Abstract
Objectives
To determine if treatment with ampicillin together with dexamethasone and indomethacin delays preterm birth induced by intra-amniotic Group B Streptococcus (GBS) in a nonhuman primate model.
Study Design
After contraction onset induced by GBS (106 cfu/ml), chronically instrumented rhesus macaques received either: no treatment (controls, n=6); ampicillin (n=4); or ampicillin + dexamethasone + indomethacin (n=5). Outcomes included the interval from contraction onset until delivery and concentrations of amniotic fluid (AF) inflammatory mediators.
Results
Mean interval (hours) from contraction onset until delivery was 33 ± 8.7 in controls, 82 ± 28.0 with ampicillin (p=0.18 vs. controls); and 213 ± 50.8 with ampicillin + dexamethasone + indomethacin (p=0.004 vs. controls). Ampicillin eradicated GBS, but uterine activity, AF cytokines, prostaglandins, and MMP-9 remained elevated. AMP/DEX/INDO suppressed IL-1β, TNF-α, PGE2 and PGF2α, but did not alter MMP expression or chorioamnionitis.
Conclusions
The combination of AMP/DEX/INDO suppressed inflammation and significantly prolonged gestation.
Keywords: Intra-amniotic infection, Preterm labor, Cytokines, Prostaglandins, Rhesus macaques
INTRODUCTION
Intrauterine infection occurs in nearly 50% of extremely preterm births, and results in a pro-inflammatory cascade that leads to preterm labor.1–3 Preterm labor complicated by intra-amniotic infection (IAI) is refractory to tocolysis and antibiotic treatment trials have been largely unsuccessful in prolonging gestation among patients with preterm labor.4–6 The failure of antibiotic trials to delay or prevent infection-induced preterm birth is likely multifactorial. Potential confounders include the inclusion of women with preterm contractions without infection or the administration of antibiotics at varying stages of intrauterine infection or that do not target the appropriate pathogens. However, a major reason why antibiotics alone do not prevent infection-associated preterm birth may be a failure to reverse the production of pro-inflammatory mediators that play a key role in the initiation of labor. Elevated concentrations of pro-inflammatory cytokines, prostaglandins and matrix metalloproteinases in human amniotic fluid (AF) are associated with IAI and preterm labor.7–10 Interleukin-1β (IL-1β) or tumor necrosis factor-alpha (TNF-α) infused into the amniotic cavity of pregnant nonhuman primates also induces preterm labor and delivery.11 Bacteria or bacterial products stimulate prostaglandin synthesis in vitro and induce matrix metalloproteinase activity in placental tissues, which may contribute to the rupture of fetal membranes.12–14
Antibiotics coupled with immunomodulators that downregulate the proinflammatory cascade might delay preterm birth associated with IAI. We previously found that immunomodulators (including indomethacin, dexamethasone, and interleukin-10) inhibited the uterine activity, cytokine and prostaglandin production induced by intra-amniotic infusion of IL-1β in a non-human primate model of preterm labor.15, 16 Indomethacin (INDO), a non-selective cyclooxygenase inhibitor, may also inhibit calcium channel currents in myometrium.17 Glucocorticoids downregulate inflammation largely by the inhibition of transcription factors (e.g., nuclear factor-κB and activated protein-1), that control proinflammatory cytokine and prostaglandin production. Since INDO and dexamethasone (DEX) cross the placenta, target multiple pro-inflammatory effectors and are used in clinical practice, we evaluated their combined use in the multifactorial management of infection-associated preterm labor.15,16,18
We also previously demonstrated that intra-amniotic inoculation of Group B streptococci (GBS) increases uterine contractility, pro-inflammatory cytokines, and prostaglandins and leads to preterm delivery in non-human primates.19 We hypothesized, in this experimental model, that prolongation of gestation would occur only when both the inflammatory response and infection were treated. To test this hypothesis, GBS IAI was produced in chronically instrumented rhesus monkeys at 135 days gestation and effects of ampicillin (AMP) alone compared to AMP with INDO and DEX on the time interval from the onset of infection-induced preterm contractions to delivery. The effect of AMP alone or AMP in combination with INDO and DEX also was examined on the production of cytokines, chemokines, prostaglandins, and matrix metalloproteinases; amniotic fluid and fetal infection; and histologic chorioamnionitis.
MATERIAL AND METHODS
Animals
Study protocols were approved by the Institutional Animal Care and Utilization Committee and guidelines for humane care followed. Timed-pregnant rhesus monkeys (Macaca mulatta) were adapted to the vest and mobile catheter protection device as previously described.20 Intrauterine surgery was performed at 123 days of gestation (range, 118–134; there were no differences in age at surgery between groups) to implant fetal ECG electrodes and catheters in the amniotic fluid, maternal femoral vein and artery, fetal jugular vein and fetal carotid artery.19 Postoperatively, animals were treated with 250 mg cefazolin sodium IV q12 hours for 5 days and either 1mg/hr terbutaline sulfate IV (Bricanyl, Merrel Dow Pharm. Inc., Kansas City, MO) or 2 μg/kg/min atosiban IV (Merck & Co., Inc., West Point, PA) for 1–5 days to control uterine irritability. All of these medications were discontinued at least 72 hours prior to experimental intra-amniotic infection. At our center, term gestation in the non-instrumented rhesus monkey population averages 167 days (range, 155–172 days).
Study Groups
Experimental intra-amniotic infection was induced in 15 animals by the intra-amniotic inoculation of 106 colony-forming-units (cfu) of a clinical isolate of Group B streptococcus (GBS, type III) at a mean gestational age of 133 days (range 126–141 days; no difference between groups). Following infection, 6 animals were observed without treatment until delivery. Four animals received maternal intravenous AMP (30 mg/kg every 6 hours for 7 days or until delivery) after the onset of regular uterine contractions and an increase in the hourly contraction area of at least twice of baseline. Five animals received intravenous AMP for 7 days and both oral INDO (50 mg given to the mother twice daily for 5 days) and maternal intravenous DEX (1 mg/kg given every 6 hours for 3 days) begun after the onset of regular uterine contractions.
Uterine Activity, Preterm Labor, Cesarean Section, and Histopathology
Uterine activity was continuously recorded from the time of surgery as the integrated area under the amniotic fluid pressure curve and reported as the hourly contraction area (HCA; mmHg●sec/hr). Preterm labor was defined as >10,000 mmHg●sec/hr associated with a change in cervical dilation or effacement as determined by digital examination under conscious sedation with ketamine. Delivery by cesarean section, was performed to collect gestational tissues when vaginal delivery was considered imminent as judged by progressive cervical change and persistent increases in HCA (>10,000 HCA for more than 2 hours). After delivery, fetuses were euthanized by barbiturate overdose followed by exsanguination and fetal necropsy. Bacterial cultures of the fetal blood, lungs, meninges, and fetal membranes were performed and selected fetal tissues were also sampled for routine histologic examination. Placentae and membranes were also systematically sampled and submitted for histopathology by a primate pathologist (MKA) as previously described.21
Quantitation of Amniotic Fluid Cytokines, Chemokines, Leukocytes, and MMP-9
Amniotic fluid (AF) was sampled daily, beginning 48 hours prior to GBS inoculation, and more frequently following inoculation until delivery. Samples were centrifuged and the supernatant frozen and stored at −20°C. Prior to freezing, ethylenediaminetetraacetic acid (EDTA, 7.9 mM) and indomethacin (0.3 mM) was added to samples saved for prostaglandin quantitation to prevent prostaglandin metabolism. Quantities of IL-1β, IL-6, IL-8, PGE2, and PGF2α were determined using commercially available human ELISA (BioSource International, Camarillo, CA) and EIA kits (Cayman Chemical, Ann Arbor, MI) previously validated for use in the rhesus monkey.16 TNF-α concentrations were determined either by rhesus monkey-specific ELISA (BioSource International, Camarillo, CA) or by bioassay measuring the viability of a subcloned WEHI mouse fibroblast cell line.19 TNF-α concentrations were similar by both assay methods. Standard gelatin zymography was used to semi-quantitate the activity of MMP-9 in AF, adapted from previously published methods.14 In brief, all AF samples (5μl volume) from each animal were loaded onto a gelatin-containing polyacrylamide gel, electrophoresed at constant voltage, digested for 24h, and stained with Coomassie blue R-250. Gels were scanned and densitometric analysis of integrated area of lysis was conducted using Scion Image (NIH). Comparisons of MMP-9 activity were conducted on percent change from average baseline to average post-inoculation sample for each animal.
Statistical Analysis
The primary study outcome was the time interval between the onset of contractions and delivery. The time interval was compared among all groups using one-way ANOVA and between two groups using Student’s t-test (unequal variances assumed). Secondary outcomes were peak quantities of AF cytokines [IL-1β, TNF-α, interleukin-6 (IL-6), and interleukin-8 (IL-8)], prostaglandin E2 (PGE2), prostaglandin F2α (PGF2α), and the activity of matrix metalloproteinase-2 (MMP-2) and -9 (MMP-9). Following infection, the peak pre-treatment concentration of cytokines and prostaglandins were compared to the post-treatment concentration within each group by Wilcoxon signed ranks test. Statistical analysis was conducted using SPSS Version 12.0 (SPSS Inc., Chicago, IL), and significance was accepted at p<0.05. Unless otherwise noted, data are presented as means ± standard error of the mean.
RESULTS
Uterine Activity and Time until Delivery
The mean gestational age of animal inoculation with GBS was of 135 days (range, 126–141 days), which did not differ among groups. Prior to GBS inoculation, the uterus was quiescent in all animals with an average HCA less than 250 mmHg●sec/hr. Consistent increases in uterine contractility occurred a mean of 28.8 ± 6.3 hours after GBS. There was no difference in the interval between inoculation and onset of contractions among the three groups.
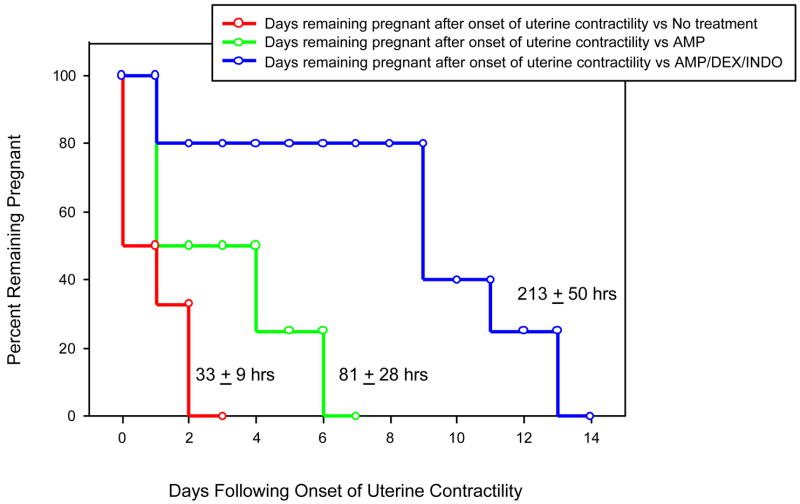
A Kaplan-Meier plot of the gestational length after the onset of contractions until delivery stratified by treatment group is presented in Figure 1. The average time between the onset of contractions and delivery was 33.2 ± 8.7 hours in monkeys not treated and 81.8 ± 28.0 hours in monkeys treated with AMP alone (p=0.18). Three (75%) of four animals treated with ampicillin alone delivered within 4 days of treatment. By contrast, a significantly longer mean interval between onset of contractions and delivery (213 ± 50.8 hours) occurred in monkeys treated with the combination of AMP/DEX/INDO (p=0.005 among groups by one-way ANOVA; p=0.02 vs. no treatment; p=0.06 vs. AMP alone by step-wise comparison). Of note, four (80%) of five animals treated with AMP/DEX/INDO had prolongations in pregnancy of 7 days or greater (range 234–374 hours) after initiation of treatment. Prolongation of pregnancy was not associated with increased maternal infectious morbidity.
Figure 1.
Kaplan-Meier plot of percent remaining pregnant following onset of contractions induced by experimental GBS intra-amniotic infection stratified by treatment regimen: no treatment (red), AMP alone (green), and AMP/DEX/INDO (blue). Below each step function for a specific treatment group, we report the mean interval from onset of uterine contractions-to-delivery ± SEM. Treatment with AMP/DEX/INDO significantly prolonged gestation following GBS-induced increases in uterine contractility (p=0.004, ANOVA.
Cytokines and Prostaglandins
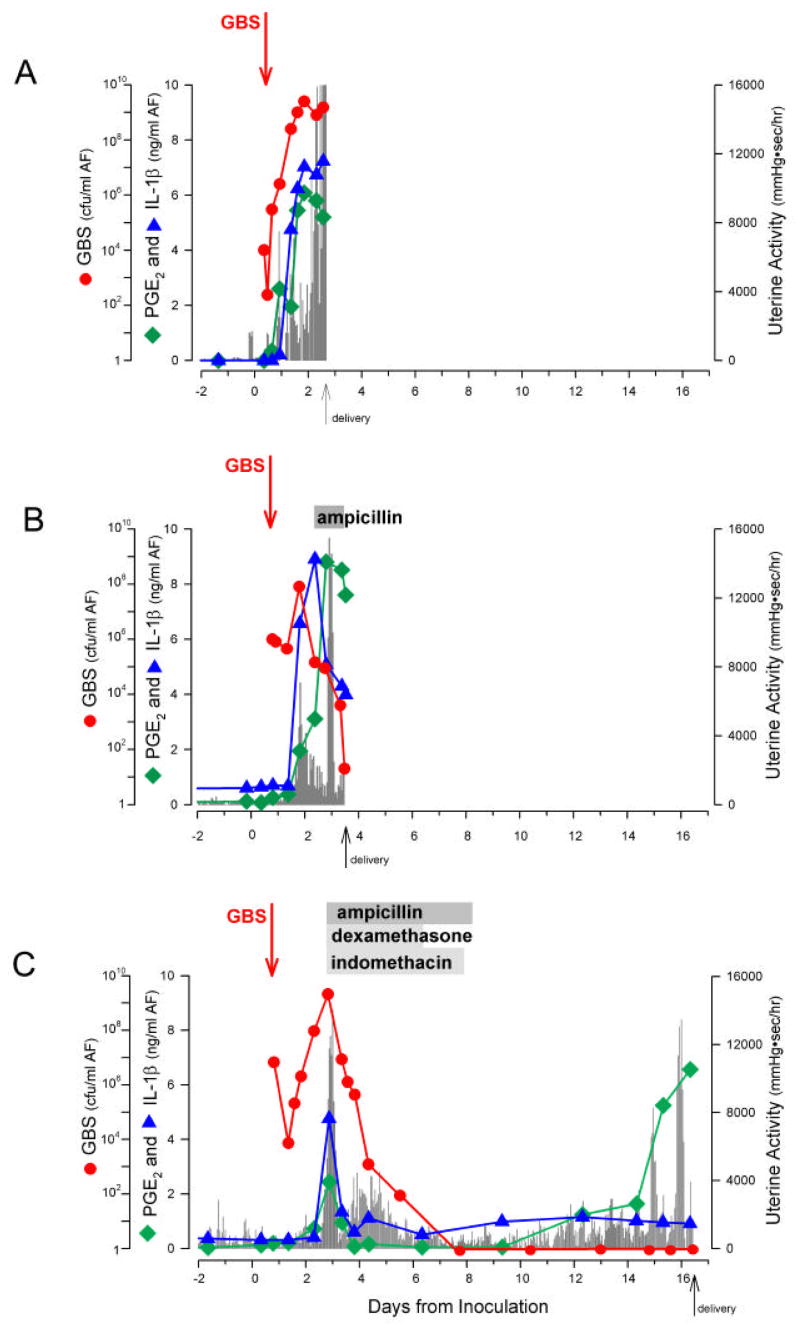
Mean amniotic fluid cytokine concentrations before and after infection and therapy in the different treatment groups are presented in Table I. A representative animal from each group is shown in Figure 2. Following GBS inoculation, there was a rapid increase in AF concentrations of IL-1β, TNF-α, IL-6, IL-8, PGE2, and PGF2α compared to pre-infection basal values, in all animals (p<0.05, Table I). These concentrations remained elevated until delivery in those animals receiving no treatment. Modest reductions in PGE2 concentrations at the time of delivery were observed, and may have been related to amnion necrosis and sloughing, as previously observed in this nonhuman primate model.19 Treatment with AMP alone resulted in lower post-treatment concentrations of IL-1β TNF-α PGE2, and PGF2α compared to post-infection and pre-treatment values although none of these achieved statistical significance. Mean IL-6 and IL-8 concentrations post-infection were similar before and after treatment with AMP. In contrast, AMP/DEX/INDO treatment was associated with significant reductions in AF concentrations of TNF-α (p=0.04), IL–6(p=0.04), PGE2 (p=0.04), and PGF2α(p=0.04), and nearly significant reductions in IL-1β (p=0.06) but not in IL-8 concentrations (p=0.68), in a comparison of peak levels pre-treatment (Table I). In summary, post-infection elevations in IL-1β, TNF-α, IL-6, PGE2, and PGF2α persisted despite AMP, and were suppressed only when DEX/INDO were added to the antibiotic regimen.
Table I.
Amniotic fluid cytokine and prostaglandin concentrations before and after experimental intra-amniotic GBS infection without treatment, treatment with ampicillin (AMP) alone, or treatment with ampicillin+dexamethasone+indomethacin (AMP/DEX/INDO)
| All (n=15) | No Treatment (n=6) | AMP Alone (n=4) | AMP/DEX/INDO (n=5) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Infection Basal |
Peak Concentration† |
Delivery |
p value* |
Pre-treatment Concentration† |
Post-treatmen t Concentration |
p value* |
Pre-treatment Concentration† |
Post-treatment Concentration |
p value* |
|
| IL-1β | 0.14 ±0.07 | 2.48 ± 0.49 | 2.53 ±0.76 | 0.72 | 2.09 ± 0.65 | 1.42 ± 0.56 | 0.18 | 5.01 ± 1.42 | 0.59 ± 0.23 | 0.06 |
| TNF-α | 0.02 ±0.01 | 3.47 ± 0.17 | 5.2 ±0.23 | 0.11 | 2.64 ± 1.08 | 1.22 ± 0.61 | 0.29 | 0.94 ± 0.51 | 0.08 ± 0.01 | 0.04 |
| IL-6 | 16.3 ±5.0 | 196.6 ± 99.6 | 116.6 ±47.6 | 0.11 | 259.6 ± 76.7 | 249.0 ± 121.1 | 0.59 | 479.0 ± 93.2 | 144.6 ± 38.8 | 0.04 |
| IL-8 | 2.8 ± 1.7 | 177.9 ± 62.1 | 166.7 ±73.3 | 0.32 | 191.0 ± 16.3 | 177.7 ± 19.4 | 0.11 | 130.5 ± 30.4 | 119.9 ± 28.5 | 0.68 |
| PGE2 | 0.54 ±0.20 | 16.6 ± 3.53 | 8.84 ±2.89 | 0.11 | 22.63 ± 12.01 | 11.32 ± 4.26 | 0.11 | 9.17 ± 3.18 | 0.18 ± 0.04 | 0.04 |
| PGF2α | 0.22 ±0.06 | 5.14 ± 1.86 | 3.73 ±1.63 | 0.47 | 2.63 ± 0.22 | 1.66 ± 0.09 | 0.11 | 3.31 ±1.20 | 0.14 ± 0.04* | 0.04 |
Concentrations are in nanogram/milliliter and presented as mean ± standard error of the mean (ng/ml).
All pre-treatment peak cytokine and prostaglandin concentrations were statistically significantly higher than basal levels (p<0.05) by Wilcoxin signed ranks test.
Wilcoxon signed ranks test, post-treatment versus pre-treatment within each treatment group.
Figure 2.
Graphs depict serial changes after experimental GBS intra-amniotic infection in uterine contractility, GBS cfu/ml, pro-inflammatory cytokines, and prostaglandins for single representative animals in each treatment group: (A) control, (B) AMP alone, and (C) AMP/DEX/INDO. The x-axis indicates days of gestation. The y-axis represents either GBS cfu/ml (left), pg/ml (IL-1β, PGE2; right), or mmHg●sec/hr (HCA; right). Graphical depictions of the changing concentrations of GBS cfu/ml (red circles), IL-1β (blue triangles), PGE2 (green squares), and uterine activity (gray bars; HCA) are indicated. Arrows indicate the time of initial GBS inoculum and administration of a specific treatment.
Matrix metalloproteinases
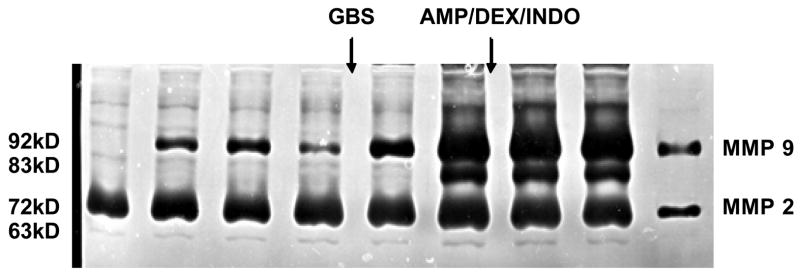
The activity of the 92-kd latent form of MMP-9 increased substantially in AF after GBS infection and preceded the subsequent appearance of the 83-kd active form as measured by zymography (Figure 3). This increase persisted and no change was observed following treatment with ampicillin or immunomodulators. Both latent and active forms of amniotic fluid MMP-2 activity remained relatively constant following infection and treatment.
Figure 3.
A representative zymogram indicating lack of suppression of either MMP-2 or MMP-9 activity (latent or active isoforms) in serial amniotic fluid samples during an intra-amniotic infection with GBS treated with AMP/DEX/INDO. Lanes 1 and 2 represent negative and positive amniotic fluid pool controls respectively. From left to right, each lane indicates MMP-2 and MMP-9 activity from progressively increasing experimental time points from a single animal. Lanes 3–4 are prior to GBS inoculation, lanes 5–6 are after GBS inoculation and prior to therapy, and lanes 7–8 are after treatment with AMP/DEX/INDO. The inactive (proenzyme) form of MMP-9 is 92 kDa and the active form is 83 kDa.
GBS cultures of amniotic fluid and fetal tissues
Following inoculation, GBS increased exponentially within amniotic fluid reaching peak values of 108–109 cfu/ml in untreated animals prior to delivery and in the two treatment groups prior to administration of antibiotics and/or immunomodulators. Maternal intravenous AMP administration either alone or in combination with immunomodulators effectively eradicated (n=6) or substantially reduced AF bacterial counts to levels below 103 cfu (n=3; persistent low levels of bacteria occurred in 2 animals given AMP alone and in 1 animal given AMP/DEX/INDO). In the absence of maternal AMP therapy, GBS was uniformly cultured from the blood and lungs of all (6 of 6) fetuses and from the meninges in 5 of 6 fetuses (83%). In contrast, maternal AMP treatment effectively eradicated GBS from fetal blood, lungs and meninges in all 9 animals given AMP with or without DEX/INDO (Table II).
Histopathology of the amniochorion, fetal lung and brain
As we previously described, experimental GBS infection caused moderate to severe acute exudative chorioamnionitis characterized by segmental or laminar infiltration of neutrophils into superficial parietal decidua, extravillous trophoblasts and chorionic connective tissue.19 Decidual cell necrosis was confined to localized areas adjacent to chorionic neutrophilic infiltration. The amnion epithelium and deeper decidua were generally unaffected. Longer intervals from inoculation until delivery were associated with more extensive infiltration by neutrophils in focal areas. AMP treatment alone was associated with little change or an actual increase in the extent of focal leukocytic infiltration. AMP/DEX/INDO treated animals had a modest decrease in the severity of leukocytic infiltration and the appearance of coagulative necrosis within the chorion trophoblast and superficial decidual layers. Coagulative necrosis refers to specific changes in the cytoplasm (increased eosinophilia) and nucleus (chromatin clumping) of dead cells prior to their removal by an inflammatory reaction.
GBS infection in fetal lungs was characterized by an acute alveolitis with widely scattered collections of neutrophils in alveoli and terminal airways but with no evidence of lung parenchyma damage. AMP/DEX/INDO treatment was associated with a reduction in alveolar neutrophils which correlated with a decrease in the AF leukocyte counts observed during eradication of GBS from the amniotic cavity. Histopathological examination of fetal meninges showed no evidence of inflammation.
COMMENT
Efforts to develop new therapies to prevent and/or delay infection-associated preterm labor have been unsuccessful to date. Antibiotic treatment trials yield inconsistent results, suggesting a lack of efficacy.4–6 Studies in humans do not elucidate the underlying mechanisms for the success or failure of an antibiotic intervention because the temporal relationships among bacterial load, inflammatory mediators, and preterm labor are not defined. This limitation was overcome in this established NHP model for experimental infection, where these relationships that culminate in preterm birth can be temporally described and the effects of interventions can be determined.
We previously examined the reasons for failure of antibiotics alone to prevent preterm delivery in the setting of intraamniotic infection. Our pilot studies in rhesus monkeys indicated that antibiotic treatment begun before the onset of uterine contractility eliminated GBS in amniotic fluid and prevented elevations in AF cytokines, prostaglandins and preterm labor.22 We now provide additional evidence that antibiotic therapy given after the onset of uterine contractions may eliminate GBS from AF but does not prevent preterm delivery or elevations in AF cytokines (IL-1β, IL-6, IL-8, TNF-α), prostaglandins, and MMP-9. It is likely that once bacterial up-regulation of IL-1β and TNF-α production reaches a threshold sufficient to initiate preterm labor, the process is no longer reversible by antibiotics alone. Thus, our data support a central role of the inflammatory response in triggering preterm labor and suggest that immunomodulators in combination with antibiotics may be more effective than antibiotics alone to manage infection-induced preterm labor.
This study is the first to demonstrate in intraamniotic infection that immunomodulators, when combined with specific antibiotic therapy, effectively delay the onset of preterm labor and delivery. Our rationale for using INDO and DEX as adjuncts to antibiotic therapy was based on our previous experimental results where INDO and other immunomodulators inhibited cytokine-induced preterm uterine contractions and blocked intrauterine prostaglandin production.15, 16 INDO is a potent and nonselective inhibitor of cyclooxygenase (COX) which readily crosses the placenta and inhibits PG synthesis in both maternal and fetal tissues.18 In addition to COX inhibition and direct inhibitory effects on voltage-gated calcium channel currents in myometrial cells, INDO has anti-oxidant and hydroxyl radical scavenging properties and also functions as a PPARγ agonist.23–25 PPARγ agonists reduce the expression of cytokines and neuronal damage in cell culture in animal models.23 INDO is one of the few tocolytic agent that in clinical trials reduces the risk of preterm delivery and low birthweight.26 Of concern are potential fetal side-effects of INDO such as constriction of the ductus arteriosis, oligohydramnios, and, infrequently, necrotizing enterocolitis and intracranial hemorrhage.27,28 Clinical experience demonstrates that ductal constriction is infrequent before 28 weeks of gestation. A recent study indicated that INDO prophylaxis in preterm, extremely low birthweight infants did not adversely affect outcomes including pulmonary function, neurosensory impairment, or death at 18 months of age.29
Although COX inhibition is an effective therapeutic approach, a more comprehensive strategy may be to inhibit the proinflammatory cascade upstream. We previously demonstrated that in chorioamnionitis, IL-1β and TNF-α are primary triggers of preterm labor.11 Observations in genetic knock-out mice support the hypothesis that both IL1β and TNF-α are necessary effectors for preterm birth.30 Thus, it may be necessary to block the actions of both IL and TNF to develop an optimal strategy to treat preterm labor. We found that maternal DEX, together with intraamniotic IL-10 administration, inhibits cytokine-mediated preterm uterine contractions but only DEX readily crosses the primate placenta16, thus providing a clinical rationale for its use in the current study.
Glucocorticoids inhibit inflammation by negating the activity of transcription factors that control proinflammatory cytokine production and by promoting anti-inflammatory T-helper type 2 responses that, in turn, increase production of anti-inflammatory cytokines IL-4 and IL-10.31,32 By inhibiting the fetal hypothalamic-pituitary-adrenal axis, DEX suppresses fetal placental estrogen biosynthesis, which also contributes to a tocolytic effect.33 The dose we used in our study is comparable to that proposed for severe preeclampsia HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome and similar to the clinical dose used for meningitis.34,35 The combination of DEX and INDO is potentially synergistic in down-regulating the pro-inflammatory cascade. It is also pertinent that DEX exerts a protective effect on potential side-effects of INDO (i.e., intraventricular hemorrhage) and on cytokine-induced damage to oligodendrocyte precursor cells.36
Maternal ampicillin (AMP) treatment, with or without DEX/INDO, effectively eradicated GBS from fetal blood, meninges and lungs in all animals. Histopathology demonstrated no parenchymal damage to the fetal lungs of treated animals. In fact, fewer neutrophils within the fetal lungs from the AMP/DEX/INDO group correlated with reduced amniotic fluid neutrophils (data not shown). Likewise, there was no evidence of meningitis or disruption of the cerebral white matter of the fetal brain in the immunomodulator treatment group, but further studies will be necessary to fully delineate the effects of treatment upon fetal brain injury. Animals treated with AMP/DEX/INDO demonstrated coagulative necrosis in chorion and decidua to a greater degree than did animals treated with AMP alone. This may reflect the extended duration in utero of the combined treatment group or to the influence of DEX. Glucocorticoids promote the uptake of damaged and apoptotic cells during the resolution or “healing” phase of inflammation37, consistent with our observed histologic findings. Infection with GBS was associated with increases in AF MMP-9 92 kD proenzyme and 83 kD enzyme production. This may contribute to an increased risk of preterm premature rupture of the membranes (PPROM) frequently seen in the setting of infection, although we did not observe PPROM in study animals. However, we did not evaluate levels of MMP inhibitors, which may negate the activity of elevated MMP concentrations.
Our intent was to seek a “proof of concept” combination of anti-microbial and anti-inflammatory therapy to prevent preterm birth and its consequences. The main strength of our experimental intra-amniotic infection model is the ability to investigate the discrete effect of antibiotics alone and in combination with anti-inflammatory treatment on gestational length and the pro-inflammatory cascade. This model has many similarities to human preterm labor. However, there are apparent important limitations of our model. First, we induced preterm labor with GBS, a bacteria very sensitive to AMP. Some Gram-negative or anaerobic bacteria may be less easily eradicated from the amniotic cavity, fetal blood, or fetal tissues. Second, antibiotic treatment was probably initiated sooner than is possible in human pregnancy. AMP was administered upon detection of regular uterine contractions as determined by an intra-amniotic pressure catheter. Many women are unaware of the early signs of preterm labor and delay notification of their obstetrician, which may be too late for them to receive the full benefit from anti-inflammatory treatment. Third, larger studies are necessary to determine the safety of prolonging pregnancy in the setting of intrauterine infection, even following anti-microbial treatment. A potentially undesirable consequence of therapy for preterm labor associated with infection is an increased risk of cerebral white matter lesions from prolonged exposure of the fetus to inflammatory cytokines in utero. Intrauterine infection is a significant risk factor for periventricular leukomalacia and cerebral palsy.38 Theoretically, prolonged exposure of the fetus to bacterial products and/or pro-inflammatory cytokines in utero could increase the risk of lung injury and white matter damage. This important area needs further detailed study.
At present, preterm labor is viewed as irreversible in the setting of infection and, thus, early diagnosis and treatment of intra-amniotic infection is not reliable or cost effective. Our data counter this nihilistic view for two reasons: infection-induced preterm labor in the non-human primate model was significantly delayed by anti-inflammatory and antibiotic administration; and recent data suggest that rapid identification of intra-amniotic infection is possible.39 In our experimental infection model, novel amniotic fluid peptides were identified in the amniotic fluid by proteomics-based analysis as early as 12 hours after infection.39 A rapid screening test for these peptides would allow the diagnosis of intra-amniotic infection at an early stage, when treatment is more likely to be successful.
Ultimately, the combined use of antibiotics, corticosteroids, cyclooxygenase inhibitors, and perhaps cytokine inhibitors may play an important role in the treatment of infection-associated labor. In addition, the eradication of intra-amniotic GBS from fetal blood and tissues by AMP suggests that antibiotics administered to women in preterm labor might benefit the fetus and, combined with immunomodulators, could ameliorate fetal lung and brain injury. The observations reported here need to be confirmed in larger studies and neonatal outcomes assessed. The study of optimal combined treatment to prevent infection-related or inflammation-related spontaneous preterm birth should become a research priority.
Acknowledgments
Supported by NIH grants AI42490, HD06159, HD18185, RR00163, and HD01264.
Footnotes
Presented, in part, at the 24th Annual Meeting, Society for Maternal-Fetal Medicine, New Orleans, LA, February 2–7, 2004
CONDENSATION
Ampicillin plus dexamethasone and indomethacin significantly delay preterm birth induced by intraamniotic Group B streptococcus in a nonhuman primate model.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chellam VG, Rushton DI. Chorioamnionitis and funiculitis in the placentas of 200 births weighing less than 2.5 kg. Br J Obstet Gynaecol. 1985;92:808–814. doi: 10.1111/j.1471-0528.1985.tb03050.x. [DOI] [PubMed] [Google Scholar]
- 2.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972–8. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 3.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79:351–7. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Stetzer BP, Mercer BM. Antibiotics and preterm labor. Clin Obstet Gynecol. 2000;43:809–17. doi: 10.1097/00003081-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Kenyon SL, Taylor DJ, Tarnow-Mordi W. Broad-spectrum antibiotics for spontaneous preterm labour: the ORACLE II randomised trial. ORACLE Collaborative Group Lancet. 2001;357:989–94. doi: 10.1016/s0140-6736(00)04234-3. [DOI] [PubMed] [Google Scholar]
- 6.King J, Flenady V. Prophylactic antibiotics for inhibiting preterm labour with intact membranes. Cochrane Database Syst Rev. 2002:CD000246. doi: 10.1002/14651858.CD000246. [DOI] [PubMed] [Google Scholar]
- 7.Romero R, Brody DT, Oyarzun E, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–23. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 8.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol. 1993;81:941–8. [PubMed] [Google Scholar]
- 9.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero R, Manogue KR, Mitchell MD, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989;161:336–41. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- 11.Sadowsky DW, Adams KM, Gravett MG, Axthelm MK, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1betta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a non-human primate model. Am J Obstet Gynecol. 2006;195:1578–89. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, Mitchell MD. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins. 1989;37:13–22. doi: 10.1016/0090-6980(89)90028-2. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell MD, Dudley DJ, Edwin SS, Schiller SL. Interleukin-6 stimulates prostaglandin production by human amnion and decidual cells. Eur J Pharmacol. 1991;192:189–91. doi: 10.1016/0014-2999(91)90090-d. [DOI] [PubMed] [Google Scholar]
- 14.Vadillo-Ortega F, Sadowsky DW, Haluska GJ, et al. Identification of matrix metalloproteinase-9 in amniotic fluid and amniochorion in spontaneous labor and after experimental intrauterine infection or interleukin-1 beta infusion in pregnant rhesus monkeys. Am J Obstet Gynecol. 2002;186:128–38. doi: 10.1067/mob.2002.118916. [DOI] [PubMed] [Google Scholar]
- 15.Sadowsky DW, Haluska GJ, Gravett MG, Witkin SS, Novy MJ. Indomethacin blocks interleukin 1beta-induced myometrial contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2000;183:173–80. doi: 10.1067/mob.2000.105968. [DOI] [PubMed] [Google Scholar]
- 16.Sadowsky DW, Novy MJ, Witkin SS, Gravett MG. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2003;188:252–63. doi: 10.1067/mob.2003.70. [DOI] [PubMed] [Google Scholar]
- 17.Sawdy R, Knock GA, Bennett PR, Poston L, Aaronson PI. Effect of nimesulide and indomethacin on contractility and the Ca2+ channel current in myometrial smooth muscle from pregnant women. Br J Pharmacol. 1998;125:1212–7. doi: 10.1038/sj.bjp.0702211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moise KJ, Jr, Ou CN, Kirshon B, Cano LE, Rognedrud C, Carpenter RJ., Jr Placental transfer of indomethacin in the human pregnancy. Am J Obstet Gynecol. 1990;162:549–54. doi: 10.1016/0002-9378(90)90427-9. [DOI] [PubMed] [Google Scholar]
- 19.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660–7. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 20.Ducsay CA, Cook MJ, Novy MJ. Simplified vest and tether system for maintenance of chronically catheterized pregnant rhesus monkeys. Lab Anim Sci. 1988;38:343–4. [PubMed] [Google Scholar]
- 21.Giannoulias D, Haluska GJ, Gravett MG, Sadowsky DW, Challis JRG, Novy MJ. Localization of prostaglandin H synthase, prostaglandin dehydrogenase, corticotropin releasing hormone and glucocorticoid receptor in rhesus monkey fetal membranes with labor and in the presence of infection. Placenta. 2005;26:289–97. doi: 10.1016/j.placenta.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Gravett MG, Sadowsky DW, Witkin SS, Novy MJ. Early but not delayed antibiotic treatment prevents preterm delivery following experimental intraamniotic infection (IAI). (Abstract). Annual Scientific Meeting, Infectious Diseases Society for Obstetrics and Gynecology; Hyannis, MA. August 7–9, 2003. [Google Scholar]
- 23.Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer’s disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J Neurosci. 2000;20:558–67. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa O, Umegaki H, Sumi D, et al. Inhibition of inducible nitric oxide synthase gene expression by indomethacin or ibuprofen in beta-amyloid protein-stimulated J774 cells. Eur J Pharmacol. 2000;408:137–41. doi: 10.1016/s0014-2999(00)00721-4. [DOI] [PubMed] [Google Scholar]
- 25.Prasad K, Laxdal VA. Hydroxyl radical-scavenging property of indomethacin. Mol Cell Biochem. 1994;136:139–44. doi: 10.1007/BF00926074. [DOI] [PubMed] [Google Scholar]
- 26.King J, Flenady V, Cole S, Thornton S. Cyclo-oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database Syst Rev. 2005:CD001992. doi: 10.1002/14651858.CD001992.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Sawdy RJ, Lye S, Fisk NM, Bennett PR. A double-blind randomized study of fetal side effects during and after the short-term maternal administration of indomethacin, sulindac, and nimesulide for the treatment of preterm labor. Am J Obstet Gynecol. 2003;188:1046–51. doi: 10.1067/mob.2003.255. [DOI] [PubMed] [Google Scholar]
- 28.Norton ME, Merrill J, Cooper BA, Kuller JA, Clyman RI. Neonatal complications after the administration of indomethacin for preterm labor. N Engl J Med. 1993;329:1602–7. doi: 10.1056/NEJM199311253292202. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt B, Asztalos EV, Roberts RS, Robertson CM, Sauve RS, Whitfield MF. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA. 2003;289:1124–9. doi: 10.1001/jama.289.9.1124. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch E, Filipovich Y, Mahendroo M. Signaling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am J Obstet Gynecol. 2006;194:1334–40. doi: 10.1016/j.ajog.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 31.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 32.Skjolaas KA, Grieger DM, Hill CM, Minton JE. Glucocorticoid regulation of type 1 and type 2 cytokines in cultured porcine splenocytes. Vet Immunol Immunopathol. 2002;87:79–87. doi: 10.1016/s0165-2427(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 33.Charles D, Pinkus JL, Fanous R, Chattoraj SC. The effect of dexamethasone on the urinary excretion of steroids during pregnancy. J Obstet Gynaecol Br Commonw. 1971;78:241–7. doi: 10.1111/j.1471-0528.1971.tb00262.x. [DOI] [PubMed] [Google Scholar]
- 34.Odio CM, Faingezicht I, Paris M, et al. The beneficial effects of early dexamethasone administration in infants and children with bacterial meningitis. N Engl J Med. 1991;324:1525–31. doi: 10.1056/NEJM199105303242201. [DOI] [PubMed] [Google Scholar]
- 35.Magann EF, Graves GR, Roberts WE, Blake PG, Morrison JC, Martin JN., Jr Corticosteroids for enhanced fetal lung maturation in patients with HELLP syndrome: impact on neonates. Aust N Z J Obstet Gynaecol. 1993;33:131–5. doi: 10.1111/j.1479-828x.1993.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 36.Feldhaus B, Dietzel ID, Heumann R, Berger B. [Corticoids protect oligodentrocyte precursor cells against cytokine-induced damage] Zentralbl Gynakol. 2004;126:282–5. doi: 10.1055/s-2004-822759. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Cousin JM, Hughes J, et al. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J Immunol. 1999;162:3639–46. [PubMed] [Google Scholar]
- 38.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA. 200;284:1417–1424. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 39.Gravett MG, Novy MJ, Rosenfeld RG, et al. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA. 2004;292:462–9. doi: 10.1001/jama.292.4.462. [DOI] [PubMed] [Google Scholar]