Abstract
Patients presenting to the Emergency Department (ED) with chest pain are triaged to early reperfusion therapies based on their initial 12-lead electrocardiogram (ECG). The standard 12- lead ECG lacks sensitivity to detect acute myocardial infarction (AMI). Electrocardiographic diagnosis of non-ST elevation myocardial infarction (non-STEMI) is especially difficult and is delayed until cardiac biomarkers turn positive, indicating onset of myocardial necrosis.
Study Aims:
The purpose of this analysis was to extract global ST-T waveform features from chest pain patients, compare these features in patients with and without AMI, and then identify features that distinguish diagnostic categories.
Methods:
Secondary analysis of data from the IMMEDIATE AIM Study, a prospective clinical trial in which patients were attached to Holter monitor devices to obtain 24 hours of continuous ECG data. Digital recordings from 176 patients were analyzed: 88 with AMI (STEMI & non-STEMI), and 88 without AMI or unstable angina. The non-acute coronary syndrome (ACS) group was further subdivided into those with non-ACS cardiac conditions such as heart failure, and those without cardiac disease who had non-cardiac chest pain. For each patient, 10 consecutive waveforms were obtained within the first 120 minutes of ED presentation. The waveforms were time-aligned to the QRS, signal averaged, baseline adjusted. ST-T waveforms were complied according to diagnostic category and pooled for further analysis. Eigenvector-lead feature coefficients (Karhunen-Loève or K-L coefficients) were obtained for each patient by taking the dot product of the ST-T wave (ST-segment or entire waveform) and the first 3 common eigenvectors, producing 24 K-L coefficients. Cumulative probability distribution function curves (CPDF) were plotted for each diagnostic category. Statistical significance of category coefficient distribution differences was determined. Multinomial regression was used to assess accuracy of feature coefficients to predict diagnostic category.
Results:
Non-STEMI and non-ACS cardiac category K-L coefficient curves were statistically different in 11 out of 24 feature curves (P<.001-.047). ST-segment (50 samples) coefficients predicted non-ACS cardiac patients 11.5% more often (P=.02) than those derived from the entire ST-T wave.
Conclusion:
Patients diagnosed with non-STEMI have distinct distribution of K-L coefficients compared with non-ACS cardiac patients. Coefficients from the first 50 samples of the ST-T wave (ST-segment), better predict diagnostic category then do coefficients derived from the entire ST-T wave. K-L coefficient feature analysis may provide early diagnostic information to distinguish non-STEMI versus non-ACS cardiac patients.
Introduction
Patients presenting to the ED with chest pain are triaged to early reperfusion therapies based on their initial ECG. Unfortunately, the standard 12-lead ECG lacks sensitivity to detect AMI. The diagnosis of non-STEMI is even more problematic and delayed until cardiac biomarkers turn positive due to the onset of myocardial necrosis. A particular challenge is the ECG distinction between AMI patients and patients with a history of cardiac disease but who are not experiencing an acute coronary syndrome. Such cardiac patients typically have an abnormal ECG and many have ST-T wave changes secondary to bundle branch block, digitalis therapy, left ventricular hypertrophy, etc., that confound the diagnosis. When “time is muscle,” it would be ideal to identify ECG features or ischemia preceding biomarker evidence of infarction to salvage myocardium and reduce occurrence of adverse clinical outcomes.
The ST-T wave represents cardiac repolarization, which is thought to be sensitive to ischemic changes in the myocardium. Conventional metrics focus on local amplitude and morphological characteristics, such as ST segment deviation (e.g. elevation or depression) and T wave morphology to ascertain presence of ischemia. In this preliminary study, we used the Karhunen-Loève representation to extract global features of the ST-T wave.
The body surface ECG signal is a complex summation of cardiac electrical potentials confounded by noise and redundancy.1 To optimize the ability to extract dynamic features of the ST-T waveform, we used the Karhunen-Loève representation because of its ability to concentrate the signal information in a minimum number of coefficients, while rejecting noise and reducing signal redundancy.2
The purpose of this study was to extract global ST-T waveform features of patients admitted to the ED with chest pain, and compare coefficients by diagnostic category. K-L coefficient distributions for non-STEMI and non-ACS cardiac diagnostic categories were identified.
Methods
This is a secondary analysis of a prospective clinical trial called the IMMEDIATE AIM study (Ischemia Monitoring & Mapping in the Emergency Department in Appropriate Triage & Evaluation of Acute Ischemic Myocardium). Enrolled in this study were 1308 patients presenting to the University of California San Francisco Emergency Department with chest pain from April 1, 2002 until December 31, 2004. Of these patients, 88 had a diagnosis of AMI. A matching number of 88 patients with a non-ACS diagnosis were randomly selected to serve as the comparison group. The AMI and non-AMI groups were further subdivided into 4 diagnostic categories (Figure1). Excluded from the IMMEDIATE AIM study were patients with left bundle branch block and ventricular paced rhythms. However, patients with right bundle branch block and other confounders such as left ventricular hypertrophy were included. Specially trained research nurses consented patients using a two-tier approach in order to apply Holter monitoring immediately upon ED presentation; first by verbal assent, then after patient stabilization, by written consent. Body surface electrodes were applied with particular attention to placement and skin preparation. Digital recordings were obtained with 10 electrodes in Mason-Likar3 configuration, producing a standard 12-lead ECG of up to 24 hours of continuous 8-channel data (channels 1 thru 8, corresponding to leads I, II, V1-V6). The Holter device recorded at a sampling rate of 180 samples per second (H12, Mortara Instruments, Inc., Milwaukee, WI), and was allowed to record to its capacity of 24 hours, or until patient discharge, whichever occurred first. Raw binary ECG data was imported into Matlab (v7.3, The MathWorks Inc., Natick, MA.) for analysis.
Figure 1.
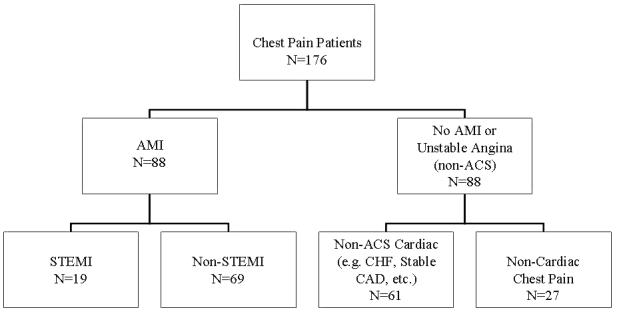
Sample tree showing diagnostic category constituent numbers.
Ten consecutive, relatively noise-free, beats occurring within the first 120 minutes of ED presentation were selected for each patient. Correct R-wave identification was confirmed visually prior to signal-averaging. Signal-averaged beats were baseline adjusted to P-R and T-P segments with Matlab cubic splines function using graphic calipers and technique modified from Meyer.4 A root-mean-square (RMS) waveform, calculated from the 8-channel data, was used to determine waveform onset and offset. For each patient recording, the J-point was identified visually. The T-wave offset was determined to be the point following the RMS T-wave peak at which the amplitude drops to 0.4% of the T-wave maximum amplitude. The ST-T wave was defined as the J-point to T-wave offset. To compensate for patient heart rate, each ST-T wave was time-normalized to 150 samples using the Matlab resample function. Time-normalized ST-T waves were compiled by diagnostic category (i.e. STEMI, non-STEMI, non-ACS cardiac, and non-cardiac). Common eigenvectors and eigenvalues of the compiled covariance matrix were calculated. The first 3 common eigenvectors were used to calculate the ST-T wave representations. The dot product of compiled ST-T waves and 3 common eigenvectors produced 24 eigenvector-lead (channel) coefficients (i.e. K-L coefficients). Cumulative probability density function curves were plotted from K-L eigenvector-lead coefficients for each diagnostic category pair (i.e. STEMI versus non-STEMI, STEMI versus non-ACS cardiac, etc.) Additionally, K-L eigenvector-lead coefficients for the first 50 samples of time-normalized ST-T waves were calculated.
Statistical Analysis
Means and standard deviations were used for central tendency measures of normally distributed variables. Medians and 25% to 75% inter-quartile ranges were used for non-normally distributed data. The independent sample t-test was used to compare means, while the Mann-Whitney was used to compare medians. The McNemar Test was used to compare proportions for variables using repeated measures. The Two-Sample Kolmogorov-Smirnov Test was used to test the likelihood that pairs of cumulative probability density functions curves were drawn from the same continuous distributions. Multinomial regression was used to test classification accuracy of coefficient predictors. For all statistics, a p-value ≤ .05 was considered significant.
Results
Sample characteristics
The AMI group was slightly older and had a higher proportion of males compared to the non-AMI group (Table 1). The groups did not differ in the proportion of ECG confounders (LVH, RBBB). Both groups had Holter monitoring initiated within the first hour of presentation to the ED.
Table 1.
Group Sample Characteristics
| AMI (n=88) |
Non-ACS Cardiac (n=88) |
Sig. of difference* | |
|---|---|---|---|
| Age (mean±SD) | 69.6±15.7 | 61.7±17.1 | p=.002 |
| Gender (% male) | 56.8 | 38.8 | p=.020 |
| Rhythm (% sinus) | 88.6 | 94.3 | NS |
| LVH (% present) | 19.3 | 14.8 | NS |
| RBBB (% present) | 10.2 | 14.7 | NS |
| Time to Holter Median minutes (25%-75% IQR) |
41.0 (29.0-56.8) |
39.0 (31.3-59.5) |
NS |
NS = not statistically significant
Differences between diagnostic categories
In comparing cumulative probability density function curves of the STEMI, non-STEMI, non-ACS cardiac, and non-cardiac diagnostic categories, 29.2% to 54.2% of the eigenvector-leads curves showed significant distribution differences by the Kolmogorov-Smirnov test. Non-STEMI versus non-ACS cardiac diagnostic categories showed significantly different distribution curves in 45.8% of the eigenvector-leads (Figure 2). Non-STEMI versus non-cardiac curves were statistically different in 8.3% of the eigenvector-leads (Table 2). There were no significant differences between the distribution curves of non-ACS cardiac patients versus non-cardiac patients.
Figure 2.
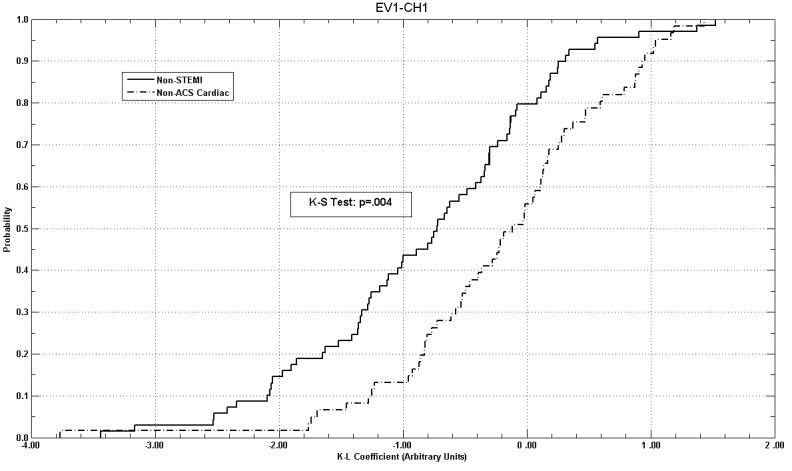
Example of cumulative probability density function curves, comparing K-L coefficient distributions from non-STEMI and non-ACS Cardiac patients in eigenvector-lead EV1-CH1. Note separation of distributions over majority of coefficient range.
Table 2.
K-L eigenvector-leads with significantly different coefficient distributions by Kolmogorov-Smirnov Test
| STEMI # Leads (%) |
Non-STEMI # Leads (%) |
|
|---|---|---|
| Non-STEMI | 13 (54.2) | not tested |
| Non-ACS Cardiac | 12 (50.0) | 11 (45.8) |
| Non-Cardiac | 7 (29.2) | 2 (8.3) |
When eigenvector-lead coefficients from both the first 50 samples of the ST-T wave (corresponding to the ST-segment), and those from the entire ST-T, were introduced into a multinomial regression model as predictors of diagnostic category, a significant improvement in prediction was seen by the 50 sample ST-segment model over the 150 sample ST-T model. Eigenvector-lead coefficients from the first 50 samples of the ST-T wave showed an 11.5% (p=.02) improvement in predicting non-ACS cardiac patient category membership, and 11.1% (p=.04) improvement in predicting non-cardiac patient diagnostic category (Table 3). Both multinomial regression models were statistically significant (p<.001).
Table 3.
Diagnostic Group Classification Prediction
| Correct Prediction (%) | ||||
|---|---|---|---|---|
| 150 sample (ST-T wave) |
50 sample (ST segment) |
% Difference | P Value* | |
| STEMI | 63.2 | 68.4 | +5.2 | NS |
| Non-STEMI | 75.4 | 76.8 | +1.4 | NS |
| Non-ACS Cardiac | 72.1 | 83.6 | +11.5 | .02 |
| Non-Cardiac | 18.5 | 29.6 | +11.1 | .04 |
Multinomial regression models (p<.001)
Discussion
In this preliminary study, we found the Karhunen-Loève approach to characterize coefficient distributions valuable to distinguish AMI patients from patients without AMI or unstable angina. The K-L analysis is a robust technique used for ECG signal redundancy reduction, filtering (noise reduction), risk stratification, waveform morphology monitoring, and classification.1,5-9 The distinction between patients with and without ACS in the present study is important because we selected ECG recordings from the first minutes of ED presentation when standard ECG diagnosis is often inconclusive. An improved ECG diagnosis is particularly significant in the ED setting because symptoms may be an unreliable indicator of ischemia leading to infarction, and one-year mortality in ED patients with missed ischemia is high.10-12 We also found differences in K-L coefficient distributions between patients with STEMI and patients with non-STEMI or non-ACS diagnoses. However, these distinctions are of less clinical importance because STEMI is an electrocardiographically-defined diagnosis that is readily apparent on the standard ECG. If ECG diagnosis could be established using the K-L approach from the initial 12-lead ECG obtained in the ED, treatment algorithms for ACS could be immediately initiated. Distinction between coefficient distributions for non-STEMI and non-ACS cardiac patients have more clinical utility because standard ST-T wave criteria may be present in both groups due to ECG confounders such as bundle branch block or left ventricular hypertrophy. Moreover, non-STEMI may be electrocardiographically silent.
Multinomial regression analysis, using diagnostic category membership as the dependent variable, and diagnostic category K-L coefficients as predictors, indicates possible repolarization feature abnormalities distinguishing members of these diagnostic categories. Of note are improvements in classification, when the first 50 samples of the ST-T wave (which approximates the ST segment), are used to in generate coefficient predictors in the regression model, in contrast to using the entire ST-T wave sample. This lends evidence to the knowledge that repolarization is a non-homogeneous temporally dynamic process in which information about the physiological state of the myocardium is time-variant.
Limitations
K-L representation is dependent upon the sample chosen to derive the common eigenvectors, used for feature-lead representation. This study used a small training sample of only 10 beats, at a single time point, which limits the ability to draw conclusions about the dynamism of the ischemic process. Analyses of the continuous Holter data from the IMMEDIATE AIM Study is ongoing to determine whether samples from longer recording periods will further improve the distinction between diagnostic categories.
In this study, diagnostic category predictions from multinomial regression are limited by a relatively small training set, which risks “over-training” of the prediction model, and may limit prediction accuracy for a larger sample of patients. Future studies need to be conducted to test the K-L approach in a different sample of patients presenting to the ED with chest pain.
Two ECG confounders, left bundle branch block and ventricular paced rhythms, were excluded from this analysis. When these ECG confounders are present in the sample used to derive K-L coefficients, diagnostic category prediction accuracy may be less than shown in the present analysis.
Conclusions
Patients with non-STEMI have a distinct distribution of K-L coefficients compared with patients without acute coronary syndrome, regardless of the presence of other cardiac abnormalities. The first 50 samples of the ST-T wave, which approximates the ST segment, produces better diagnostic category prediction than when the entire ST-T wave is used. K-L coefficient feature analysis may provide early diagnostic information to distinguish patients with non-STEMI versus those with non-ACS cardiac conditions.
Acknowledgments
Funding
This study was supported by a grant from the National Heart, Lung, & Blood Institute (RO1HL69753), by the General Clinical Research Center, University of California, San Francisco, and the Cardiovascular Research & Teaching Institute, University of Utah.
Additional funding provided by NIH – Ruth L. Kirschstein National Research Service Award training grant (F31NR009615-02)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laguna P, Moody GB, Garcia J, Goldberger AL, Mark RG. Analysis of the ST-T complex of the electrocardiogram using the Karhunen--Loeve transform: adaptive monitoring and alternans detection. Med Biol Eng Comput. 1999;37:175–89. doi: 10.1007/BF02513285. [DOI] [PubMed] [Google Scholar]
- 2.Lux RL, Evans AK, Burgess MJ, Wyatt RF, Abildskov JA. Redundancy reduction for improved display and analysis of body surface potential maps. I. Spatial compression. Circ Res. 1981;49:186–96. doi: 10.1161/01.res.49.1.186. [DOI] [PubMed] [Google Scholar]
- 3.Mason RE, Likar I. A new system of multiple-lead exercise electrocardiography. Am Heart J. 1966;71:196–205. doi: 10.1016/0002-8703(66)90182-7. [DOI] [PubMed] [Google Scholar]
- 4.Meyer CR, Keiser HN. Electrocardiogram baseline noise estimation and removal using cubic splines and state-space computation techniques. Comput Biomed Res. 1977;10:459–70. doi: 10.1016/0010-4809(77)90021-0. [DOI] [PubMed] [Google Scholar]
- 5.Lux RL. Principal components analysis: an old but powerful tool for ECG analsysis. International Journal of Bioelectromagnetism. 2003;5:342–345. [Google Scholar]
- 6.Garcia J, Sornmo L, Olmos S, Laguna P. Automatic detection of ST-T complex changes on the ECG using filtered RMS difference series: application to ambulatory ischemia monitoring. IEEE Trans Biomed Eng. 2000;47:1195–201. doi: 10.1109/10.867943. [DOI] [PubMed] [Google Scholar]
- 7.Zabel M, Acar B, Klingenheben T, Franz MR, Hohnloser SH, Malik M. Analysis of 12-lead T-wave morphology for risk stratification after myocardial infarction. Circulation. 2000;102:1252–7. doi: 10.1161/01.cir.102.11.1252. [DOI] [PubMed] [Google Scholar]
- 8.Laguna P, Moody GB, Mark RG. Analysis of the cardiac repolarization period using the KL transform: applications on the ST-T database Computers in Cardiology 1994. 1994:233–236. [Google Scholar]
- 9.Garcia J, Lander P, Sornmo L, Olmos S, Wagner G, Laguna P. Comparative study of local and Karhunen-Loeve-based ST-T indexes in recordings from human subjects with induced myocardial ischemia. Comput Biomed Res. 1998;31:271–92. doi: 10.1006/cbmr.1998.1481. [DOI] [PubMed] [Google Scholar]
- 10.Drew BJ, Schindler DM, Zegre J, Fleischmann KE, Lux RL. Estimated body surface potential maps in emergency department patients with unrecognized transient myocardial ischemia. Journal of Electrocardiology. 2007;(Supplement) doi: 10.1016/j.jelectrocard.2007.05.032. publication pending. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fesmire FM, Smith EE. Continuous 12-lead electrocardiograph monitoring in the emergency department. Am J Emerg Med. 1993;11:54–60. doi: 10.1016/0735-6757(93)90061-f. [DOI] [PubMed] [Google Scholar]
- 12.Caldwell MA, Pelter MM, Drew BJ. Chest pain is an unreliable measure of ischemia in men and women during PTCA. Heart Lung. 1996;25:423–9. doi: 10.1016/s0147-9563(96)80042-2. [DOI] [PubMed] [Google Scholar]


