molecules and thus OPN likely promotes atherosclerosis via its well demonstrated ability to promote inflammatory cell migration [15,16,42,43].
Our study has a number of limitations which should be noted. Firstly all our studies we carried out in HUVECs in vitro. We utilized HUVECs in our studies as this enabled us to confirm findings in cells extracted from a number of different patients as compared to limiting studies to one commercial cell line. Further studies in other endothelial cells and animal models would support our findings, although information from mice models can be difficult to relate to humans. Secondly, the degree of change in each individual adhesion molecule was quite small with upregulation of between 1.2 and 1.8 fold (Figure 2). The number of adhesion molecules altered though supports the importance of these findings, which translated to a very significant increase in monocyte adhesion (Figure 4). Ideally monocyte adhesion experiments should be carried out under laminar flow conditions however we did not have access or experience with these techniques.
In conclusion the findings of this study demonstrate the ability of OPG to stimulate up-regulation of adhesion molecules in TNF-α activated endothelial cells suggesting one potential mechanism for the association between OPG and atherosclerosis complications.
Acknowledgments
This project is supported in part by a bursary from the British Journal of Surgery and grant numbers RO1 HL080010-01 from The National Institute of Health and 379600 from the National Health and Medical Research Council Australia. JG is supported by Practitioner Fellowships from the NHMRC, Australia (431503). The authors would like to thank Dr. Rajiv Khanna from the Tumor Immunology Laboratory, Queensland Institute of Medical Research for kindly providing the THP-1 cell line as well as Boehringer-Ingelheim for supplying the monoclonal anti-ICAM-1 antibody. We also thank Drs Maria Nataatmadja and Allison Sutherland from the Department of Medicine, University of Queensland for their assistance with cell culture techniques.
Funding – The British Journal of Surgery, The National Health and Medical Research Council (379600) and The National Institute of Health (R01 HL080010-01) supported this work. JG is supported by Practitioner Fellowships from the NHMRC, Australia (431503).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bini A, Mann KG, Kudryk BJ, Schoen FJ. Noncollagenous bone matrix proteins, calcification, and thrombosis in carotid artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19:1852–61. doi: 10.1161/01.atv.19.8.1852. [DOI] [PubMed] [Google Scholar]
- 2.Stary HC, Chandler B, Dinsmore RE, Fuster V, Glagov S, Insull W, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, American Heart Association Atheroscler. Thromb Vasc Biol. 1995;15:1512–31. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 3.Abedin M, Tintut Y, Demer LL. Vascular Calcification. Mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–70. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 4.Schoppet M, Al-Fakhri N, Franke FE, Katz N, Barth PJ, Maisch B, et al. Localization of osteoprotegerin, tumor necrosis factor-related apoptosis-inducing ligand, and receptor activator of nuclear factor-κB ligand in monckeberg's sclerosis and atherosclerosis. J Clin Endocrinol Metab. 2004;89:4104–12. doi: 10.1210/jc.2003-031432. [DOI] [PubMed] [Google Scholar]
- 5.Fitzpatrick LA, Severson A, Edwards WD, Ingram RT. Diffuse calcification in human coronary arteries. Association of osteopontin with atherosclerosis. J Clin Invest. 1994;94:1597–604. doi: 10.1172/JCI117501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golledge J, McCann M, Mangan S, Lam A, Karan M. Osteoprotegerin and osteopontin are expressed at high concentrations within symptomatic carotid atherosclerosis. Stroke. 2004;35:1636–41. doi: 10.1161/01.STR.0000129790.00318.a3. [DOI] [PubMed] [Google Scholar]
- 7.Ueland T, Jemtland R, Godang K, Kjekshus J, Hognestad A, Omland T, et al. Prognostic value of osteoprotegerin in heart failure after acute myocardial infarction. J Am Coll Cardiol. 2004;44:1970–6. doi: 10.1016/j.jacc.2004.06.076. [DOI] [PubMed] [Google Scholar]
- 8.Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, et al. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109:2175–80. doi: 10.1161/01.CIR.0000127957.43874.BB. [DOI] [PubMed] [Google Scholar]
- 9.Ueland T, Yndestad A, Oie E, Florholmen G, Halvorsen B, Froland SS, et al. Dysregulated osteoprotegerin/RANK ligand/RANK axis in clinical and experimental heart failure. Circulation. 2005;111:2461–68. doi: 10.1161/01.CIR.0000165119.62099.14. [DOI] [PubMed] [Google Scholar]
- 10.Anand DV, Lahiri A, Lim E, Hopkins D, Corder R. The relationship between plasma osteoprotegerin levels and coronary artery calcification in uncomplicated type 2 diabetic subjects. J Am Coll Cardiol. 2006;47:1850–57. doi: 10.1016/j.jacc.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 11.Minoretti P, Falcone C, Calcagnino M, Emanuele E, Buzzi MP, Coen E, et al. Prognostic significance of plasma osteopontin levels in patients with chronic stable angina. Eur Heart J. 2006;27:802–07. doi: 10.1093/eurheartj/ehi730. [DOI] [PubMed] [Google Scholar]
- 12.Kurata M, Okura T, Watanabe S, Fukuoka T, Higaki J. Osteopontin and carotid atherosclerosis in patients with essential hypertension. Clin Sci (Lond) 2006;111:319–24. doi: 10.1042/CS20060074. [DOI] [PubMed] [Google Scholar]
- 13.Matsui Y, Rittling SR, Okamoto H, Inobe M, Jia N, Shimizu T, Akino M, et al. Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1029–34. doi: 10.1161/01.ATV.0000074878.29805.D0. [DOI] [PubMed] [Google Scholar]
- 14.Isoda K, Kamezawa Y, Ayaori M, Kusuhara M, Tada N, Ohsuzu F. Osteopontin transgenic mice fed a high-cholesterol diet develop early fatty-streak lesions. Circulation. 2003;107:679–81. doi: 10.1161/01.cir.0000055739.13639.d7. [DOI] [PubMed] [Google Scholar]
- 15.Zhu B, Suzuki K, Goldberg HA, Rittling SR, Denhardt DT, McCulloch CA, et al. Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin. J Cell Physiol. 2004;198:155–67. doi: 10.1002/jcp.10394. [DOI] [PubMed] [Google Scholar]
- 16.O'Regan A, Berman JS. Osteopontin: a key cytokine in cell-mediated and granulomatous inflammation. Int J Exp Pathol. 2000;81:373–90. doi: 10.1046/j.1365-2613.2000.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moran CS, McCann M, Karan M, Norman P, Ketheesan N, Golledge J. Association of osteoprotegerin with human abdominal aortic aneurysm progression. Circulation. 2005;111:3119–25. doi: 10.1161/CIRCULATIONAHA.104.464727. [DOI] [PubMed] [Google Scholar]
- 18.Bennett BJ, Scatena M, Kirk EA, Rattazzi M, Varon RM, Averill M, et al. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE−/− mice. Arterioscler Thromb Vasc Biol. 2006;26:2117–24. doi: 10.1161/01.ATV.0000236428.91125.e6. [DOI] [PubMed] [Google Scholar]
- 19.Jander S, Sitzer M, Schumann R, Schroeter M, Siebler M, Steinmetz H, et al. Inflammation in high-grade carotid stenosis. A possible role for macrophages and T cells in plaque destabilization. Stroke. 1998;29:1625–30. doi: 10.1161/01.str.29.8.1625. [DOI] [PubMed] [Google Scholar]
- 20.Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol. 2003;41:15S–22S. doi: 10.1016/s0735-1097(02)02834-6. [DOI] [PubMed] [Google Scholar]
- 21.Call JT, Deliargyris EN, Newby LK. Focusing on inflammation in the treatment of atherosclerosis. Cardiol Rev. 2003;12:194–200. doi: 10.1097/01.crd.0000111822.34362.71. [DOI] [PubMed] [Google Scholar]
- 22.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–94. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 23.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 24.Hansen LA, Poulsen OM, Wurtz H. Endotoxin potency in the A549 lung epithelial cell bioassay and the limulus amebocyte lysate assay. J Immunol Methods. 1999;226:49–58. doi: 10.1016/s0022-1759(99)00047-2. [DOI] [PubMed] [Google Scholar]
- 25.Gao B, Wang Y, Tsan MF. The heat sensitivity of cytokine-inducing effect of lipopolysaccharide. J Leukoc Biol. 2006;80:359–66. doi: 10.1189/jlb.1205738. [DOI] [PubMed] [Google Scholar]
- 26.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cell derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–56. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haraldsen G, Kvale D, Lien B, Farstad IN, Brandtzaeg P. Cytokine-regulated expression of E-selectin, Intercellular adhesion molecule -1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human intestinal microvascular endothelial cells. J Immunol. 1996;156:2558–65. [PubMed] [Google Scholar]
- 28.Grabner R, Till U, Heller R. Flow cytometric determination of E-selectin, vascular cell adhesion molecule-1, and intercellular cell adhesion moleule-1 in formaldehyde-fixed endothelial cell monolayers. Cytometry. 2000;40:238–44. doi: 10.1002/1097-0320(20000701)40:3<238::aid-cyto9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Ding C, Cantor CR. A high-throughput gene expression analysis technique using competitive PCR and matrix-assisted laser desorption ionization time-of-flight MS. PNAS. 2003;100:3059–64. doi: 10.1073/pnas.0630494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elvidge GP, Price TS, Glenny L, Ragoussis J. Development and evaluation of real competitive PCR for high-throughput quantitative applications. Anal Biochem. 2005;339:231–41. doi: 10.1016/j.ab.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150–6. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 33.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 34.Oldberg A, Franzen A, Heinegard D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986;83:8819–23. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haylock DN, Nilsson SK. Osteopontin: a bridge between bone and blood. Br J Haematol. 2006;134:467–74. doi: 10.1111/j.1365-2141.2006.06218.x. [DOI] [PubMed] [Google Scholar]
- 36.Jono S, Ikari Y, Shioi A, Mori K, Miki T, Hara K, et al. Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation. 2002;106:1192–4. doi: 10.1161/01.cir.0000031524.49139.29. [DOI] [PubMed] [Google Scholar]
- 37.Asanuma Y, Chung CP, Oeser A, Solus JF, Avalos I, Gebretsadik T, et al. Serum osteoprotegerin is increased and independently associated with coronary-artery atherosclerosis in patients with rheumatoid arthritis. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.04.049. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clancy P, Oliver L, Jayalath R, Buttner P, Golledge J. Assessment of a serum assay for quantification of abdominal aortic calcification. Arterioscler Thromb Vasc Biol. 2006;26:2574–6. doi: 10.1161/01.ATV.0000242799.81434.7d. [DOI] [PubMed] [Google Scholar]
- 39.Malyankar UM, Scatena M, Suchland KL, Yun TJ, Clark EA, Giachelli CM. Osteoprotegerin is an avb3-induced, NF-kB-dependent survival factor for endothelial cells. J Biol Chem. 2000;275:20959–62. doi: 10.1074/jbc.C000290200. [DOI] [PubMed] [Google Scholar]
- 40.Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thruston G, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-αand has a crucial role in the induction of inflammation. Nature Medicine. 2006;12:235–39. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 41.Niculescu HGRF, Vlaicu R. Tumor necrosis factor-alpha in human arterial wall with atherosclerosis. Atherosclerosis. 1991;89:247–54. doi: 10.1016/0021-9150(91)90066-c. [DOI] [PubMed] [Google Scholar]
- 42.O'Regan AW, Chupp GL, Lowry JA, Goetschkes M, Mulligan N, Berman JS. Osteopontin is associated with T cells in sarcoid granulomas and has T cell adhesive and cytokine-like properties in vitro. J Immunol. 1999;162:1024–31. [PubMed] [Google Scholar]
- 43.Giachelli CM, Lombardi DM, Johnson RJ, Murry CE, Almeida M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am J Pathol. 1998;152:353–58. [PMC free article] [PubMed] [Google Scholar]