Abstract
Background
Epidemiologic studies reveal that posttraumatic stress disorder (PTSD) is highly comorbid with both conduct disorder and major depression in men. The genetic and environmental etiology of this comorbidity has not been examined.
Methods
Data were analyzed from 6,744 middle aged male-male monozygotic and dizygotic twins from the Vietnam Era Twin Registry. Conduct disorder, major depression, and PTSD were assessed via telephone interview using the Diagnostic Interview Schedule for the DSM-III-R in 1992. Structural equation modeling was used to estimate additive genetic, shared environmental and individual-specific environmental effects common and specific to conduct disorder, major depression, and PTSD.
Results
The association between conduct disorder and PTSD was explained primarily by common shared environmental influences; these explained 10%(95% CI: 6-17%) of the variance in PTSD. The association between major depression and PTSD was largely explained by common genetic influences; these explained 19%(95% CI: 11-26%) of the variance in PTSD.
Conclusion
Our findings suggest different etiologic mechanisms explain the association of conduct disorder and major depression with PTSD in male veterans. If replicated in other populations, results suggest research aimed at identifying of specific genetic and environmental factors that influence PTSD may benefit from starting with those that have been more consistently and strongly associated with major depression and conduct disorder.
Introduction
Epidemiologic studies of military veterans and civilians reveal that posttraumatic stress disorder (PTSD) is highly comorbid with both conduct disorder and major depression in men 1-5. Pre-existing conduct problems increase risk for both exposure to potentially-traumatic events and for developing PTSD among individuals exposed to such events.6,7 A history of juvenile conduct disorder is also highly prevalent among adults with major depression.8 Evidence suggests major depression is both a risk factor for and a consequence of PTSD.9-11 Thus, the relation of conduct disorder and major depression with PTSD is complex. Major depression appears to increase risk for PTSD and vice versa; conduct disorder is associated with increased risk of both disorders. However, the association among conduct disorder, major depression, and PTSD could also be explained by a shared diathesis of genetic or environmental origin. This study used a twin design to test competing explanations for the etiology of the association among conduct disorder, major depression, and PTSD in 3,372 male-male twin pairs from the Vietnam Era Twin (VET) Registry.
Twin studies offer a unique way to test competing explanations for the association among phenotypes, such as conduct disorder, major depression, and PTSD. The twin method exploits the different level of genetic relatedness between monozygotic (MZ) and dizygotic (DZ) twin pairs to estimate the contribution of genetic and environmental factors to individual differences in an outcome of interest. 12 Multivariate twin studies partition the covariance among phenotypes into an additive genetic component and two types of environmental components that are common to those phenotypes. The first is a shared environmental effect that is correlated between twins and makes siblings from the same family similar to each other. The second is a non-shared or individual specific environmental effect that is uncorrelated between siblings and includes measurement error. Variance that is not shared among phenotypes is partitioned into additive genetic, shared environmental, and non-shared environmental components that are specific to a phenotype.
This study uses a twin design to assess the relative contributions of genetic, shared environmental and individual-specific environmental influences to the comorbidity of conduct disorder, major depression, and PTSD. Genetic influences explain a substantial proportion of the variance in conduct disorder, 13,14 major depression, 15,16 and PTSD 17,18 in men and, therefore, might explain their covariation. Prior research on a subsample of the veterans included in the present study suggested that common genetic influences contribute to the association between PTSD and major depression. 19,20 However, prior studies did not test whether common genetic influences remained statistically significant after controlling for genetic and environmental influences on conduct disorder, which is associated with increased risk of both major depression and PTSD.6,22 Thus, one possible explanation is that common genetic influences contribute to the association among conduct disorder, major depression, and PTSD. This possibility has not been examined.
A second possible explanation is that environmental factors account for the association among phenotypes. Twin studies suggest that environmental influences account for about 50 to 70% of the variance in conduct disorder, 13,14 60% of the variance in major depression, 15,16,21 and 70% of the variance in PTSD. 18 Shared environmental influences also contribute to some conduct disorder symptoms 13 but not to PTSD. 17,18 These three disorders share in common many putative shared environmental risk factors, such as an adverse childhood environment 23 and low family socio-economic status. 7,24,25 Non-shared environmental risk factors, such as childhood abuse, have also been associated with increased risk for conduct disorder, major depression, and PTSD individually. 26,27
To inform research and practice, it is critical to establish the etiology of the association among conduct disorder, major depression, and PTSD. Information on etiology could be used to inform decisions about disorder classification. Proposals for a quantitative hierarchical organization for DSM-V have grouped major depression and PTSD under a sub-class of internalizing-distress-disorders. 28 A common genetic diathesis may be considered evidence in support of such a classification. Moreover, the absence of such an association between PTSD and conduct disorder would further build that case by demonstrating the discriminant validity of the association. Evidence of a common genetic diathesis would suggest that, for example, genes influencing major depression contribute to variation in PTSD or vice versa. Such information is valuable in the search for specific genes and neurobiological systems involved in these phenotypes. Alternatively, if environmental factors influence the association among phenotypes, then prevention efforts would be guided to target those factors.
Methods and Materials
Sample
Participants were drawn from the VET Registry. This Registry is a nationally distributed cohort consisting of male-male twin pairs born between 1939 and 1957 in which both siblings served on active military duty during the Vietnam War era. 29 Zygosity was assessed by twenty questions about sibling similarity and supplemented with blood typing data obtained from military records. Zygosity determination by such methods has been shown to have 95% accuracy. 30 Registry members are representative of all twins who served in the military during the Vietnam War on a variety of sociodemographic and other variables. 31,32 The data used in the present study were from the 1992 Harvard Twin Study of Drug Abuse and Dependence. The response rate was 79.6% including the 3,372 complete pairs who comprise the sample for the present investigation. In 1992 the mean age of respondents was 44.6 years (S.D. + 2.8, range 36-55 years); 90.4% were non-Hispanic white, 4.9% were African-American, 2.7% were Hispanic, 1.3% were Native American and 0.7% were “other.” Approximately one-third reported high school as their highest degree attained and 38.6% were college graduates. Over ninety-two percent were employed full-time and 1.8% part-time. Seventy-five percent had been married at the time of the study and 11% were never married. Registry members lived in all 50 states of the United States. The majority of participants were MZ twins (55.6%).
Measures
Lifetime diagnoses of PTSD, conduct disorder, and major depression were obtained using the Mental Health Diagnostic Interview Schedule Version III - revised (DIS-III-R). 33 The DIS-III-R is a structured psychiatric interview for epidemiological research that leads to clinical diagnoses based on the Diagnostic and Statistical Manual Third Edition Revised (DSM-III-R). Experienced lay interviewers from the Institute for Survey Research at Temple University were trained by one of the project investigators to administer the telephone interview. The interview was administered after the respondent had given verbal informed consent. Details of the interview procedure, types of traumatic events reported, and PTSD diagnostic data was reported previously. 11 Reliability of diagnoses of the twins was assessed by re-interviewing a subset of 146 participants using a different interviewer at a mean interval between interviews of 466 days (+/- 50.5). Earlier analyses of the VET data reported fair to good test-retest reliability of diagnostic measures. 6,34,35 These reliability estimates are similar to those found in other community samples.
Statistical Analysis
Univariate and multivariate logistic regression were conducted to estimate odds ratios (OR) and 95% confidence intervals (CI) for the associations of PTSD diagnosis with conduct disorder and major depression diagnosis using STATA 7. The Huber-White robust variance estimator was used to adjust estimates of standard errors from non-independent observations. 36
The twin method exploits the different level of genetic relatedness between monozygotic (MZ) and dizygotic (DZ) twin pairs to estimate the contribution of genetic and environmental factors to the association between phenotypes. MZ pairs are genetically identical whereas DZ pairs are genetically no more alike than ordinary full siblings. In a univariate twin model, the variance for a phenotype, such as PTSD, is partitioned into the variance due to additive genetic and two types of environmental influences: nonshared or individual-specific environmental influences and shared environmental influences. In a multivariate genetic model, the intrapair covariance or correlation between two phenotypes, such as PTSD and major depression, is partitioned into the additive genetic, shared environmental, and nonshared environmental influences. MZ and DZ correlations are compared across twins and phenotypes: that is, one twin’s major depression symptoms are correlated with the co-twin’s PTSD symptoms. If the cross-twin cross-phenotype correlations are greater in MZ than in DZ pairs, this implies that genetic factors contribute to the phenotypic correlation between the two phenotypes. To test this hypothesis, we first compared polychoric correlations using lifetime diagnostic symptom counts of PTSD, conduct disorder, and major depression separately for MZ and DZ pairs. The polychoric correlation estimates the correlation between the normally distributed underlying liability rather than the observed variables. 37 The polychoric correlations provide more reliable parameter estimates and confidence intervals than tetrachoric correlations based on dichotomous diagnostic variables. However, the authors also conducted analyses using diagnoses. Parameter estimates were similar; confidence intervals were much wider. These analyses are available from the first authors on request. The polychoric correlation and accompanying asymptotic weight covariance matrices computed using PRELIS 2 served as the input data for the subsequent twin modeling analysis. 38
The multivariate twin method is a form of structural equation modeling (SEM), an extension of most notably multiple regression and factor analysis, that examines multiple relationships between dependent and independent variables. This method partitions covariation among traits, such as conduct disorder, major depression, and PTSD, into several sub-components that can not be directly observed, named latent variables. In the current report, the latent variables represent additive genetic, shared environmental and nonshared environmental effects common and specific to conduct disorder, major depression, and PTSD. Measurement error is included in the nonshared environmental components, which are computed after removing the additive genetic and shared environmental effects. We tested a series of nested sub-models with the aim of identifying the most parsimonious model that provided a good fit to the data. Sub-models were tested according to a priori hypotheses by constraining the non-significant genetic or environmental parameter estimates to zero. The fit of each sub-model was evaluated using a goodness-of-fit chi-squared test. A significant chi-squared test (p<0.05) suggests a worse fit for the sub-model compared to the full model. For non-nested models, a model with smaller value of Akaike Information Criterion (AIC), 39 defined as −2 (maximum log likelihood)+2(number of parameters), suggests a better fit. The best-fitting model was determined by a balance of parsimony and goodness-of-fit based on both a chi-square test and AIC.
We fitted a triangular decomposition model to the polychoric correlation and corresponding asymptotic weight covariance matrices in MZ and DZ pairs using the SEM software Mx. 39 This model assumed that the additive genetic latent variable associated with conduct disorder not only influenced conduct disorder, but also influenced major depression and PTSD. After controlling for the genetic effects on conduct disorder, we tested whether the residual additive genetic latent variable influenced major depression and PTSD. The remaining residual genetic effect on PTSD was represented by the third genetic latent variable. The same variance decomposition technique was applied to shared and nonshared environmental effects, respectively. A graphical presentation of this model is found in Figure 1 in the Results section. The one-way arrows represent additive genetic (A), shared environmental (C), and nonshared environmental (E) influences on the three disorders. The technical aspects of this model can be found in detail elsewhere. 40,41 In twin analysis, MZ and DZ pairs are not assumed to differ in their concordance for pertinent shared environmental risk factors, named equal environment assumption (EEA). Previous research supports this EEA for psychiatric disorders in the VET Registry, 42 and is consistent with findings in other twin samples. 43
Results
The prevalence of conduct disorder, major depression and PTSD was 7.9%, 9.2%, and 9.7%, respectively. Logistic regression analysis indicated that conduct disorder and major depression were strongly associated with each other as well as with PTSD in this sample. The odds of having PTSD was 3.5 times (95% CI: 2.8, 4.4) greater for those with a history of conduct disorder as for those without; the odds of having PTSD was also 7 times (95% CI: 5.8, 8.6) greater for those with a history of major depression as for those without. When conduct disorder and major depression were jointly included as predictors in one regression model, the odds of having lifetime PTSD was 2.9 times (95% CI: 2.3,3.6) more likely among those with a history of conduct disorder and 6.5 times (95% CI: 5.3,7.9) more likely among those with a history of major depression.
All of the cross-twin within-variable polychoric correlations in MZ pairs were greater than the corresponding correlations in DZ pairs (correlation coefficients on diagonal in Table 1), suggesting genetic effects on the variance of each disorder individually. Similarly, the cross-twin cross-variable polychoric correlations between conduct disorder and major depression as well as between major depression and PTSD were greater in MZ pairs compared to DZ pairs suggesting a substantial role for genetic influences in their covariance. However, the cross-twin cross-variable polychoric correlations between conduct disorder and PTSD were equal for MZ and DZ twin pairs, meaning that shared environmental influences largely contribute to their covariance.
Table 1.
Cross-twin tetrachoric correlations for conduct disorder, major depression and PTSD by zygosity.
| MZ Twin 2 | DZ Twin 2 | |||||
|---|---|---|---|---|---|---|
| Twin 1 | Conduct disorder | Major depression | PTSD | Conduct disorder | Major depression | PTSD |
| Conduct disorder | 0.41 | 0.23 | 0.18 | 0.32 | 0.12 | 0.14 |
| Major depression | 0.24 | 0.39 | 0.32 | 0.16 | 0.14 | 0.15 |
| PTSD | 0.12 | 0.22 | 0.28 | 0.15 | 0.15 | 0.20 |
Note: Cross-twin within-phenotype correlations are in bold.
The process of searching the best-fitting model is summarized in Table 2 using the model-fitting indices. The full model gave a good fit (p=0.06, AIC=-3.3). Models 2 and 3 that fixed genetic or shared environmental variances and covariances of all three disorders to zero produced a very poor fit to the data. The goodness-of-fit chi-square tests were statistically significant and the AIC values were greater than the AIC for Model 1. Model 4 that fixed common genetic covariance between conduct disorder and PTSD to zero as well as fixed genetic variance specific to PTSD to zero provided an excellent fit to the data. The goodness-of-fit chi-square test was not statistically significant and the AIC value was smaller than that for Model 1. Similarly, an alternative model (Model 5) that fixed common shared environmental liability to major depression and PTSD to zero as well as fixed shared environmental variance specific to major depression and PTSD to zero also provided an excellent fit to the data. Finally, Model 6, which simultaneously assumed no common genetic covariance between conduct disorder and PTSD, no common shared environmental covariance between major depression and PTSD, and no genetic and shared environmental variances specific to PTSD, was gave a better fit than any of the other models (p=0.23, AIC=-13.0). This model was the most parsimonious and the best-fitting model.
Table 2.
Results of model fitting of conduct disorder, major depression, and posttraumatic stress disorder.
| Additive Genetic Factor | Shared Environmental Factor | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model Number | Common | Common | Specific | Common | Common | Specific | Fit of Model | ||||||||
| CD | MD | PTSD | MD | PTSD | PTSD | CD | MD | PTSD | MD | PTSD | PTSD | x2 (df) | p | AIC | |
| 1. Full Model | 20.7 (12) | 0.06 | -3.3 | ||||||||||||
| 2. | X | X | X | X | X | X | 72.8 (18) | 0.00 | 36.8 | ||||||
| 3. | X | X | X | X | X | X | 41.4 (18) | 0.00 | 5.4 | ||||||
| 4. | X | X | 21.0 (14) | 0.10 | -7.0 | ||||||||||
| 5. | X | X | X | 20.8 (15) | 0.14 | -9.2 | |||||||||
| 6. | X | X | X | X | X | 21.0 (17) | 0.23 | -13.0 | |||||||
Note: X = Factor loading fixed to zero; CD = conduct disorder, MD = major depression, PTSD = posttraumatic stress disorder.
Best fitting model shown in bold. Individual-specific environmental influences common and specific to each phenotype were freely estimated in all models.
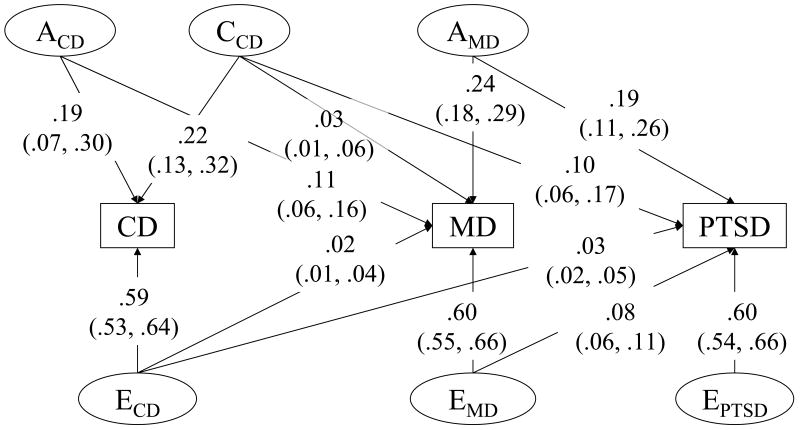
The genetic and environmental variance-covariance estimates for the best-fitting model (Model 6, Table 2) were presented in Figure 1. Under this model, 19% of the variance in conduct disorder was explained by genetic influences, 22% by shared-environmental influences, and 59% by individual-specific environmental influences. For major depression, 35% (0.11+0.24) of the variance was explained by genetic influences, 3% by shared environmental influences, and 62% by individual-specific environmental influences.
Figure 1.
Variance components and 95% confidence intervals for the best fitting model of conduct disorder (CD), major depression (MD), and posttraumatic stress disorder (PTSD). Acd = additive genetic influences on conduct disorder; Ccd = shared environmental influences on conduct disorder; Ecd = individual-specific environmental influences on conduct disorder; Amd = additive genetic influences on major depression; Emd = individual-specific environmental influences on major depression; Eptsd = individual-specific environmental influences on PTSD.
The association between conduct disorder and PTSD was explained by common shared environmental influences; 10% of the variance in PTSD overlapped with shared environmental variance in conduct disorder. No shared environmental variance specific to PTSD was found after controlling for shared environmental influences overlapping with conduct disorder. Equivalently, the shared environmental correlation was unity between conduct disorder and PTSD. Of the individual-specific environmental variance of PTSD (71%), only 3% of the variance in PTSD overlapped with the individual-specific environmental variance in conduct disorder. Taken together, 13% of the total variance in PTSD overlapped with the environmental variance in conduct disorder. The genetic covariance between conduct disorder and PTSD was not statistically significant from zero.
However, the association between major depression and PTSD was almost entirely explained by common genetic influences; 19% of the variance in PTSD overlapped with the genetic variance in major depression after controlling for shared genetic covariance between conduct disorder and major depression. There were no additional genetic influences specific to PTSD above and beyond that shared with major depression. This means that the genetic correlation between major depression and PTSD was unity after adjusting for the genetic correlation between conduct disorder and major depression. Of the total individual-specific environmental variance of PTSD, 8% of the variance in PTSD overlapped with individual-specific environmental variance in major depression. A total of 27% (0.19+0.08) of the variance in PTSD overlapped with genetic and individual-specific environmental variance in major depression. Individual-specific environmental effects specific to PTSD were responsible for 60% of variance in PTSD. The shared environmental covariance between major depression and PTSD was not statistically different from zero after adjusting for the shared environmental effect associated with conduct disorder.
In addition, the association between conduct disorder and major depression was explained primarily by common genetic influences; 11% of the variance in major depression overlapped with the genetic variance in conduct disorder. This common genetic covariance was independent of the common genetic covariance between major depression and PTSD. However, 2% and 3% of the variance in MD overlapped with shared environmental and individual-specific environmental variance in conduct disorder, respectively. No additional shared environmental influences on MD were found after controlling for shared environmental influences on conduct disorder.
Discussion
This study focused on the etiology of the comorbidity among PTSD, conduct disorder and major depression. Our findings suggest that differential etiologic mechanisms explain the association between conduct disorder and major depression with PTSD in this population of male veterans. Shared environmental risk factors were almost entirely responsible for the association between PTSD and conduct disorder. In contrast, the association between PTSD and MD was primarily accounted for by shared genetic influences. These shared genetic influences were different from those causing the association between conduct disorder and major depression. The magnitude of the covariance (genetic plus individual-specific environmental) between major depression and PTSD was about twice as large as that for the covariance (shared plus individual-specific environmental) between conduct disorder and PTSD. This suggests the etiology of PTSD is more closely related to major depression than conduct disorder. This study extends previous findings from the bivariate analysis for conduct disorder and PTSD 6 and for major depression and PTSD, 19,20 respectively, by revealing the differential etiology of the associations among these three disorders. It is important to point out that the majority of the variance in PTSD is explained by non-shared environmental risk. This is consistent with previous findings. 19
The association between conduct disorder and PTSD was largely explained by shared environmental factors. Although this study is not able to specify what these shared environmental factors might be, our results suggest these factors are present in environments shared between siblings such as those in the family, school, and neighborhood. This is supported by other studies showing an adverse and unstable family environment in childhood and low socioeconomic status increase risk for both disorders independently. 23-25 Although the association between an adverse or unstable family environment and conduct disorder may be genetically mediated via parental externalizing psychopathology, emerging evidence also supports environmentally mediated effects of family environment on conduct disorder44-46 and possibly PTSD. Moreover, a small but significant proportion of the association between conduct disorder and PTSD is also explained by common individual-specific environmental factors. Evidence suggests individuals with externalizing problems, such as conduct disorder, are more likely to be exposed to potentially-traumatic events, which in turn increase risk for PTSD.7 Individual-specific environmental risk factors that predispose people to conduct disorder and PTSD may also include lifestyle factors such as substance abuse that increases risk for trauma exposure and PTSD through direct and indirect mechanisms.
The common genetic liability to major depression and PTSD implies that genes associated with major depression are good candidates for PTSD and vice versa. Initial molecular genetic studies, although there are few for PTSD, provide preliminary support for this suggestion. Caspi et al. first demonstrated a significant interaction between the short version of a functional polymorphism in the promoter region of the serotonin transporter gene (SLC6A4) and negative life events in the development of major depression. 47 This polymorphism has also been associated with PTSD in a Korean sample. 48 Gene-hunters focused on identifying genes associated with PTSD may benefit from starting with those that have been more consistently and strongly associated with major depression.
The majority of the variance in all three disorders was accounted for by individual-specific environmental influences. Individual-specific environmental influences common to conduct disorder and major depression also explained a significant, albeit small, proportion of the variance in PTSD. Such influences suggest the association of conduct disorder and major depression with PTSD is not entirely explained by shared familial (genetic or environmental) influences. Significant individual-specific environmental influences on phenotypic covariance are consistent with a causal relationship between phenotypes; 49 conduct disorder may be a risk factor for PTSD. They could also result from shared measurement error or environmental risk factors uncorrelated between siblings. Our study does not specify which factors make up such influences; however, previous work with this sample suggests they could include type or severity of trauma exposure, level of education at entry into the military or age at entry into the military. 11 Individual-specific environmental influences may also contain gene by environment interaction; in variance components models variance explained by the interaction between additive genetic and individual-specific environmental influences is contained in the term for the individual-specific environment.
Our findings are subject to several limitations. First, the sample consisted entirely of male Vietnam era veterans. The etiology of the association of CD and MD with PTSD may not generalize to civilians, females or other cohorts. Second, selection into military service probably excluded those with severe, early onset antisocial behavior or psychopathology. This would result in only those with mild to moderate psychopathology being included in the sample. Thus, the association among phenotypes in this sample may be underestimated. Third, our study relied on retrospective self-reported lifetime psychiatric diagnoses. Misclassification of MD and PTSD contributes to measurement error, usually resulting in an underestimate of the role of additive genetic factors and an overestimate of the role of individual-specific environmental factors in etiology. Thus, the contribution of genetic influences to the association between MD and PTSD in this study is likely to be an underestimate. If measurement error is uncorrelated among phenotypes, this would lead to an underestimate of non-shared environmental influences. Data were cross-sectional and could not distinguish subjects with current disorders from those with temporally separate disorders over the lifespan. Additionally, patterns of comorbidity and our results may have been influenced by DSM diagnostic decision/hierarchy rules. Finally, our data are based on the DSM-III-R and results may not be the same under DMS-IV.
Our finding of a differential etiology of conduct disorder and major depression with PTSD has implications for theory, research, and practice. This study is only one in a series of investigations that support the role of a shared etiology for many of the common psychiatric disorders. 50-52 This study demonstrates that both internalizing and externalizing factors, indexed by major depression and conduct disorder in this work, contribute to the etiology of PTSD. Taken together, these findings support R. Krueger’s view that research on the etiology of psychiatric disorders will benefit from focusing on core processes underlying multiple forms of psychopathology rather than on discrete disorders. 53,54 Much research has focused on identifying risk factors for PTSD; research aimed at understanding the structure and etiology of PTSD comorbidity is sorely needed.
In practical terms, investigators interested in identifying causal environmental or genetic risk factors for posttraumatic psychopathology may benefit from focusing on those factors that appear to be robust across disorders and from using comorbidity to identify subtypes of psychiatric disturbance with a common etiology. 55 Such research may benefit from identifying coherent patterns of PTSD comorbidity, such as those proposed by Miller and colleagues in their work on developing an internalizing/externalizing typology of posttraumatic response. 56 Research aimed at identifying patterns of PTSD comorbidity and their etiologic substrates may inform where PTSD best fits in terms of either the current DSM-IV classification or the more recently proposed dimensional or hierarchical models put forward for DSM-V. The identification of environmental factors and specific genes that are common risk factors for the development of multiple mental disorders is necessary to inform the development of interventions that will ultimately improve population mental health.
Acknowledgments
Dr. Fu is supported in part by NCI-K07CA104119 and American Foundation for Suicide Prevention. Dr. Koenen is supported in part by NIMH-K08MH070627. Additional funding was provided by the National Institute on Drug Abuse and the Robert Wood Johnson Foundation. The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance in the conduct of this study, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible.
Footnotes
Financial Disclosures: No financial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davidson JR, Hughes D, Blazer DG, George LK. Post-traumatic stress disorder in the community: An epidemiological study. Psychol Med. 1991;21:713–721. doi: 10.1017/s0033291700022352. [DOI] [PubMed] [Google Scholar]
- 2.Helzer JE, Robins LN, McEvoy L. Post-traumatic stress disorder in the general population: Findings of the epidemiological catchment area survey. N Engl J Med. 1987;317:1630–1634. doi: 10.1056/NEJM198712243172604. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 4.Kulka R, Schlenger W, Fairbank J, Hough R, Jordan K, Marmar C, et al. Trauma and the Vietnam War Generation: Report of the findings from the National Vietnam Veterans Readjustment Study. New York: Brunner/ Mazel; 1990. [Google Scholar]
- 5.Orsillo SM, Weathers FW, Litz BT, Steinberg HR, Huska JA, Keane TM. Current and lifetime psychiatric disorders among veterans with war zone-related posttraumatic stress disorder. Journal of Nervous and Mental Disease. 1996;184:307–313. doi: 10.1097/00005053-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Koenen KC, Fu QJ, Lyons MJ, Toomey R, Goldberg J, Eisen SA, et al. Juvenile conduct disorder as a risk factor for trauma exposure and posttraumatic stress disorder. J Trauma Stress. 2005;18:23–32. doi: 10.1002/jts.20010. [DOI] [PubMed] [Google Scholar]
- 7.Koenen KC, Moffitt TE, Poulton R, Martin J, Caspi A. Early childhood factors associated with the development of post-traumatic stress disorder: results from a longitudinal birth cohort. Psychol Med. 2006:1–12. doi: 10.1017/S0033291706009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Arch Gen Psychiatry. 2003;60:709–717. doi: 10.1001/archpsyc.60.7.709. [DOI] [PubMed] [Google Scholar]
- 9.Breslau N, Davis G, Andreski P, Federman B, Anthony JC. Adversity, stress, and psychopathology. London, Oxford: University Press; 1998. Epidemiological findings on posttraumatic stress disorder and co-morbid disorders in the general population; pp. 319–328. [Google Scholar]
- 10.Breslau N, Davis GC, Peterson EL, Schultz LR. A second look at comorbidity in victims of trauma: The posttraumatic stress disorder-major depression connection. Biological Psychiatry. 2000;48:902–909. doi: 10.1016/s0006-3223(00)00933-1. [DOI] [PubMed] [Google Scholar]
- 11.Koenen KC, Harney R, Lyons MJ, Wolfe J, Simpson JC, Goldberg J, et al. A twin registry study of familial and individual risk factors for trauma exposure and posttraumatic stress disorder. Journal of Nervous and Mental Disease. 2002;190:209–218. doi: 10.1097/00005053-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics. New York: Worth Publishers; 2001. [Google Scholar]
- 13.Lyons MJ, True WR, Eisen S, Goldberg J, Meyer JM, Faraone SV, et al. Diffential heritability of adults and juvenile antisocial traits. Arch Gen Psychiatry. 1995;52:906–915. doi: 10.1001/archpsyc.1995.03950230020005. [DOI] [PubMed] [Google Scholar]
- 14.Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: A meta-analysis of twin and adoption studies. Psychol Bull. 2002;128:490–529. [PubMed] [Google Scholar]
- 15.Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, et al. A registry-based twin study of depression in men. Arch Gen Psychiatry. 1998;55:468–472. doi: 10.1001/archpsyc.55.5.468. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 17.Stein MB, Jang KJ, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder: A twin study. Am J Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- 18.True WJ, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- 19.Koenen KC, Fu QJ, Ertel K, Lyons MJ, Eisen SA, True W, Goldberg J, Tsuang M. Common genetic liability to major depression and posttraumatic stress disorder in men. J Affect Disord. 2007 doi: 10.1016/j.jad.2007.04.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koenen KC, Lyons MJ, Goldberg J, Simpson J, Williams WM, Toomey R, et al. A high risk twin study of combat-related PTSD comorbidity. Twin Res. 2003;6:218–226. doi: 10.1375/136905203765693870. [DOI] [PubMed] [Google Scholar]
- 21.Fergusson DM, Horwood LJ, Ridder EM. Show me the child at seven: the consequences of conduct problems in childhood for psychosocial functioning in adulthood. J Child Psychol Psychiatry. 2005;46:837–849. doi: 10.1111/j.1469-7610.2004.00387.x. [DOI] [PubMed] [Google Scholar]
- 22.Kendler KS, Prescott CA. A population-based twin study of lifetime major depression in men and women. Arch Gen Psychiatry. 1999;56:39–44. doi: 10.1001/archpsyc.56.1.39. [DOI] [PubMed] [Google Scholar]
- 23.Foley DL, Eaves LJ, Wormley B, Silberg JL, Maes HH, Kuhn J, et al. Childhood adversity, monoamine oxidase a genotype, and risk for conduct disorder. Arch Gen Psychiatry. 2004;61:738–744. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- 24.Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- 25.Gilman SE, Kawachi I, Fitzmaurice GM, Buka L. Socio-economic status, family disruption and residential stability in childhood: relation to onset, recurrence and remission of major depression. Psychol Med. 2003;33:1341–1355. doi: 10.1017/s0033291703008377. [DOI] [PubMed] [Google Scholar]
- 26.Jaffee SR, Moffitt TE, Caspi A, Fombonne E, Poulton R, Martin J. Differences in early childhood risk factors for juvenile-onset and adult-onset depression. Arch Gen Psychiatry. 2002;59:215–222. doi: 10.1001/archpsyc.59.3.215. [DOI] [PubMed] [Google Scholar]
- 27.Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry. 1999;156:1223–1229. doi: 10.1176/ajp.156.8.1223. [DOI] [PubMed] [Google Scholar]
- 28.Watson D. Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. J Abnorm Psychol. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- 29.Eisen S, True WR, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin Registry: Method of construction. Acta Genet Med Gemellol. 1987;36:61–66. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- 30.Eisen S, Neuman R, Goldberg J, Rice J, True WR. Determining zygosity in the Vietnam Era Twin Registry: An approach using questionnaires. Clin Genet. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg J, True WR, Eisen SA, Henderson WG, Robinette CD. The Vietnam Era Twin (VET) Registry: Ascertainment bias. Acta Ganet Med Gemellol. 1987;36:67–78. doi: 10.1017/s0001566000004608. [DOI] [PubMed] [Google Scholar]
- 32.Henderson W, Eisen S, Goldberg J, True WR, Barnes JT, Vitek ME. Vietnam Twin Registry: A resource for medical research. Public Health Report. 1990;105:368–373. [PMC free article] [PubMed] [Google Scholar]
- 33.Robins LN, Helzer JE, Cottler L, Golding E. National Institute of Mental Health diagnistic interview schedule version III - revised. St. Louis, MO: Department of Psychiatry, Washington University; 1988. [Google Scholar]
- 34.Slutske WS, Eisen S, Xian H, True WR, Lyons MJ, Goldberg J, et al. Long - term reliability and validity of alcoholism diagnoses and symptoms in a large national telephone interview survey. Alcoholism: Clinical & Experimental Research. 1998;22:553–558. doi: 10.1111/j.1530-0277.1998.tb04292.x. [DOI] [PubMed] [Google Scholar]
- 35.True WR, Xian H, Scherrer JF, Madden PAF, Bucholz KK, Heath AC, Eisen SA, Lyons MJ, Goldberg J, Tsuang MT. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch Gen Psychiatry. 1999;56:655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- 36.Stata. Computer software. College Station, TX: Stata Corporation; 2001. Stata statistical software (Version 7.0) [Google Scholar]
- 37.Falconer DS. Quantitative genetics. Edinburgh, UK: Oliver and Boyd; 1960. [Google Scholar]
- 38.Joreskog KG, Sorbom D. PRELIS2 User's Reference Guide. Chicago, Illinois: Scientific Software International; 1993. [Google Scholar]
- 39.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- 40.Neale MC, Boker SM, Xie G, Maes H. Mx: statistical modeling. VCU Box 900126, Richmond: VA 23298: Department of Psychiatry; 2002. [Google Scholar]
- 41.Neale MC, Maes H. Methodology for genetic studies of twins and families. Dordecht, The Netherlands: Kluwer Academic Publishers; 2000. [Google Scholar]
- 42.Xian H, Scherrer JF, Eisen SA, True WR, Heath AC, Goldberg J, et al. Self-Reported zygosity and the equal-environments assumption for psychiatric disorders in the Vietnam Era Twin Registry. Behav Genet. 2000;30:303–310. doi: 10.1023/a:1026549417364. [DOI] [PubMed] [Google Scholar]
- 43.Kendler KS, Gardner CO., Jr Twin studies of adult psychiatric and substance dependence disorders: are they biased by differences in the environmental experiences of monozygotic and dizygotic twins in childhood and adolescence? Psychol Med. 1998;28:625–633. doi: 10.1017/s0033291798006643. [DOI] [PubMed] [Google Scholar]
- 44.Koenen KC, Moffitt TE, Poulton R, Martin J, Caspi A. Early childhood factors associated with the development of post-traumatic stress disorder: results from a longitudinal birth cohort. Psychol Med. 2007;37:181–192. doi: 10.1017/S0033291706009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim-Cohen J, Caspi A, Rutter M, Tomas MP, Moffitt TE. The caregiving environments provided to children by depressed mothers with or without an antisocial history. Am J Psychiatry. 2006;163:1009–18. doi: 10.1176/ajp.2006.163.6.1009. [DOI] [PubMed] [Google Scholar]
- 46.Kim-Cohen J, Moffitt TE, Taylor A, Pawlby SJ, Caspi A. Maternal depression and children's antisocial behavior: nature and nurture effects. Arch Gen Psychiatry. 2005;62:173–181. doi: 10.1001/archpsyc.62.2.173. [DOI] [PubMed] [Google Scholar]
- 47.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig I, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 48.Lee HJ, Lee MS, Kang RH, Kim H, Kim SD, Kee BS, et al. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depress Anxiety. 2005;21:135–139. doi: 10.1002/da.20064. [DOI] [PubMed] [Google Scholar]
- 49.Purcell S, Koenen KC. Environmental mediation and the twin design. Behav Genet. 2005;35:491–498. doi: 10.1007/s10519-004-1484-9. [DOI] [PubMed] [Google Scholar]
- 50.Fu Q, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, et al. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence. Arch Gen Psychiatry. 2002;59:1125–1132. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- 51.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 52.Khan AA, Jacobson KC, Gardner CO, Prescott CA, Kendler KS. Personality and comorbidity of common psychiatric disorders. Br J Psychiatry. 2005;186:190–196. doi: 10.1192/bjp.186.3.190. [DOI] [PubMed] [Google Scholar]
- 53.Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- 54.Krueger RF, Watson D, Barlow DH. Introduction to the special section: toward a dimensionally based taxonomy of psychopathology. J Abnorm Psychol. 2005;114:491–493. doi: 10.1037/0021-843X.114.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller MW, Kaloupek DG, Dillon AL, Keane TM. Externalizing and internalizing subtypes of combat-related PTSD: a replication and extension using the PSY-5 scales. J Abnorm Psychol. 2004;113:636–645. doi: 10.1037/0021-843X.113.4.636. [DOI] [PubMed] [Google Scholar]
- 56.Miller MW, Greif JL, Smith AA. Multidimensional Personality Questionnaire profiles of veterans with traumatic combat exposure: externalizing and internalizing subtypes. Psychol Assess. 2003;15:205–215. doi: 10.1037/1040-3590.15.2.205. [DOI] [PubMed] [Google Scholar]