Abstract
Stilbazulenyl nitrone (STAZN) is a potent antioxidant that, in a rat model of transient focal cerebral ischemia, confers significant enduring functional and morphological neuroprotection. This study investigated the influence of dose and time of administration on the neuroprotective effects of STAZN in the intraluminal-suture model of middle cerebral artery occlusion (MCAo).
Dose-Response
At 2 and 4h after the onset of MCAo, animals received intravenously either STAZN (low dose=0.07 mg/kg, n=8), (medium dose=0.7 mg/kg, n=9), (high dose=3.5 mg/kg, n=9), an equivalent volume of vehicle (30% Solutol HS15 and 70% isotonic saline, 0.37 ml/kg, n=5), or saline (0.37 ml/kg, n=5). Only the medium dose improved scores (p<0.05) on a standardized neurobehavioral test at 1, 2 and 3d after MCAo. Only the medium dose reduced the total infarction (51%, p=0.014) compared to controls. These results indicate that STAZN exhibits maximal neuroprotection at the 0.7 mg/kg dose.
Therapeutic Window
STAZN (0.6 mg/kg) dissolved in dimethylsulfoxide was given intra-peritoneally at 2 and 4h (n=11), 3 and 5h (n=10), 4 and 6h (n=10), or 5 and 7h (n=7) after the onset of MCAo. Additional doses were given at 24 and 48h. Vehicle (dimethylsulfoxide, 2.0 ml/kg, n=6) was administered at 3, 5, 24 and 48h. STAZN treatment initiated at 2 or 3h after the onset of MCAo improved neurological scores (p<0.001) and reduced total infarction (42.2%, p<0.05) compared to controls.
Keywords: stroke, cerebral ischemia, reperfusion injury, free radicals, antioxidant, neuroprotection
1. INTRODUCTION
An abundance of evidence has established that free radicals and the oxidative stress that they engender contribute to ischemia-induced injury (Fiskum, Rosenthal et al. 2004; Crack and Taylor 2005). This fact has provided the rationale for the testing of various antioxidant therapeutics in animal models of cerebral ischemia (Margaill, Plotkine et al. 2005; Weinberger 2006). We have previously demonstrated the marked, enduring neuroprotective effects of a novel antioxidant free-radical scavenger, stilbazulenyl nitrone (STAZN), in transient focal cerebral ischemia (Ginsberg, Becker et al. 2003; Ley, Vigdorchik et al. 2005). The successful translation of antioxidant therapy from animal models to the clinic, however, is not always straightforward (Committee. 2000; Green and Ashwood 2005). For example, although free radicals and oxidative stress are implicated in most human diseases, a recent meta-analysis of antioxidant supplements, such as vitamin E, vitamin A and β-carotene, found that antioxidant therapy did not confer benefit, but rather increased all-cause mortality in a variety of pathologies including neurological, cardiovascular, ocular, renal, endocrinological, gastrointestinal and dermatological diseases (Bjelakovic, Nikolova et al. 2007). Thus, in order to translate the potential of antioxidants to reduce free-radical-mediated damage into clinically significant neuroprotection, a more detailed understanding of the subtleties of antioxidant therapy may be required (Sena, Wheble et al. 2007). Here we report on the effects of dose and time of administration of STAZN on its neuroprotective efficacy in a rat model of transient focal cerebral ischemia produced by intraluminal occlusion of the middle cerebral artery (MCAo) for 2h, followed by recirculation (Belayev, Alonso et al. 1996). Neurobehavioral score was assessed sequentially, and quantitative histopathology was performed at 3 days.
2. RESULTS
Dose Response Series
In this series, rats received 2-h MCAo and were treated with STAZN or vehicle i.v. at 2h and 4h after onset of ischemia. STAZN dosing-groups were either 0.07, 0.7, or 3.5 mg/kg.
Physiological Variables
These are shown in Table 1. Physiological variables were generally similar in the four treatment groups at all times studied. Exceptions, however, were cranial and rectal temperature measurements at 2h after onset of MCAo, which were higher in the pooled controls than in STAZN-treated rats (Table 1, p<0.05). This was not the case prior to MCAo or at subsequent times during the 3-day survival period (Table 1).
Table 1.
Physiological Variables – Dose-response series
| Pooled Controls (n=10) | STAZN (0.07 mg/kg) (n=8) | STAZN (0.7 mg/kg) (n=8) | STAZN (3.5 mg/kg) (n=9) | |
|---|---|---|---|---|
| Before MCAo (15 min) | ||||
| Cranial temperature (°C) | 36.9 ± 0.2 | 36.9 ± 0.2 | 36.9 ± 0.2 | 36.7 ± 0.3 |
| Rectal temperature (°C) | 36.7 ± 0.6 | 36.8 ± 0.5 | 36.7 ± 0.3 | 36.7 ± 0.4 |
| Arterial pH | 7.44 ± 0.03 | 7.45 ± 0.03 | 7.49 ± 0.13 | 7.45 ± 0.02 |
| PaO2, mm Hg | 129 ± 24 | 102 ± 12 | 119 ± 33 | 113 ± 11 |
| PaCO2, mm Hg | 38.1 ± 2.0 | 37.9 ± 1.5 | 37.2 ± 2.3 | 38.6 ± 3.0 |
| MABP, mm Hg | 115 ± 10 | 110 ± 10 | 108 ± 10 | 113 ± 9 |
| Plasma glucose, mg/dL | 141 ± 14 | 137 ± 14 | 146 ± 26 | 137 ± 21 |
| Body weight | 316 ± 18 | 306 ± 16 | 309 ± 14 | 307 ± 12 |
| During MCAo (15 min) | ||||
| Cranial temperature (°C) | 36.8 ± 0.3 | 36.9 ± 0.3 | 36.9 ± 0.2 | 36.9 ± 0.3 |
| Rectal temperature (°C) | 37.0 ± 0.5 | 37.0 ± 0.3 | 36.9 ± 0.3 | 36.9 ± 0.2 |
| Arterial pH | 7.43 ± 0.03 | 7.42 ± 0.05 | 7.43 ± 0.02 | 7.47 ± 0.03 |
| PaO2, mm Hg | 128 ± 19 | 108 ± 9 | 121 ± 18 | 125 ± 14 |
| PaCO2, mm Hg | 40.0 ± 2.3 | 39.9 ± 2.2 | 37.8 ± 1.6 | 37.9 ± 1.6 |
| MABP, mm Hg | 126 ± 13 | 128 ± 11 | 121 ± 14 | 132 ± 11 |
| Plasma glucose, mg/dL | 155 ± 15 | 149 ± 16 | 176 ± 14 | 157 ± 22 |
| After MCAo (2h) | ||||
| Cranial temperature (°C) | 38.2 ± 0.8 | 37.2 ± 0.4* | 37.1 ± 0.6* | 37.2 ± 0.8* |
| Rectal temperature (°C) | 37.9 ± 0.5 | 37.1 ± 0.6* | 37.5 ± 0.8* | 37.3 ± 0.8* |
| MABP, mm Hg | 103 ± 15 | 104 ± 12 | 102 ± 12 | 98 ± 6 |
| During 3 day survival | ||||
| Rectal temperature (°C) – 1 day | 37.6 ± 0.6 | 37.6 ± 0.5 | 37.5 ± 0.8 | 37.6 ± 0.4 |
| Rectal temperature (°C) – 2 days | 37.5 ± 0.5 | 37.1 ± 0.7 | 37.3 ± 1.0 | 37.2 ± 0.5 |
| Rectal temperature (°C) – 3 days | 37.2 ± 0.9 | 37.1 ± 0.9 | 36.9 ± 2.0 | 37.2 ± 0.6 |
Value are mean ± S.D.
Different from vehicle group (p<0.05, one-way ANOVA followed by Holm-Sidak test).
Neurological Score
Prior to MCAo, the total neuroscore was zero in every rat. When re-tested at ~105 min of MCAo, each rat of the entire series showed a neuroscore of 11, indicating a severe neurological deficit (Figure 1). Neuroscores in control rats treated with saline or with Solutol vehicle did not differ; hence, these groups were pooled for analysis. Neuroscores during MCAo and following treatment are shown in Figure 1. As early as 3.75h after onset of ischemia (i.e., 1.75 h after the first treatment), differences among groups tended to emerge. Repeated-measures ANOVA revealed a highly significant difference among treatment groups post-ischemia (F(3,32) = 4.106, p<0.014). At 24h, 48h, and 72h post-ischemia, animals treated with STAZN at a dose of 0.7 mg/kg showed substantially improved neuroscores relative to vehicle/saline-treated rats (p<0.05, Holm-Sidak test); this was not the case, however, at lower (0.07 mg/kg) or higher (3.5 mg/kg) STAZN doses (Figure 1).
Figure 1.
Neuroscores in rats during MCA occlusion (1h) and at various survival times after onset of ischemia. STAZN or control treatments were administered at 2h and 4h after onset of ischemia. Values shown are means ± SEM. *, STAZN 0.7 mg/kg group significantly different from corresponding vehicle/saline group, p<0.05 (two-way repeated-measures ANOVA followed by Holm-Sidak test). Numbers of rats: pooled saline/vehicle controls, n=10; STAZN 0.07 mg/kg, n=8; STAZN 0.7 mg/kg, n=8; STAZN 3.5 mg/kg, n=9.
Histopathology
Hematoxylin-and-eosin-stained coronal sections of paraffin-embedded saline- or vehicle-treated brains with MCAo showed prominent confluent zones of pan-necrosis; microscopic examination revealed necrotic neurons, astrocytic proliferation, and variable cavitation. This appearance was altered in brains of animals treated with STAZN. Figure 2 presents histological sections obtained at a central coronal level in representative rats from each of the 4 treatment groups.
Figure 2.

Representative H&E-stained paraffin-embedded brain sections at a central coronal level (Level 4 – see Figure 3) from rats treated with A) saline and B) STAZN (0.7 mg/kg). (Infarct volumes in these animals were at the median of their respective groups.) The images were constituted as montages of low-power (1x) microscopic fields.
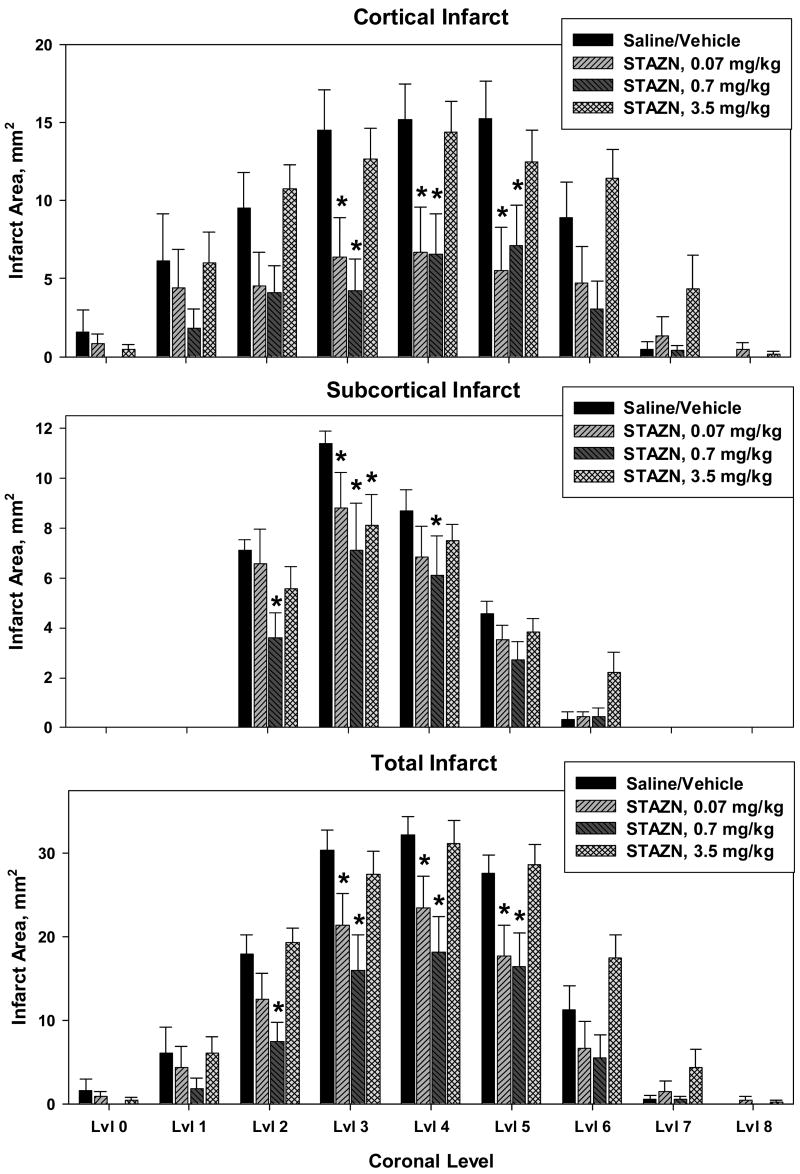
Quantitative results of histopathological analysis are shown in Figure 3. Infarct areas in control rats treated with saline or with Solutol vehicle did not differ statistically; hence, these groups were pooled for analysis. STAZN treatment (either 0.07 or 0.7 mg/kg doses) reduced cortical infarction at all 3 central coronal levels. Subcortical infarction was also modestly reduced, particularly at the STAZN dose of 0.7 mg/kg. Total infarct areas (Figure 3, bottom panel) were substantially reduced by STAZN treatment at 0.07 or 0.7 mg/kg, while the 3.5 mg/kg dose was ineffective.
Figure 3.
Measurements of areas of cortical infarct (top), subcortical infarct (middle), and total infarction (corrected for brain swelling, lower panel) in rats with 2-h MCAo treated with saline/vehicle control, or with STAZN at 0.07, 0.7, and 3.5 mg/kg doses at 2h and 4h following the onset of ischemia. Values shown are means ± SEM. Results were analyzed by two-way repeated-measures ANOVA, which revealed a significant effect of treatment-group for cortical infarct (F(3,32)=3.144, p=0.039) and total infarct (F(e,e2)=4.005, p=0.016). *, significantly different from corresponding saline/vehicle control value, p<0.05, Holm-Sidak test. Numbers of rats are given in legend to Figure 1.
Integrated infarct volumes in the four treatment groups are shown in Figure 4. ANOVA revealed a significant inter-group difference for both cortical infarction (p=0.041) and total infarct volume (p=0.014). Total infarct volume (corrected for brain swelling (Ley, Vigdorchik et al. 2005)), averaged 148 ± 55 mm3 (mean ± SD) in the pooled controls. STAZN treatment at the 0.7 mg/kg dose reduced the volume of total infarction to 75 ± 60 mm3 – a mean reduction of 51%.
Figure 4.
Integrated infarct volumes in rats with MCAo treated with saline/vehicle control or with STAZN. *, significantly different from corresponding value in saline/vehicle group, p<0.05, one-way ANOVA followed by Holm-Sidak test. Numbers of rats are given in legend to Figure 1.
Brain swelling, computed as
averaged 9.8 ± 3.8% (SD) in saline/vehicle controls but fell to 3.8 ± 4.7% in rats treated with STAZN at a dose of 0.7 mg/kg – a 61% mean reduction. ANOVA revealed a significant overall difference (p=0.032) among treatment groups.
Therapeutic-Window Series
In this series, rats received 2-h MCAo and were treated with STAZN, 0.6 mg/kg, or vehicle i.p. at treatment times ranging from 2h + 4h after onset of ischemia, to 5h + 7h.
Physiological Variables
These are shown in Table 2. All treatment groups had similar values for each physiological variable at each measurement-time.
Table 2.
Physiological Variables – Therapeutic window series
| DMSO (3h, 5h, 24h, 48h) (n=6) | STAZN (2h, 4h, 24h, 48h) (n=11) | STAZN (3h, 5h, 24h, 48h) (n=10) | STAZN (4h, 6h, 24h, 48h) (n=10) | STAZN (5h, 7h, 24h, 48h) (n=9) | |
|---|---|---|---|---|---|
| Before MCAo (15 min) | |||||
| Cranial temperature (°C) | 36.2 ± 0.2 | 36.3 ± 0.2 | 36.4 ± 0.1 | 36.2 ± 0.2 | 36.2 ± 0.2 |
| Rectal temperature (°C) | 36.4 ± 0.1 | 36.5 ± 0.2 | 36.5 ± 0.2 | 36.4 ± 0.2 | 36.5 ± 0.2 |
| Arterial pH | 7.42 ± 0.02 | 7.44 ± 0.03 | 7.43 ± 0.03 | 7.44 ± 0.03 | 7.44 ± 0.03 |
| PaO2, mm Hg | 97 ± 28 | 109 ± 22 | 115 ± 23 | 111 ± 21 | 112 ± 23 |
| PaCO2, mm Hg | 39.6 ± 1.2 | 38.4 ± 1.2 | 40.0 ± 2.5 | 39.3 ± 1.8 | 39.0 ± 1.9 |
| MABP, mm Hg | 109 ± 8 | 98 ± 12 | 99 ± 15 | 97 ± 8 | 97 ± 14 |
| Plasma glucose, mg/dL | 140 ± 24 | 153 ± 21 | 167 ± 25 | 162 ± 20 | 146 ± 33 |
| Body weight | 319 ± 13 | 323 ± 17 | 320 ± 26 | 312 ± 15 | 301 ± 19 |
| During MCAo (15 min) | |||||
| Cranial temperature (°C) | 36.6 ± 0.3 | 36.6 ± 0.2 | 36.7 ± 0.4 | 36.5 ± 0.2 | 36.4 ± 0.2 |
| Rectal temperature (°C) | 36.6 ± 0.4 | 36.7 ± 0.3 | 36.7 ± 0.5 | 36.6 ± 0.3 | 36.5 ± 0.4 |
| Arterial pH | 7.42 ± 0.03 | 7.43 ± 0.02 | 7.42 ± 0.03 | 7.43 ± 0.04 | 7.43 ± 0.02 |
| PaO2, mm Hg | 93 ± 20 | 107 ± 10 | 101 ± 18 | 104 ± 21 | 102 ± 18 |
| PaCO2, mm Hg | 38.7 ± 2.1 | 39.8 ± 1.5 | 40.0 ± 2.5 | 40.1 ± 3.4 | 40.0 ± 1.1 |
| MABP, mm Hg | 118 ± 8 | 122 ± 8 | 116 ± 13 | 116 ± 11 | 114 ± 14 |
| Plasma glucose, mg/dL | 138 ± 18 | 143 ± 24 | 153 ± 27 | 144 ± 26 | 140 ± 17 |
| After MCAo ( 2h) | |||||
| Cranial temperature (°C) | 36.4 ± 0.8 | 36.4 ± 0.7 | 36.5 ± 0.8 | 36.8 ± 1.0 | 36.6 ± 0.4 |
| Rectal temperature (°C) | 37.9 ± 0.8 | 37.6 ± 0.7 | 37.5 ± 0.7 | 37.9 ± 1.0 | 37.6 ± 0.6 |
| MABP, mm Hg | 109 ± 11 | 117 ± 5 | 114 ± 7 | 115 ± 11 | 107 ± 8 |
| During 3 day survival | |||||
| Rectal temperature (°C) 1 day | 38.6 ± 0.7 | 37.7 ± 0.6 | 38.0 ± 0.8 | 37.7 ± 0.6 | 37.6 ± 0.6 |
| Rectal temperature (°C) 2 days | 37.6 ± 0.9 | 37.6 ± 0.3 | 37.2 ± 0.9 | 37.1 ± 0.6 | 37.4 ± 1.1 |
| Rectal temperature (°C) 3 days | 36.7 ± 1.1 | 37.0 ± 0.6 | 36.8 ± 1.4 | 36.2 ± 1.8 | 35.9 ± 2.7 |
Value are mean ± S.D.
Neurological score
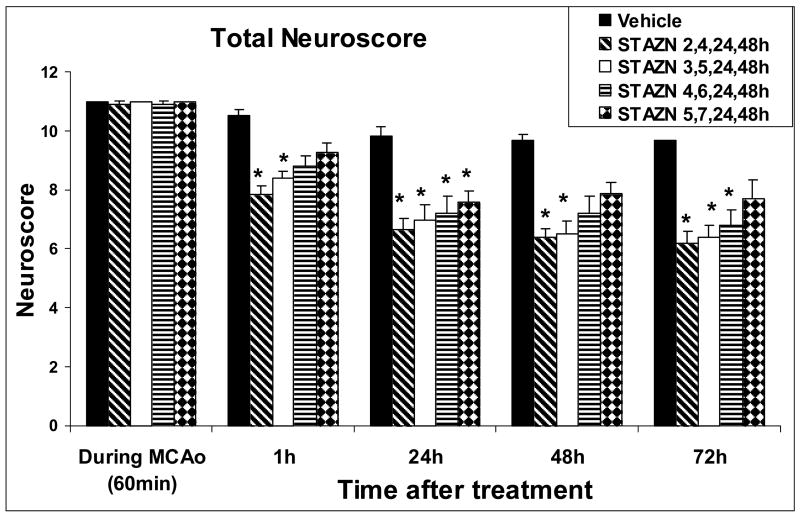
Prior to MCAo, all rats had a normal total neuroscore of zero. When re-tested at 60 min of MCAo, all rats displayed a severe neurological deficit (total neuroscore 11 in 42 of 44 rats and 10 in the remaining 2 cases; Figure 5). STAZN treatment led to rapid and sustained improvement in total neuroscore (ANOVA by ranks, p<0.001), which varied according to the delay-to-treatment (Figure 5). In rats whose STAZN treatment was begun at 2 or 3 hours after onset of MCAo, total neuroscore improved by 23% within 1h of initial dosing, and continued improvements were sustained at 24, 48, and 72 hours. Groups in which initiation of STAZN treatment was delayed by 4 or 5h, by contrast, showed lesser, more inconsistent degrees of neurological improvement relative to vehicle-controls.
Figure 5.
Total neuroscore, therapeutic-window series. Compared to vehicle-treated rats, animals treated with STAZN beginning at 2 or 3h following MCAo onset showed improvements of neuroscore beginning within 1h of initial dosing and sustained throughout the 72-hour survival period (Values shown are means ± SEM. *, different from corresponding vehicle value, p<0.05, ANOVA on ranks followed by Dunn or Holm-Sidak test). Numbers of rats: Vehicle group, n=6; STAZN 2,4,24,48h, n=11; STAZN 3,5,24,48h, n=10; STAZN 4,6,24,48h, n=10; STAZN 5,7,24,48h, n=9.
Histopathology
STAZN treatment initiated at 2 or 3 hours after onset of MCAo was associated with 58.6% and 42.2% mean reductions in the volumes of cortical and total infarction, respectively, relative to vehicle-treated controls (Figure 6). The volume of hemispheric swelling averaged 10.8% in the 44 animals of this series and was unaffected by STAZN treatment.
Figure 6.
Infarct volumes, therapeutic-window series. Rats treated with STAZN beginning at 2h or 3h after onset of MCAo, but not at 4 or 5h, showed significant reductions in volumes of cortical and total infarction compared to vehicle-treated animals (Values shown are means ± SEM. *, p<0.05 for pooled 2-h and 3-h groups compared to vehicle-treated rats, ANOVA followed by Holm-Sidak test). Numbers of rats are given in legend to Figure 5.
3. DISCUSSION
The results of this study establish a dose-response relationship and define the therapeutic window for the neuroprotective effects of STAZN in a rat model of transient focal cerebral ischemia. These two sets of results provide important insights into the scope of the neuroprotection conferred by STAZN and support the well-known role of oxygen radicals in contributing to the pathophysiological cascade of molecular events leading to ischemic tissue damage (Schaller, Graf et al. 2003). Earlier studies demonstrated that STAZN (0.7 mg/kg, given after 2 hours of ischemia, at the onset of reperfusion) was highly neuroprotective in the MCA suture-occlusion model of transient focal cerebral ischemia (Ginsberg, Becker et al. 2003), and that the neuroprotective effect persisted at 30 days (Ley, Vigdorchik et al. 2005). The present study confirms our earlier findings for the intermediate dose of STAZN (0.7 mg/kg). The neurobehavioral scoring system used here, based on the methods of Bederson et al (Bederson, Pitts, et al. 1986) and De Ryck et al (De Ryck, van Reempts, et al 1989), is a well-validated instrument that our laboratory has employed in almost two dozen prior studies. Significant neurobehavioral improvement began within 24 hours of the initial STAZN dose (given 2 h after ischemia) and persisted at 3 days (Figure 1). The histological results in STAZN-treated animals were concordant with the neurological outcome, with a marked increase in preserved brain tissue at 3 days compared to saline/vehicle controls (Figures 3, 4).
The significant neurological improvements seen in animals treated with 0.7 mg/kg of STAZN were abrogated by a tenfold decrease in the dose or a fivefold increase (Figure 1). Likewise, the significant reduction in total infarct volume seen with the 0.7 mg/kg dose compared to saline/vehicle controls was not observed with the other STAZN doses (Figure 4). However, when discrete coronal levels were considered, both the low dose (0.07 mg/kg) and the middle dose (0.7 mg/kg) showed significantly reduced infarction compared to saline/vehicle controls (Figure 3). No neuroprotective effects were observed with the high dose of STAZN (3.5 mg/kg).
In summary, the dose-response curve for the neuroprotective effects of STAZN reveals modest neuroprotection at the lowest dose (0.07 mg/kg), maximal functional and morphological protection at the middle dose (0.7 mg/kg), and no significant neuroprotective effects observed at the highest dose (3.5 mg/kg). Various neuroprotective antioxidants of diverse structures and activities also display a biphasic dose-response relationship and afford less neuroprotection at higher doses than at lower doses. Examples include ebselen in a permanent MCAO model (Green and Ashwood 2005); triliazad mesylate in cultured retina cells (Levin, Clark et al. 1996); dipyrimadole, vitamin E and N-acetyl cysteine in cultured hippocampal neurons (Farinelli, Greene et al. 1998); and α-phenyl butyl nitrone (PBN), azulenyl nitrone (AZN), dimethylthiourea (Castagne, Lefevre et al. 1999), and BXT-51072, a glutathione peroxidase mimetic (Castagne and Clarke 2000), in axotomized retinal ganglion cells.
The term hormesis has been used to define the phenomenon whereby an intermediate dose can produce the opposite effect of a higher or lower dose. Calabrese and co-workers found more than 5000 hormetic dose-response relationships published for over 900 chemical and physical agents.(Calabrese and Blain 2005) Given the vast array of chemical and physical agents and their different mechanisms of action, hormetic effects are unlikely to be the result of a single molecular mechanism and more likely to be related to a disruption of homeostasis (Hayes 2007). It may be helpful to view the effects of antioxidants generally and the neuroprotective effects of STAZN in particular in this context. Deficiencies in antioxidants, especially in the face of elevated oxidative stress, are deleterious (Shenkin 2006), and yet excessive doses of antioxidant supplementation confer no benefit and, indeed, may be potentially detrimental (Virtamo, Pietinen et al. 2003), (Bjelakovic, Nikolova et al. 2007). Free radicals and reactive oxygen species themselves, while potentially harmful at levels which overwhelm antioxidant defenses, act as cellular messengers necessary for normal function, and a brief increases may activate protective mechanisms such as are seen in pre-conditioning and post-conditioning (Valko, Leibfritz et al. 2007). Thus, the efficacy of free-radical-scavenging antioxidants may be limited to their ability to restore levels of free radicals and oxidative stress to within narrow homeostatic limits (Castagne, Lefevre et al. 1999; Hayes 2007).
The suture-occlusion model employed here produces substantial, consistent cortical-plus-subcortical infarction that closely resembles, in extent and severity, the large hemispheric infarcts resulting from proximal MCA and internal carotid artery occlusions in patients. Essential to the consistency of the model is the close monitoring and control of physiological variables, including brain temperature. Brain-temperature control is particularly important as it is a modulator of the extent of ischemic brain injury (Ginsberg and Busto 1998). In the present series, when animals were re-anesthetized (after neuroscore assessment) for suture removal at the end of MCAo, mean cranial and rectal temperatures in animals about to receive vehicle/saline were slightly higher than in rats about to receive STAZN (Table 1, p<0.05). This was not the case prior to MCAo, or at any later survival time (Table 1). Temperature did not affect the extent of tissue salvage achieved with STAZN (0.7 mg/kg) as compared to controls seen in earlier studies (Ginsberg, Becker et al. 2003; Ley, Vigdorchik et al. 2005). Furthermore, the STAZN dose-response findings in the present study were not confounded by temperature because there were no significant temperature differences between STAZN treatment groups.
The failure to administer treatment within the therapeutic window observed in animal studies is one reason cited for the failure of many clinical trials of diverse neuroprotective therapeutics to demonstrate effectiveness (Fisher 1999; Fisher 2001; Fisher 2003; Labiche and Grotta 2004; Fisher, Albers et al. 2005). In rats whose STAZN treatment was begun at 2 or 3 hours after onset of MCAo, neurological behavior improved significantly and was associated with 58.6% and 42.2% reductions of cortical and total infarct size, respectively, relative to vehicle-treated controls. The width the therapeutic window of STAZN is similar to that of other neuroprotectants, although other studies often employed a shorter duration of ischemia (Di Fabio, Conti et al. 1999; Yrjanheikki, Tikka et al. 1999; Piao, Kim et al. 2003; Yu, Kim et al. 2005; Xu, Croslan et al. 2006). Relatively few therapeutics, such as albumin, have shown efficacy when administered more than 3 hours after the onset of a 2-hour ischemic insult (Belayev, Liu et al. 2001; Mary, Wahl et al. 2001; Williams, Berti et al. 2004). In preclinical and initial clinical studies, neurological improvement was observed when NXY-059, a disulfonyl-substituted α-phenyl nitrone, was given up to 6 hours after the onset of ischemia (Kuroda, Tsuchidate, et al. 1999; Zivin 2007) However, the recently completed SAINT II multicenter clinical trial failed to confirm a significant benefit of NXY-059 for the treatment of stroke compared to controls (Shuaib 2007). In contrast to NXY-059, STAZN is a much more lipophilic nitrone that would therefore be expected to have higher blood-brain barrier penetration (Ley, Vigdorchik et al. 2005); in addition, STAZN is a much more potent free-radical scavenger that effectively inhibits lipid peroxidation (Becker, Ley et al. 2002; Mojumdar, Becker et al. 2004).
In summary, significant neuroprotective effects of STAZN shown in this study suggest its promise for the treatment of ischemic stroke -- a leading cause of death and disability for which no established neuroprotectant therapy is currently available. Thus, STAZN warrants further study, the design of which should be informed by the present results: a) STAZN is a potent antioxidant – low doses were sufficient to confer neuroprotection (0.7 mg/kg) while doses five times greater abrogated the neuroprotective effects; and b) STAZN is protective when initial administration is deferred by up to 3 hours after onset of temporary ischemia. Proper design of pre-clinical and clinical trials may allow STAZN to realize its potential to help mitigate the devastating consequences of ischemic stroke.
4. Experimental Procedure
Chemical Synthesis of STAZN
STAZN was prepared from commercially available guaiazulene following a previously published procedure (in the laboratory of Dr. Becker).(Becker, Ley et al. 2002)
Animal Preparation
Fasted Male Sprague-Dawley rats weighing 313 ± 17 g (S.D.) were used in these experiments. All studies were approved by the University of Miami’s Animal Use Committee. Baseline neurological behavior scores were obtained to confirm normal neurological function (described below). Animals were placed in a jar and anesthesia was induced with 3% halothane, 70% nitrous oxide, and a balance of oxygen. Atropine sulfate, 0.15 mg/kg i.p., was given to diminish secretions during orotracheal intubation (2.1mm O.D. × 45mm B&D Insyte catheter tubing, Becton Dickinson Infusion Therapy Systems Inc., Sandy, UT). Femoral arteries and veins were cannulated with PE-50 polyethylene tubing. Animals were ventilated with a rodent respirator (Stoelting Co., Wood Dale, IL) on a mixture of 70% nitrous oxide, 1.0–1.5% halothane, and a balance of oxygen passed through a humidifier containing Mucomyst-10 (acetylcysteine) in water (1:100 v/v). Pancuronium bromide (initial dose, 0.75 mg/kg i.v.; and 0.35 mg/kg i.v. every half-hour) was given for immobilization. Arterial blood pressure (Model RS3400 polygraph; Gould, Inc, Valley View, OH) was controlled by adjusting the level of halothane. Arterial blood gases (pO2, pCO2, pH) (Model ABL 330, Radiometer America, Inc, Westlake, OH) were controlled by adjusting the ventilator rate and volume. Plasma glucose was measured (Model 2300 Stat; Yellow Springs Instrument Co, Inc, Yellow Springs, OH). Rectal temperature was maintained at 36.8 ± 0.4°C (S.D.) by a heating pad beneath the animal (CMA/150 Temperature Controller, CMA/Microdialysis AB, Stockholm, Sweden). Cranial temperature was monitored by a thermocouple probe (Omega Engineering, Stamford, CT) implanted in the left temporalis muscle and was maintained at 36.9 ± 0.3°C (S.D.) by a warming lamp placed near the head. Full details are presented in previous publications (Belayev, Alonso et al. 1996), (Belayev, Liu et al. 2001).
Middle Cerebral Artery Occlusion
The proximal middle cerebral artery (MCA) was transiently occluded for 2 hours by the widely used intraluminal-suture occlusion model. To favor consistent and clinically relevant infarct size, the suture was coated with poly-L-lysine solution prior to use (Belayev, Alonso et al. 1996).
The right common carotid artery (CCA) was carefully exposed. The occipital branch of the external carotid artery (ECA) was coagulated and the internal carotid artery (ICA) was isolated. A 4-cm length of 3-0 monofilament, poly-L-lysine-coated nylon suture was inserted into the ECA and advanced retrogradely until the bifurcation of the CCA from whence it was advanced a distance of 20–22 mm into the ICA to occlude the MCA (Belayev, Alonso et al. 1996). A ligature was tied around the ICA, the incisions were closed with surgical staples, and the animal was extubated and returned to its cage. The animal was subjected to neurobehavioral testing (described below) 105 minutes after insertion of the suture (see below). A total score ≥ 10 was observed in all animals, confirming a profound neurological deficit. Animals were then briefly re-anesthetized and the MCA-suture withdrawn after 2 h of MCA occlusion. Incisions were closed and the rats were returned to their cages.
Drug Treatment
Dose response series
In this series, STAZN or vehicle was administered at 2 hours after the onset of MCA occlusion (i.e., at onset of recirculation) and again at 4 hours. STAZN, which is highly lipophilic and not soluble in water, was dissolved in a vehicle of 30% Solutol HS 15 (Strickley 2004) and 70% isotonic saline to allow intravenous administration. Animals were randomized to receive either low-dose STAZN (0.07 mg/kg, n=8), mid-dose STAZN (0.7 mg/kg, n=9), high-dose STAZN (3.5 mg/kg, n=9), or an equivalent volume of vehicle (0.37 ml/kg, n=5) or isotonic saline (0.37 ml/kg, n=5). Randomization to treatment group was performed by an investigator who was not involved in the conduct of the animal studies.
Therapeutic-window series
In another series of rats, conducted prior to the development of Solutol-vehicle, the drug (STAZN, 0.6 mg/kg) was dissolved in 2.0 ml/kg of dimethylsulfoxide (DMSO) and administered intra-peritoneally at either 2 and 4h (n=11), 3 and 5h (n=10), 4 and 6h (n=10) or 5 and 7h (n=7) after the onset of MCA occlusion. In each of the above groups, additional doses of STAZN were given at 24 and 48h. Control animals received vehicle (DMSO, 2.0 ml/kg; n=6) at 3 h and 5 h after onset of MCAo and additional doses were given at 24 and 48h.
Neurobehavioral Evaluation
Animals were tested with a standardized neurobehavioral exam before, during (105 min) and after MCAo (90 min, 1, 2 and 3 days) to confirm the initial neurological deficit and to monitor behavior for 3 days. All testing was performed by an observer blinded to the treatment-group allocation. The neurobehavioral battery, which we have previously described in detail (Belayev, Alonso et al. 1996), consisted of two tests used to evaluate various aspects of neurological function: 1) the postural reflex test developed by Bederson et al (Bederson, Pitts, et al. 1986) to examine upper body posture while the animal is suspended by the tail; and 2) the forelimb placing test developed by De Ryck et al (De Ryck, van Reempts, et al. 1989) to examine sensorimotor integration in forelimb placing responses to visual, tactile, and proprioceptive stimuli. The total neurological score ranged from a normal of score of 0 to a maximal possible score of 12.
Histopathology
Animals were deeply anesthetized with halothane 3 days after MCAo. After mid-sternal thoracotomy, a catheter was inserted into an incision at the apex of the left ventricle and ligated at the root of the aorta. The right atrium was incised and the animal was perfused with isotonic saline for 3–5 min followed by FAM (40% formaldehyde, glacial acetic acid and absolute methanol, 1:1:8 by volume) for 20 min at a pressure of 100–120 mmHg. The animals were decapitated and the heads were placed in a refrigerator for 24 h, after which the brain was removed and immersed in FAM for 24 h at 4°C. Brains were embedded in paraffin, and ten-μm-thick coronal sections were cut at 9 standard intervals. These were stained by hematoxylin and eosin (H & E) and examined by brightfield microscopy.
Morphometry and image-analysis
Electronic images of the sections were made with a high-resolution CCD camera. The area of infarction, a central region of coagulation necrosis delimited by generalized tissue pallor, was measured with an MCID image-analysis system (Imaging Research, Inc., St. Catherines, Canada). The areas of the ipsolateral and contralateral cerebral hemispheres were also measured. The corresponding tissue volumes were calculated by numerical integration of the areas from all sections using Simpson’s method.(Zhao, Ginsberg et al. 1996) Image-analysis was conducted by an operator blinded to the treatment-group assignment.
Acknowledgments
This investigation was supported by Grants from the NIH NS46295 and NS05820 to (M.D.G.)
Abbreviations
- NXY-059
disodium 4-[(tert-butylimino)methyl] benzene-1,3-disulfonate N-oxide
- STAZN
stilbazulenyl nitrone
- MCA
middle cerebral artery
- DMSO
dimethyl sulfoxide
- MCAo
middle cerebral artery occlusion
- FAM
(40% formaldehyde, glacial acetic acid and absolute methanol, 11:8 by volume)
- PBN
α-phenyl-N-tert-butyl nitrone
- AZN
azulenyl nitrone
Footnotes
Disclosure: Drs. Ginsberg and Becker are shareholders in Cognitrone, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker DA, Ley JJ, et al. Stilbazulenyl nitrone (STAZN): a nitronyl-substituted hydrocarbon with the potency of classical phenolic chain-breaking antioxidants. J Am Chem Soc. 2002;124(17):4678–84. doi: 10.1021/ja011507s. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, et al. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Belayev L, Alonso OF, et al. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27(9):1616–22. doi: 10.1161/01.str.27.9.1616. discussion 1623. [DOI] [PubMed] [Google Scholar]
- Belayev L, Liu Y, et al. Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001;32(2):553–60. doi: 10.1161/01.str.32.2.553. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, et al. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. Jama. 2007;297(8):842–57. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Blain R. The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview. Toxicol Appl Pharmacol. 2005;202(3):289–301. doi: 10.1016/j.taap.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Castagne V, Clarke PG. Neuroprotective effects of a new glutathione peroxidase mimetic on neurons of the chick embryo's retina. J Neurosci Res. 2000;59(4):497–503. doi: 10.1002/(SICI)1097-4547(20000215)59:4<497::AID-JNR4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Castagne V, Lefevre K, et al. An optimal redox status for the survival of axotomized ganglion cells in the developing retina. Neuroscience. 1999;93(1):313–20. doi: 10.1016/s0306-4522(99)00138-4. [DOI] [PubMed] [Google Scholar]
- Committee, T. I. S. Tirilazad mesylate in acute ischemic stroke: A systematic review. Tirilazad International Steering Committee. Stroke. 2000;31(9):2257–65. doi: 10.1161/01.str.31.9.2257. [DOI] [PubMed] [Google Scholar]
- Crack PJ, Taylor JM. Reactive oxygen species and the modulation of stroke. Free Radic Biol Med. 2005;38(11):1433–44. doi: 10.1016/j.freeradbiomed.2005.01.019. [DOI] [PubMed] [Google Scholar]
- De Ryck M, Van Reempts J, Borgers M, et al. Photochemical stroke model: flunarizine prevents sensorimotor deficits after neocortical infarcts in rats. Stroke. 1989;20:1383–1390. doi: 10.1161/01.str.20.10.1383. [DOI] [PubMed] [Google Scholar]
- Di Fabio R, Conti N, et al. Substituted analogues of GV150526 as potent glycine binding site antagonists in animal models of cerebral ischemia. J Med Chem. 1999;42(18):3486–93. doi: 10.1021/jm980576n. [DOI] [PubMed] [Google Scholar]
- Farinelli SE, Greene LA, et al. Neuroprotective actions of dipyridamole on cultured CNS neurons. J Neurosci. 1998;18(14):5112–23. doi: 10.1523/JNEUROSCI.18-14-05112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30(12):2752–8. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Fisher M. Recommendations for clinical trial evaluation of acute stroke therapies. Stroke. 2001;32(7):1598–606. doi: 10.1161/01.str.32.7.1598. [DOI] [PubMed] [Google Scholar]
- Fisher M. Recommendations for advancing development of acute stroke therapies: Stroke Therapy Academic Industry Roundtable 3. Stroke. 2003;34(6):1539–46. doi: 10.1161/01.STR.0000072983.64326.53. [DOI] [PubMed] [Google Scholar]
- Fisher M, Albers GW, et al. Enhancing the development and approval of acute stroke therapies: Stroke Therapy Academic Industry roundtable. Stroke. 2005;36(8):1808–13. doi: 10.1161/01.STR.0000173403.60553.27. [DOI] [PubMed] [Google Scholar]
- Fiskum G, Rosenthal RE, et al. Protection against ischemic brain injury by inhibition of mitochondrial oxidative stress. J Bioenerg Biomembr. 2004;36(4):347–52. doi: 10.1023/B:JOBB.0000041766.71376.81. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Becker DA, et al. Stilbazulenyl nitrone, a novel antioxidant, is highly neuroprotective in focal ischemia. Ann Neurol. 2003;54(3):330–42. doi: 10.1002/ana.10659. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Busto R. Combating hyperthermia in acute stroke: a significant clinical concern. Stroke. 1998;29(2):529–34. doi: 10.1161/01.str.29.2.529. [DOI] [PubMed] [Google Scholar]
- Green AR, Ashwood T. Free radical trapping as a therapeutic approach to neuroprotection in stroke: experimental and clinical studies with NXY-059 and free radical scavengers. Curr Drug Targets CNS Neurol Disord. 2005;4(2):109–18. doi: 10.2174/1568007053544156. [DOI] [PubMed] [Google Scholar]
- Hayes DP. Nutritional hormesis. Eur J Clin Nutr. 2007;61(2):147–59. doi: 10.1038/sj.ejcn.1602507. [DOI] [PubMed] [Google Scholar]
- Labiche LA, Grotta JC. Clinical trials for cytoprotection in stroke. NeuroRx. 2004;1(1):46–70. doi: 10.1602/neurorx.1.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S, Tsuchidate R, Smith ML, et al. Neuroprotective effects of a novel nitrone, NXY-059, after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1999;19:778–787. doi: 10.1097/00004647-199907000-00008. [DOI] [PubMed] [Google Scholar]
- Levin LA, Clark JA, et al. Effect of lipid peroxidation inhibition on retinal ganglion cell death. Invest Ophthalmol Vis Sci. 1996;37(13):2744–9. [PubMed] [Google Scholar]
- Ley JJ, Vigdorchik A, et al. Stilbazulenyl nitrone, a second-generation azulenyl nitrone antioxidant, confers enduring neuroprotection in experimental focal cerebral ischemia in the rat: neurobehavior, histopathology, and pharmacokinetics. J Pharmacol Exp Ther. 2005;313(3):1090–100. doi: 10.1124/jpet.105.083386. [DOI] [PubMed] [Google Scholar]
- Margaill I, Plotkine M, et al. Antioxidant strategies in the treatment of stroke. Free Radic Biol Med. 2005;39(4):429–43. doi: 10.1016/j.freeradbiomed.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Mary V, Wahl F, et al. Enoxaparin in experimental stroke: neuroprotection and therapeutic window of opportunity. Stroke. 2001;32(4):993–9. doi: 10.1161/01.str.32.4.993. [DOI] [PubMed] [Google Scholar]
- Mojumdar SC, Becker DA, et al. Kinetic studies on stilbazulenyl-bis-nitrone (STAZN), a nonphenolic chain-breaking antioxidant in solution, micelles, and lipid membranes. J Org Chem. 2004;69(9):2929–36. doi: 10.1021/jo030390i. [DOI] [PubMed] [Google Scholar]
- Piao CS, Kim JB, et al. Administration of the p38 MAPK inhibitor SB203580 affords brain protection with a wide therapeutic window against focal ischemic insult. J Neurosci Res. 2003;73(4):537–44. doi: 10.1002/jnr.10671. [DOI] [PubMed] [Google Scholar]
- Schaller B, Graf R, Jacobs AH. Ischaemic tolerance: a window to endogenous neuroprotection? Lancet. 2003;362:1007–1008. doi: 10.1016/S0140-6736(03)14446-7. [DOI] [PubMed] [Google Scholar]
- Sena E, Wheble P, et al. Systematic review and meta-analysis of the efficacy of tirilazad in experimental stroke. Stroke. 2007;38(2):388–94. doi: 10.1161/01.STR.0000254462.75851.22. [DOI] [PubMed] [Google Scholar]
- Shenkin A. The key role of micronutrients. Clin Nutr. 2006;25(1):1–13. doi: 10.1016/j.clnu.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Lees KR, Grotta J, Lyden P, Dávalos A, Davis SM, Diener HC, Wasiewski W, Ashwood T, Hardemark HG, Emeribe U. SAINT II: results of the second randomized, multicenter, pacebo-controlled, double-blind study of NXY-059 treatment in patients with acute ischemic stroke. International Stroke Conference Oral Presentations. 2007;38(471) [Google Scholar]
- Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21(2):201–30. doi: 10.1023/b:pham.0000016235.32639.23. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Virtamo J, Pietinen P, et al. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. Jama. 2003;290(4):476–85. doi: 10.1001/jama.290.4.476. [DOI] [PubMed] [Google Scholar]
- Weinberger JM. Evolving therapeutic approaches to treating acute ischemic stroke. J Neurol Sci. 2006;249(2):101–9. doi: 10.1016/j.jns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Berti R, et al. Delayed treatment of ischemia/reperfusion brain injury: extended therapeutic window with the proteosome inhibitor MLN519. Stroke. 2004;35(5):1186–91. doi: 10.1161/01.STR.0000125721.10606.dc. [DOI] [PubMed] [Google Scholar]
- Xu Z, Croslan DR, et al. Extended therapeutic window and functional recovery after intraarterial administration of neuregulin-1 after focal ischemic stroke. J Cereb Blood Flow Metab. 2006;26(4):527–35. doi: 10.1038/sj.jcbfm.9600212. [DOI] [PubMed] [Google Scholar]
- Yrjanheikki J, Tikka T, et al. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96(23):13496–500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YM, Kim JB, et al. Inhibition of the cerebral ischemic injury by ethyl pyruvate with a wide therapeutic window. Stroke. 2005;36(10):2238–43. doi: 10.1161/01.STR.0000181779.83472.35. [DOI] [PubMed] [Google Scholar]
- Zhao W, Ginsberg MD, et al. Depiction of infarct frequency distribution by computer-assisted image mapping in rat brains with middle cerebral artery occlusion. Comparison of photothrombotic and intraluminal suture models. Stroke. 1996;27(6):1112–7. doi: 10.1161/01.str.27.6.1112. [DOI] [PubMed] [Google Scholar]
- Zivin JA. Clinical trials of neuroprotective therapies. Stroke. 2007;38(2 Suppl):791–3. doi: 10.1161/01.STR.0000252090.44428.82. [DOI] [PubMed] [Google Scholar]