Abstract
Egfr/Ras signaling promotes vein cell fate specification in the developing Drosophila wing. While the importance of Ras signaling in vein determination has been extensively documented, the mechanisms linking Ras activity to vein differentiation remain unclear. We found that Ras signaling regulates both the levels and subcellular localization of the cell adhesion molecule DE-cadherin/Shotgun (Shg) in the differentiating wing epithelium. High Ras activity in presumptive vein cells directs the apical localization of Shg containing adherens junctions, whereas low Ras activity in intervein cells allows Shg to relocalize basally. These alterations in Shg-mediated adhesion control cell shape changes that are essential for vein morphogenesis. While decapentablegic (Dpp) acts downstream of Ras to maintain vein cell identity in the pupal wing, our results indicate that Ras controls Shg localization via a Dpp-independent mechanism. Ras, therefore, regulates both the transcriptional responses necessary for vein cell identity, and the cell adhesive changes that determine vein and intervein cell morphology.
Keywords: Drosophila, Cell adhesion, Adherens junction, Wing development, Wing vein, E-cadherin, Ras, Egfr
Introduction
Genetically specified positional cues direct the morphogenesis and differentiation of developing tissues. It is far from clear, however, how these signals are translated by developing cells into the morphological changes necessary for organ formation. In the Drosophila wing, multiple signaling pathways converge to elaborate a precise pattern of vein and intervein cells (Sotillos and De Celis, 2005). Veins form hollow, fluid-filled tubes between the two epithelial wing layers that carry nutrients to living cells of the adult wing, and act as rigid support structures that are necessary for flight. While the mechanisms by which vein cells are specified and positioned within the wing field are well understood (Crozatier et al., 2004; De Celis, 2003; De Celis and Diaz-Benjumea, 2003), we know little about the forces that morphologically distinguish vein and intervein cell types.
Current models propose that expression of rhomboid (rho) in vein primordia is the first step in the process of vein cell fate determination. Rho is a Golgi-localized serine protease that cleaves and activates Epidermal growth factor receptor (Egfr) ligands (Lee et al., 2001; Urban et al., 2001; Urban et al., 2002). Signaling downstream of the transmembrane Egfr proceeds canonically through the Ras/Raf/MAP Kinase (MAPK) pathway to affect activity of transcription factors in the nucleus. rho-expressing cells, therefore, have the highest levels of Ras/MAPK activity, and ultimately adopt vein cell fates. A wealth of genetic data supports the hypothesis that Ras/MAPK signaling is required for the initial specification of vein cells. Loss-of-function mutations in Egfr or downstream components of the Ras/MAPK pathway result in loss of veins, whereas ectopic veins develop when Ras/MAPK signaling proteins are mutationally activated (Brunner et al., 1994; Clifford and Schupbach, 1989; Diaz-Benjumea and Hafen, 1994; Guichard et al., 1999; Karim and Rubin, 1998; Prober and Edgar, 2000; Sturtevant and Bier, 1995; Sturtevant et al., 1993). Despite this genetic data, the functional consequences of Ras activity in these cells (changes in gene transcription or protein function) that direct vein cell differentiation are not well understood.
In recent years, developmental signaling pathways have been shown to regulate cadherin-mediated adhesion between cells to effect cell shape changes and tissue morphogenesis (for example (Jaiswal et al., 2006; Ulrich et al., 2005). Cadherins are transmembrane proteins that mediate primarily homophilic cell interactions (Nose et al., 1988). In polarized epithelial cells, cadherins are found in sub-apical adherens junction complexes in association with β-catenin/Armadillo (Arm), α-catenin and components of the actin cytoskeleton (D’Souza-Schorey, 2005). Cadherin function is required to maintain tissue integrity in innumerable developmental contexts (Lecuit, 2005), while the regulated loss of cadherin activity is critical as cells undergo epithelial to mesenchymal transitions (Huber et al., 2005). Differential levels of cadherin-mediated adhesion can also cause groups of cells to sort into functional units (Nose et al., 1988). For example, in the chicken spinal cord, functionally related pools of motoneurons cluster together, and their cadherin-mediated adhesive differences are necessary for motor pool sorting (Price et al., 2002). It has been demonstrated that changes in adhesivity often result from signaling between cells. In response to Wnt and BMP signaling in the mouse epidermis, for example, E-cadherin is downregulated to allow for hair follicle invagination (Jamora et al., 2003). The Sonic Hedghog pathway has also been shown to increase N-cadherin-mediated cell adhesion during differentiation of vertebrate neuroepithelial cells (Jarov et al., 2003). Altered cell adhesion, therefore, is often an important functional consequence of long and short range signaling between cells.
In Drosophila, there is a particularly striking link between Egfr/Ras signaling and the adhesion molecule Drosophila E-cadherin (DE-cadherin), encoded by the gene shotgun (shg). During embryonic optic placode formation, Egfr signaling downregulates Shg to allow for invagination of placode cells (Dumstrei et al., 2002). In the developing Drosophila trachea, Egfr activity upregulates shg expression to maintain epithelial integrity of the elongating tracheal tubes (Cela and Llimargas, 2006). In the eye, Egfr activity leads to increased levels of Shg adhesivity between photoreceptors (Brown et al., 2006; Mirkovic and Mlodzik, 2006). In these contexts, Egfr has been shown to affect both shg transcription, and the post-translational levels of Shg protein.
Here we provide evidence that the Egfr/Ras pathway regulates not only shg expression, but also protein localization during the process of Drosophila wing vein specification and differentiation. Shg-mediated adhesive differences are first seen in the wing during larval stages, when presumptive vein cells express higher levels of shg. As the wing differentiates during pupal stages, and vein and intervein cells become morphologically distinct, Shg-mediated adhesion is necessary for formation of the vein structure. At this stage of development, Ras signaling regulates the apical/basal distribution of Shg and the adherens junction complex of proteins. Surprisingly, this effect on cell adhesion is independent from the effect of Ras on vein cell fate. In this way, we define a regulatory network linking the Ras developmental signaling pathway to the cell adhesive changes necessary to differentiate the wing vein structure.
Materials and Methods
Fly Strains
w; +; Actin 5c>CD2>Gal4, UAS-GFPNLS (Neufeld et al., 1998; Pignoni and Zipursky, 1997)
hs-FLP122; +; UAS-RasV12 (Karim and Rubin, 1998)
w; +; FRT(82B), rasc40b/TM6B (Schnorr and Berg, 1996)
w; +; FRT(82B). Ub-GFP
w: UAS-P35; + (Hay et al., 1994)
w; engrailed-Gal4; +
w; +; FRT(82B), M(3)95A, Ub-GFP/TM6B (Andersson et al., 1994)
w; shg-lacz; + (Tanaka-Matakatsu et al., 1996)
arm-lacZ
w; +; rho-lacz (Freeman et al., 1992)
dsrf-lacZ (Montagne et al., 1996)
w; +; rhove vn1
w; apGal4 (Calleja et al., 1996)
w; +; tubulin-Gal80ts (McGuire et al., 2003)
FRT(42D), shgR69; tubulin-shg (Pacquelet et al., 2003)
UAS-dad (Tsuneizumi et al., 1997)
UAS-TkvQ235D (Nellen et al., 1996)
UAS-RasV12,S35 (Karim and Rubin, 1998)
UAS-RasV12,G37 (Karim and Rubin, 1998)
UAS-rhomboid
UAS-λ-torpedo (activated Egfr) (Queenan et al., 1997)
UAS-Raf-DN 3.1
UAS-Raf-GOF
UAS-SEM 8.7
UAS-MKP3 (Rintelen et al., 2003)
w; shgR69 (Tepass et al., 1996)
hs-FLP122; FRT(42D), shgR69, pwn
w; FRT(42D), Ub-GFP
hs-FLP122; UAS-shg (D33) (Sanson et al., 1996)
1096-Gal4
Details of how the UAS-shg-IR, UAS-Egfr-IR, UAS-dlg-IR and UAS-mew-IR constructs were generated are described at NIGFLY (see http://www.shigen.nig.ac.jp/fly/nigfly/). Base pair 712-1210 (499) from CG3722-RA was used for UAS-shg-IR, base pair 72-571 (499) from CG10079-RB was used for UAS-Egfr-IR, base pair 3046-3520 (474) from CG1725-RB was used for UAS-dlg-IR, and base pair 373-995 (623) from CG1771-RB was used for UAS-mew-IR. In each case, no exact matches to other coding regions in the Drosophila genome are indicated. Information concerning potential off-target sites associated with these UAS-RNAi transgenes can be found at the site listed above. All genetic experiments were conducted at 25°C unless otherwise specified.
Overexpression analysis
GFP-marked clones of cells overexpressing various UAS-regulated transgenes were generated using the Flp/Gal4 method (Neufeld et al., 1998; Pignoni and Zipursky, 1997; Struhl and Basler, 1993). Larvae were staged from hatching and raised at a density of 50 per vial at 25°C. At approximately 72 hours after egg deposition (AED) animals were heat-shocked 8–12 minutes in a 37°C water bath. Wing discs were dissected from wandering larvae (approximately 120 hours AED). The apGal4, UAS-GFPNLS/CyO; tubulin-Gal80ts (apGalts) system was used to express UAS-regulated transgenes in the dorsal pupal wing epithelium. Animals were raised at 18°C and shifted to 30°C at 0 hours after puparium formation (APF). At 30°C, Gal80ts was inactivated, thereby activating Gal4-mediated transgene expression. Wings were dissected 30–33 hours later, which is equivalent to 36 hours at 25°C. Throughout the text, pupal wing age will be expressed in 25°C hour equivalents.
Mitotic recombination
Mitotic recombination was induced using the Flp/FRT method (Xu and Rubin, 1993) by a 45 minute heat shock in a 37°C room at 48 or 72 hours AED. Wing discs were analyzed at 120 hours AED. en-Gal4 was used to express P35 in the wing to increase survival of rasc40b mutant cells. 1096-Gal4 was used to express P35 in the wing to increase survival of shgR69 mutant cells.
Immunocytochemistry
Larval discs and pupal wings were fixed in 4% paraformaldehyde/PBS for 20 minutes at room temperature. Samples were placed in blocking solution (0.1% Triton-X/4% Normal Goat Serum/PBS) over-night at 4°C before incubation with primary antibodies over-night at 4°C in the same solution. Primary antibodies used were rat anti-Shotgun (Oda et al., 1994) (DCAD2, 1:20 discs, 1:100 pupal wings), mouse anti-Armadillo (Developmental Studies Hybridoma Bank, 1:100), mouse anti-dpERK (Sigma, 1:200), mouse anti-β-Galactosidase (Cappel, 1:10,000), mouse anti-DSRF (Geneka Biotechnology, 1:500), mouse anti-β-PS-integrin (Developmental Studies Hybridoma Bank, 1:100), mouse anti-Discs-large (Developmental Studies Hybridoma Bank, 1:100), rat anti-α-catenin (Oda et al, 1993, 1:100). Alexa 488-, 568-, and 633-conjugated secondary antibodies were used (Molecular Probes, 1:1500). Nuclei were stained with Hoechst 33258 (Acros, 1:1000). Discs were mounted in Fluoroguard (Biorad) and imaged on a Leica TCS SP confocal microscope. Optical cross-sections through pupal wings are projections of xz images representing 8–15 μm. Stacks of images were compiled and analyzed using Image J and Adobe Photoshop.
Electron Microscopy
Dissected wings were fixed by immersion in 2% glutaraldehyde, 2% paraformaldehyde, 2% acrolein, 2% DMSO in 0.05M sodium phosphate buffer (pH = 7.2) on ice for two hours. Wings were rinsed three times in 0.1M sodium cacodylate buffer (pH = 7.2), and then post-fixed in 2% osmium tetroxide in 0.1M sodium cacodylate. The tissue was washed three times in distilled water, and block stained in 1% uranyl acetate at 5°C overnight. Wings were then dehydrated through a graded series of ethanol to propylene oxide, infiltrated over two days through a graded mixture of propylene oxide/Epon-Araldite resin to complete resin. After 24 hours in complete resin, wings were embedded in Epon-Araldite resin and cured for 36 hours at 60° C. Thin sections (60 to 80 nm) were cut on a Leica Ultracut UCT and the sections were stained with uranyl acetate followed by bismuth subnitrate. Sections were analyzed on a Zeiss 902 transmission electron microscope, and images were captured on a Hammamatsu CCD digital camera.
Results
Ras activity regulates shg expression
GFP-marked clones of wild-type cells generated using the Flp/Gal4 method form irregular shapes with uneven borders, suggesting that they mix freely with neighboring cells (Fig. 1A). In contrast, clones of cells expressing a mutationally activated form of Drosophila Ras, RasV12 (Karim and Rubin, 1998), are round and have smooth borders (Prober and Edgar, 2000; Prober and Edgar, 2002), suggesting that they have altered adhesive properties and resist mixing with neighboring wild-type cells (Fig. 1B). This phenotype is similar to that of clones overexpressing the intercellular adhesion mediator DE-cadherin/Shg (Dahmann and Basler, 2000). To determine whether endogenous Ras normally regulates affinity between cells, we used FLP/FRT-mediated mitotic recombination to generate rasc40b mutant clones (Schnorr and Berg, 1996). P35 was expressed via en-Gal4 to increase the survival of rasc40b mutant cells, which grow slowly and die rapidly. Clones of cells lacking ras function were also round with smooth borders (Fig. 1C), suggesting that endogenous ras is required for normal adhesion between cells.
Fig. 1. Ras signaling affects cell affinity and shg expression in the developing wing.

Clones of cells expressing GFP are irregularly shaped (A). In contrast, clones of cells expressing RasV12 are rounded (B), suggesting they have altered adhesive properties and an inability to mix freely with neighboring wildtype cells. (C) The FLP/FRT technique was used to generate homozygous rasc40b mutant clones, marked by loss of GFP (arrows). Two copies of GFP mark wild-type twinspots, while heterozygous cells are marked by one copy of GFP. ras mutant clones survive poorly and are significantly rounded compared to their wildtype twinspots. (D) Large clones of cells lacking ras function were generated using the FLP/FRT, Minute technique (GFP-negative cells). These ras mutant cells have greatly reduced levels of shg expression, as measured by a shg-lacZ reporter construct. (E) Clones of cells expressing constitutively active RasV12 (GFP-positive cells) have higher levels of shg-lacZ expression.
Since Ras appeared to affect cell affinity in the developing wing, we tested whether Ras regulates cell adhesion by modulating shg levels. Using the Minute technique, we generated large clones of rasc40b mutant cells in the wing disc. These mutant cells expressed much less shg as measured using a shg-lacZ reporter, which is known to accurately reflect shg transcription patterns (Fig. 1D) (Tanaka-Matakatsu et al., 1996). Conversely, clones of cells expressing RasV12 had elevated levels of shg-lacz expression (Fig. 1E). Cross-sectional images revealed that these wing disc clones also had elevated levels of Shg protein (not shown). Shg protein is normally found together with β-catenin/Arm and α-catenin in adherens junction complexes in epithelial cells (Peifer, 1993). α- and β-catenin were similarly present at higher levels in RasV12 expressing cells (not shown). This suggests that RasV12 promotes the formation of adherens junction complexes. Since RasV12 had no effect on an arm-lacz reporter (not shown), we infer that RasV12 increases cell adhesion in the developing wing by inducing shg transcription, and that increased Shg levels then recruit cytoplasmic pools of Arm to adherens junctions at the cell membrane, resulting in more adherens junction complexes and thus stronger cell-cell adhesion.
Patterns of shg expression correlate with patterns of MAPK activity and vein cell identity
Genetic analyses have demonstrated that low levels of Ras activity are required for growth and survival of all cells in the developing wing (Bier, 1998; Diaz-Benjumea and Hafen, 1994; Prober and Edgar, 2000). In addition, high Ras activity levels are found in presumptive vein cells and in stripes of cells on either side of the D/V boundary (corresponding to presumptive wing margin cells) during the late third larval instar. An antibody that recognizes the activated form of MAPK (di-phospho-ERK) reveals this pattern (Fig. 2A and (Gabay et al., 1997). While increased di-phospho-ERK (dp-ERK) has been reported along the wing margin and in all presumptive vein cells (Guichard et al., 1999; Martin-Blanco et al., 1999), we generally observed elevated levels only along the wing margin and in veins L3 and L4 (Fig. 2A). Interestingly, we observed elevated levels of shg-lacz (Fig. 2B), Shg protein (Fig. 2C), and Arm protein (Fig. 2D) in a similar spatio-temporal pattern. Cells with high levels of Arm and Shg co-localized with cells expressing rho-lacz, and with cells not expressing the intervein marker dsrf-lacz (not shown). Thus, high levels of endogenous Ras activity in presumptive vein and wing margin cells correlate with high levels of shg mRNA and Shg and Arm protein.
Fig. 2. Patterns of endogenous shg expression correlate with MAPK activity.
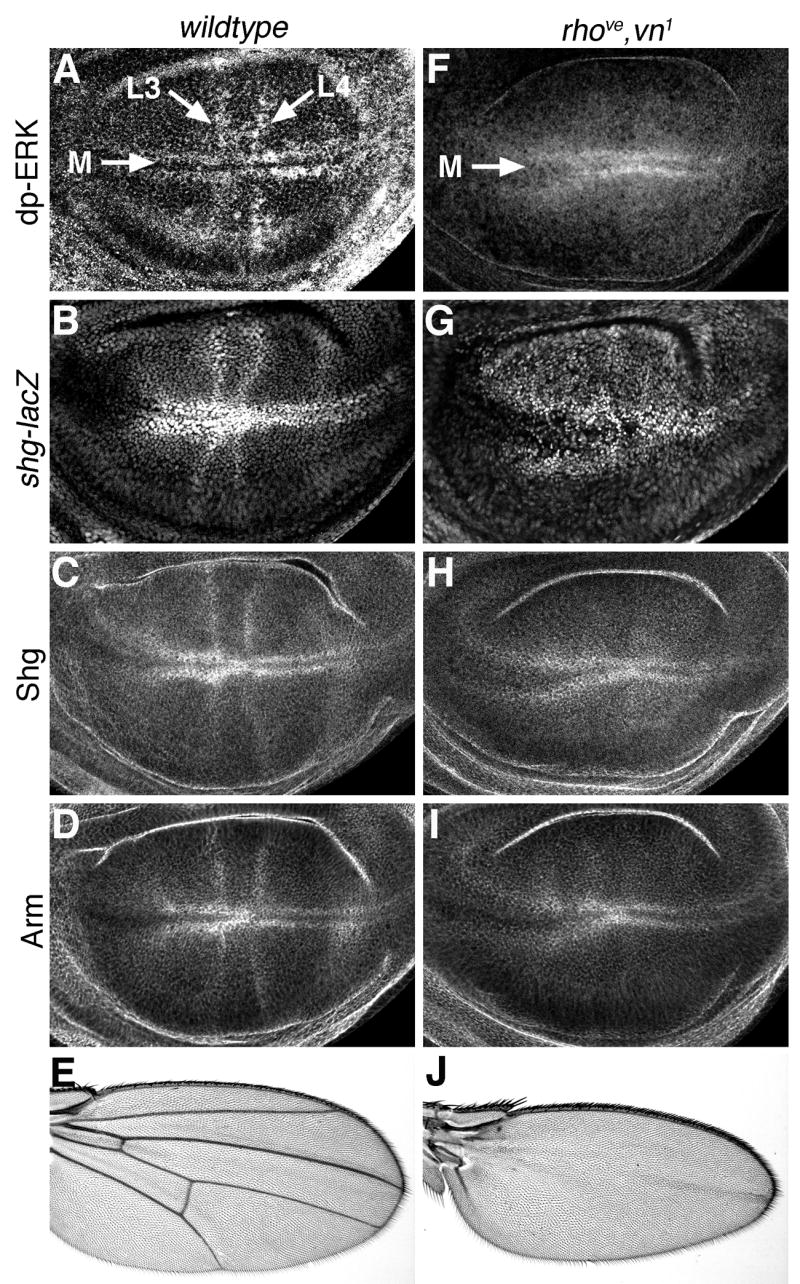
(A) Ras/MAPK activity, as measured by di-phospho-ERK (dp-ERK) levels, is highest in presumptive vein and margin cells of the developing wing blade. Longitudinal veins L3 and L4, as well as the wing margin (M) are indicated. (B) shg expression is similarly highest in presumptive wing vein cells, as measured by a shg-lacZ reporter construct. Shg protein (C) and Arm protein (D) are also present in similar patterns. Late third instar wing discs from rhove,vn1 larvae have high MAPK activity in presumptive margin cells, but lack dp-ERK staining in presumptive veins (F). Similar patterns are present for shg-lacz (G), Shg protein (H), and Arm protein (I). Compared to wildtype adult wings (E), rhove,vn1 wings have a normal margin, but lack veins (J).
To determine whether this correlation depends on Ras activity, we examined Shg and Arm levels in rhove,vn1 mutant animals. rhove and vn1 are wing-specific hypomorphic alleles of rho and the Egfr ligand vein, respectively. rhove,vn1 larvae have normal levels of MAPK activity in wing margin cells, but lack high MAPK activity in presumptive vein cells (Fig. 2F) (Guichard et al., 1999; Sturtevant et al., 1993). Adult rhove,vn1 flies have normal wing margins but lack all veins (Fig. 2J). Wing discs from homozygous rhove,vn1 larvae had elevated levels of shg-lacz, Shg and Arm in presumptive wing margin cells, as in wild-type discs, but lacked elevated shg-lacz, Shg, and Arm in cells that would normally form veins (Fig. 2G–I). Thus, activation of MAPK in presumptive vein cells is required for the increased Shg and Arm levels normally observed in these cells. These correlations suggest that Ras signaling normally increases levels of cell-cell adhesion in presumptive wing margin and vein cells during the late third larval instar.
Shg localization is dynamically regulated during wing vein differentiation
As development proceeds, and vein and intervein cells become morphologically distinct, the pattern of Shg localization becomes increasingly refined. During larval stages, highest levels of Shg were observed in presumptive vein cells (Fig. 3A), and the majority of this protein was located near the apical cell surface (sub-apical), where epithelial adherens junctions typically are located (Fig. 3A′). After puparium formation (APF), disc elongation creates a bi-layered epithelial wing structure with the basal surfaces of dorsal and ventral wing cells apposed. At 24 hours APF (shortly after dorsal/ventral re-apposition) high levels of sub-apical Shg were found in broad bands of presumptive vein cells (Fig. 3B,B′). Extremely high levels of Shg were also found in sensory structures along the wing margin and vein L3. At 30 hours APF, Shg began to accumulate near the basal surface of intervein cells, while in vein cells, Shg remained near the apical surface (Fig. 3C,C′). By 36 hours APF this pattern was further refined. In intervein cells, the majority of Shg was found basally, at points of contact between the dorsal and ventral wing surfaces, while Shg was maintained sub-apically in vein cell (Fig. 3D,D′). Similar spatio-temporal patterns of protein localization were seen with other components of the adherens junction, namely Arm and α-catenin (data not shown). This suggests that by 36 hours APF, adherens junction protein complexes localize to different sub-cellular compartments in vein and intervein cells.
Fig. 3. Shg localization is dynamically regulated during wing development.

(A) In late third instar wildtype wing discs, Shg is found at highest levels in presumptive vein cells. Longitudinal veins L3 and L4, as well as the wing margin (M) are indicated. (A′) An optical cross section through the imaginal disc wing pouch indicates that the majority of Shg is near the apical cell surface (arrow). Arrowhead indicates the basal cell surface. (B–D) Pupal wings stained for Shg are shown. Proximal is to the left and anterior is up. Longitudinal vein L4 is indicated. (B′–C′) Optical cross sections through vein L4 and the posterior margin of pupal wings are shown. Posterior is to the left and vein L4 is indicated. At these stages of development the pupal wing is composed of two cell layers (dorsal and ventral) apposed at their basal cell surfaces. Arrows in B′ indicate the apical surfaces, while the arrowhead marks the basal surfaces. (B,B′) 24 hours after puparium formation (24 hours APF) Shg levels are highest in broad bands of presumptive vein cells, and the majority of Shg is sub-apically localized (B′). At 30 hours APF, Shg is found in more restricted bands of vein cells (C,C′), while Shg is also beginning to accumulate basally in intervein cells (C′). By 36 hours APF, sub-apical Shg is concentrated in narrow bands of vein cells (D, arrow D′), while high levels of Shg are found basally in intervein regions (arrowhead D′). Shg is also found at high levels in sensory organs of the margin and vein L3.
Since a dramatic shift in sub-cellular localization of adherens junction-associated proteins was observed in intervein cells during the early stages of pupal wing development, we tested whether other junctional proteins were similarly affected. In 24 hour APF pupal wings, Discs-large (Dlg), a critical component of the septate junction (Woods et al., 1996), is found more basolaterally than adherens junctions, but still near the apical surface of epithelial cells (Fig. 4A). In contrast, β-Integrin is found at the basal surface of wing epithelial cells (Fig. 4C) (Brower and Jaffe, 1989; Fristrom et al., 1993). Between 24 and 36 hours APF, localization of these proteins in the wing epithelium did not change (Fig. 4B,D). Thus the apical/basal polarity of both vein and intervein wing cells remains constant during these early pupal stages, but the distibution of adherens junction components within the basolateral domain of intervein cells is altered.
Fig. 4. Apical/Basal polarity of intervein cells is maintained during early pupal stages.
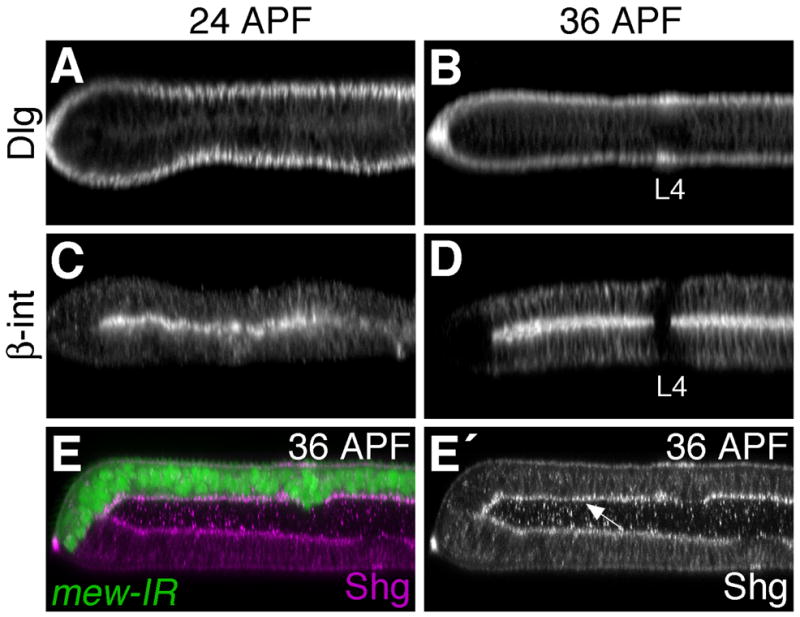
Discs large (A,B) and β-integrin (C,D), found apically and basally respectively, do not change their pattern of localization between 24 and 36 hours APF. (E) Loss of integrin function does not affect Shg localization. apGal4 was used to disrupt multiple edematous wings (mew) (the α-PS1-integrin subunit) function in dorsal wing cells (GFP-positive) using a UAS-mew-IR transgene. Wings were dissected at 36 hours APF and stained for Shg. While loss of mew function from early larval stages results in wing blistering (the dorsal and ventral wing surfaces are not apposed), the pattern of Shg localization is unaffected. Shg is still found near the basal surface of intervein cells (arrow E′).
Transcriptional regulation of shg is not essential for wing vein patterning or differentiation
Our results demonstrate that during the course of wing vein development, shg-mediated adhesion between cells is regulated both transcriptionally (vein cells express more shg) and post-translationally (Shg distribution differs between vein and intervein cells). To determine whether the transcriptional regulation of shg plays a critical role during the specification and morphogenesis of wing veins, we examined shg mutant flies that were rescued with a tubulin-shg transgene (Pacquelet et al., 2003). With the endogenous shg gene eliminated, and a tubulin promoter driving shg transcription, each cell expresses an essentially equivalent level of shg. In this way, Ras signaling cannot affect shg transcription. Examining Shg localization in wing discs of this genotype showed uniform levels of Shg across the wing pouch epithelium (although less Shg was found at the wing margin) (Fig. S1A). Optical cross-sections through the pouch region revealed no differences in Shg levels where presumptive wing veins would normally be found (Fig. S1B, compare to Fig. 3A′). At 36 hours APF, Shg levels in the rescued wings were generally elevated, but the vein/intervein and apical/basal patterns of Shg localization were similar to wildtype (Fig. S1C). Surprisingly, a small number of shg mutant animals were rescued to adulthood by the tubulin-shg transgene, and the veination pattern in their wings was normal (Fig. S1D). This indicates that the regulation of shg expression by Egfr/Ras signaling is dispensible for wing vein patterning and morphogenesis. In addition, it suggests that post-translational effects on Shg sub-cellular localization play the critical role during wing vein formation.
Egfr/Ras signaling regulates Shg localization
Since Ras’ ability to regulate shg transcription is dispensible during vein formation, we asked whether Ras activity effects Shg subcellular localization. Until 24 hours APF, Shg and the other components of the adherens junction are located near the apical surface in both vein and intervein cells (Fig. 3B′). After this time, vein and intervein cells differ in their subcellular localization of Shg. To ask what effect Ras signaling has on Shg localization, we devised a system to express RasV12 in wing cells only after pupariation. In this way, the effects of RasV12 on cell proliferation and wing disc patterning during larval development were avoided. In order to temporally control expression in the wing, we combined the apGal4 driver (Calleja et al., 1996) with the temperature sensitive tubulin-Gal80ts transgene (McGuire et al., 2003). This transgene combination will be referred to as apGalts. Animals of the genotype apGalts; UAS-RasV12/UAS-GFP were raised at 18°C until puparium formation. At this temperature Gal80ts prevented activation of the UAS transgenes by Gal4. At approximately 0 hours APF, animals were shifted to the Gal80ts non-permissive temperature (30°C) and UAS-transgene expression was activated. Based on GFP fluorescence, transgene activation via apGalts was detectable within four hours of the temperature shift, and reached maximal levels by 24 hours (not shown). Wings were subsequently dissected at 30–33 hours APF, which is equivalent to 36 hours APF at 25°C.
We distinguished between vein and intervein cells in pupal wings through localization of the Drosophila Serum Response Factor (DSRF). DSRF is a transcription factor encoded by the gene blistered, whose expression is restricted to intervein territories, and promotes intervein cell fate (Montagne et al., 1996). RasV12 expressing dorsal wing epithelia completely lacked DSRF expression (Fig. 5D), indicating that every cell had adopted a vein cell fate. Concomitantly, these cells had extremely high levels of sub-apical Shg (Fig. 5C), and constricted apical surfaces compared to intervein cells (compare Fig. 5G, 5H). The RasV12-expressing dorsal epithelial cells also assumed an exaggerated pseudo-stratified cytoarchitecture (the cells expanded along the apical-basal axis). Expression of RasV12 after 0 hours APF, therefore, promotes vein identity and dramatically affects Shg localization and cell shape.
Fig. 5. Egfr/Ras signaling regulates Shg localization.
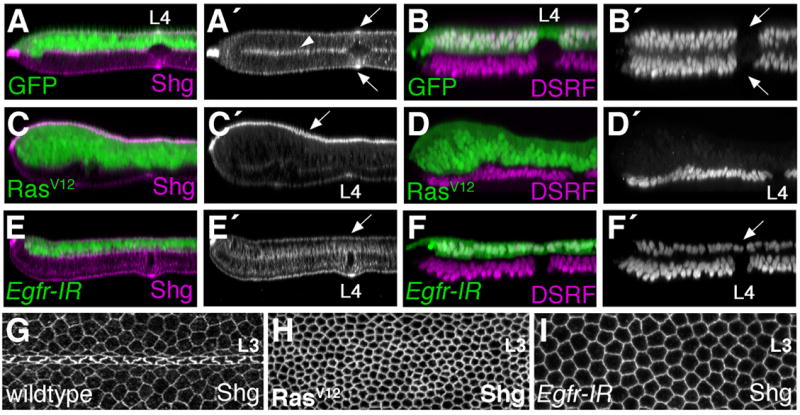
(A–F,H,I) Using apGAL4 in combination with tubulin-GAL80ts, UAS-transgenes were activated in dorsal wing cells beginning at 0 hours APF. Wings were dissected at 36 hours APF and stained for either Shg (A,C,E,H,I) or the intervein marker DSRF (B,D,F). Optical cross-sections through the L4 vein region (A–F) or apical L3 (G–I) are shown. (A,B) In wings expressing GFP, dorsal and ventral cells are similar in appearance. Both dorsal and ventral vein cells (arrows B′) lack DSRF expression and have high levels of apical Shg (arrows A′). Intervein cells express DSRF and localize Shg to both apical and basal cell surfaces (arrowhead marks the basal surfaces). (C.D) RasV12 expression causes all dorsal wing cells to adopt the vein cell fate. DSRF expression is absent from RasV12, GFP positive cells (D). In these ectopic vein cells, Shg levels are elevated and the majority of the protein is apically localized (arrow C′). (E,F) Expression of an Egfr-IR transgene eliminates vein cells. DSRF localizes to all Egfr-IR expressing cells (arrow F′), and the apical accumulation of Shg in vein regions is not observed (arrow E′). (G–I) Apical surfaces of 36 hour APF pupal wings. Consistently magnified regions of the dorsal L3 wing vein are shown and Shg is visualized. (G) Wildtype vein and intervein cells are morphologically distinct. RasV12 expression (H) promotes apical constriction (as in vein cells), whereas Egfr-IR expression (I) promotes cell flattening (as in intervein cells).
Loss of Egfr signaling during early pupal stages also affected vein/intervein identity and Shg localization, but in a manner opposite to RasV12. An Egfr inverse repeat transgene (UAS-Egfr-IR), in combination with the apGalts system, was used to deplete Egfr from 0–36 hours APF. In these wings, DSRF was expressed uniformly throughout the dorsal epithelium, indicating a loss of vein cells (Fig. 5F). Egfr-IR expressing cells failed to adopt a vein cell morphology, restricting the vein lumen to the ventral half of the wing epithelium, and flattening the cells apico-basally (Fig. 5E,I). The sub-apical accumulation of Shg in vein regions was also prevented, as every Egfr-IR expressing cell adopted an intervein pattern of Shg localization (Fig. 5E). During early pupal stages, therefore, Egfr/Ras signaling is both necessary and sufficient for vein cell identity and sub-apical Shg localization.
Shg localization and vein cell fate are regulated independently
In the above experiments, however, it is unclear whether the effects on Shg localization result from Egfr/Ras signaling directly, or as a downstream consequence of vein cell fate induction. Initially, we considered whether Ras indirectly affects Shg through integrins. One striking difference between vein and intervein cells is that integrins localize to the basal surface of intervein cells, but are absent from vein regions (Fig. 4D) (Fristrom et al., 1993). Since RasV12, when expressed via the apGalts system, significantly reduced β-integrin levels throughout the wing (not shown), we hypothesized that integrins were required for the basal localization of Shg in intervein cells. To test this idea, we used an inverse repeat transgene specific for the α-PS1-integrin subunit (encoded by the gene multiple edematous wings (mew)) to deplete mew expression in the pupal wing. Expressing UAS-mew-IR via apGal4, eliminated integrin-mediated adhesion from early larval stages and blocked apposition of dorsal and ventral wing surfaces at 36 hours APF. However, Shg localization was normal, indicating that α-PS1-integrin and dorsal/ventral apposition are not required for the basolateral redistribution of Shg in pupal intervein cells (Fig. 4E).
To further address whether Ras could affect Shg localization independent of vein cell identity, we manipulated Decapentaplegic (Dpp) signaling in the pupal wing. Several studies have shown that the secreted morphogen Dpp acts downstream of Egfr/Ras signaling during pupal stages to maintain vein cell fate (de Celis, 1997; Sotillos and De Celis, 2005). Expression of a mutationally activated form of the Dpp receptor Thickvein (TkvQ235D), transformed intervein cells into vein cells during pupal stages, as assayed by loss of DSRF (Fig. 6B). In these wings, Shg levels were elevated, but the protein was not preferentially concentrated at the apical cell surface (Fig. 6A). In addition, TkvQ235D expressing wings were dramatically blistered. Since RasV12 and TkvQ235D had different effects on Shg localization and vein cell morphology, we next inhibited Dpp signaling in RasV12 expressing cells to generate RasV12 expressing cells with intervein identity. Daughters against Dpp (Dad) inhibits the Dpp signaling pathway (Tsuneizumi et al., 1997), and blocked vein cell identity, as assayed by DSRF, when expressed in pupal wings (Fig. 6D, (Sotillos and De Celis, 2005)). Dad expressing cells did not adopt a vein cell morphology, and so the vein lumen was restricted to the ventral half of the wing (Fig. 6C). As has previously been shown in adult wings (Sotillos and De Celis, 2005), Dad inhibited the ability of RasV12 to direct vein cell identity. Pupal wings expressing both RasV12 and Dad had a relatively normal pattern of vein and intervein cell identities (Fig. 6F). These RasV12 expressing intervein cells, however, had elevated amounts of apically localized Shg (Fig. 6E). Thus, Ras signaling can affect the levels and localization of Shg independent of its effect on vein cell identity and Dpp signaling.
Fig. 6. Shg localization and vein cell fate are regulated independently.
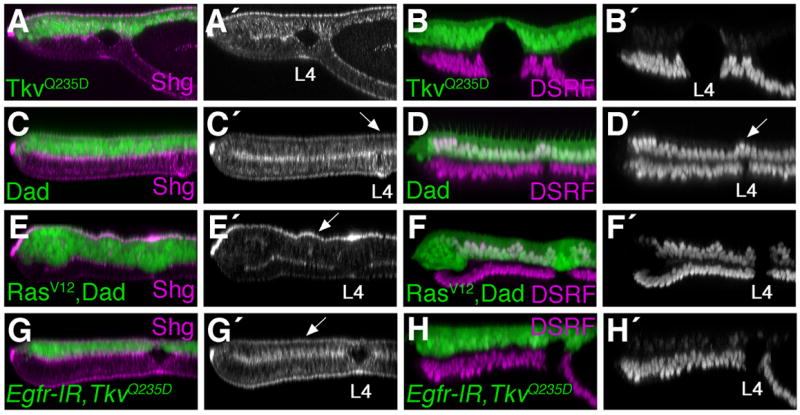
The apGalts system was used to express indicated transgenes from 0–36 hour APF. Wings were stained for Shg (A,C,E,G) or DSRF (B,D,F.H) and optical cross-sections through the L4 vein region are shown. An activated version of the Dpp receptor Thickvein (TkvQ235D) results in vein cell identity (B). While Shg is elevated in TkvQ235D expressing cells, the protein is not preferentially concentrated at the apical surface (A). Dad expression eliminates vein cell identity (D). Cells in the dorsal L4 vein region maintain DSRF expression (arrow D′) and adopt the intervein pattern of Shg localization (arrow C′). Expression of RasV12 together with Dad maintains the normal pattern of vein and intervein cell identity (F), but Shg levels are elevated and apically localized in RasV12, dad expressing intervein cells (arrow E′). In contrast, apical localization of Shg is not observed in Egfr-IR, TkvQ235D expressing vein cells (arrow G,H).
Since Ras activation affected Shg localization in intervein cells, we performed the reciprocal experiment and inhibited Egfr/Ras signaling in vein cells. It has been demonstrated that Tkv activation in the absence of Egfr signaling results in ectopic wing veins, although the effects are less penetrant than with Tkv alone (Sotillos and De Celis, 2005). Using the apGalts system, Egfr-IR expression together with TkvQ235D resulted in ectopic wing vein tissue. The dorsal epithelium lacked DSRF expression (Fig. 6H), but Shg was not found concentrated at the apical cell surface. Instead Egfr-IR expressing vein cells adopted an intervein pattern of Shg localization (Fig. 6G). These results demonstrate that in the pupal wing, Egfr/Ras signaling regulates Shg localization, and vein cell identity via different mechanisms.
To better understand the mechanism by which Egfr/Ras signaling affects vein cell fate and/or morphology (DSRF and/or Shg), we tested known Ras effectors in our pupal wing assay. Analysis of Ras effector-loop mutants (Kinashi et al., 2000; Pacold et al., 2000; Prober and Edgar, 2002; Rodriguez-Viciana et al., 1997), suggested that Ras acts through the Raf/MAPK pathway (rather than through PI3-Kinase or RalGDS) to control Shg localization and vein cell fate. The effects of RasV12,S35 (in general, a Raf/MAPK-specific version of activated Ras) on pupal wing development were indistinguishable from RasV12 (Fig. S2E,F). However, RasV12,S35 is not absolutely Raf/MAPK-specific (Rodriguez-Viciana et al., 2004), diminishing the certainty of this conclusion. We went on to test additional components of the Raf/MAPK signaling cassette and, with a few exceptions, most of the experiments supported the hypothesis that Ras acts through the canonical Rho/Egfr/Ras/Raf/MAPK pathway to regulate both vein identity and cell adhesion (see Fig. S2). Notably, MKP3, an inhibitor of MAPK (Gomez et al., 2005; Muda et al., 1996; Rintelen et al., 2003), suppressed the effects of RasV12 on Shg localization and DSRF expression (Fig. S2Q,R). However, in each instance we saw effects on both DSRF and Shg. Therefore, the point at which the signaling pathway diverges to control vein identity and cell morphology independently remains unresolved.
Ras signaling affects adherens junction morphology
To more carefully examine Ras’ effect on epithelial cell adhesion, we imaged cell junctions directly using transmission electron microscopy (TEM). The apGALts system was used to express RasV12 or TkvQ235D from 0–36 hours APF, at which time the wings were dissected and prepared for imaging. Intervein regions had adherens junctions spaced at regular intervals near the apical surface (Fig 7B). In addition, basal adhesive contacts that resembled adherens junctions were observed between the dorsal and ventral wing epithelia (Fig. 7A) (Fristrom et al., 1993). RasV12 expression greatly expanded the apico-basal width of the dorsal wing epithelium compared to the ventral control (Fig. 7C), as we had previously observed (Fig. 5C,D). Basal contacts between the two epithelial surfaces were disrupted, and large blisters were found throughout the wing (Fig. 7C). When the apical surface was examined, dramatically enlarged adherens junctions were found in the RasV12 expressing cells (Fig. 7D). The enlarged junctions were also more closely spaced than in wildtype intervein tissue, suggesting that RasV12 causes pupal wing cells to apically contrict. This agrees with our immunohistochemical data (Fig. 5C,H). However, since both increased levels of Shg protein, and the apical constriction of epithelial cells would be predicted to affect adherens junction size and/or morphology, it is unclear which RasV12 effect is dominant in this context. In contrast, no difference in adherens junction morphology was observed between the dorsal and ventral wing epithelia of TkvQ235D expressing wings (Fig. 7F). These images illustrate the profound effect Ras signaling has on adhesion between cells, and adherens junction morphology in particular. Furthermore, as our light micrographs suggested, this indicates that while RasV12 and TkvQ235D have similar effects on vein cell fate, their effects on cell adhesion are distinct.
Fig. 7. Ras signaling affects adherens junction morphology.
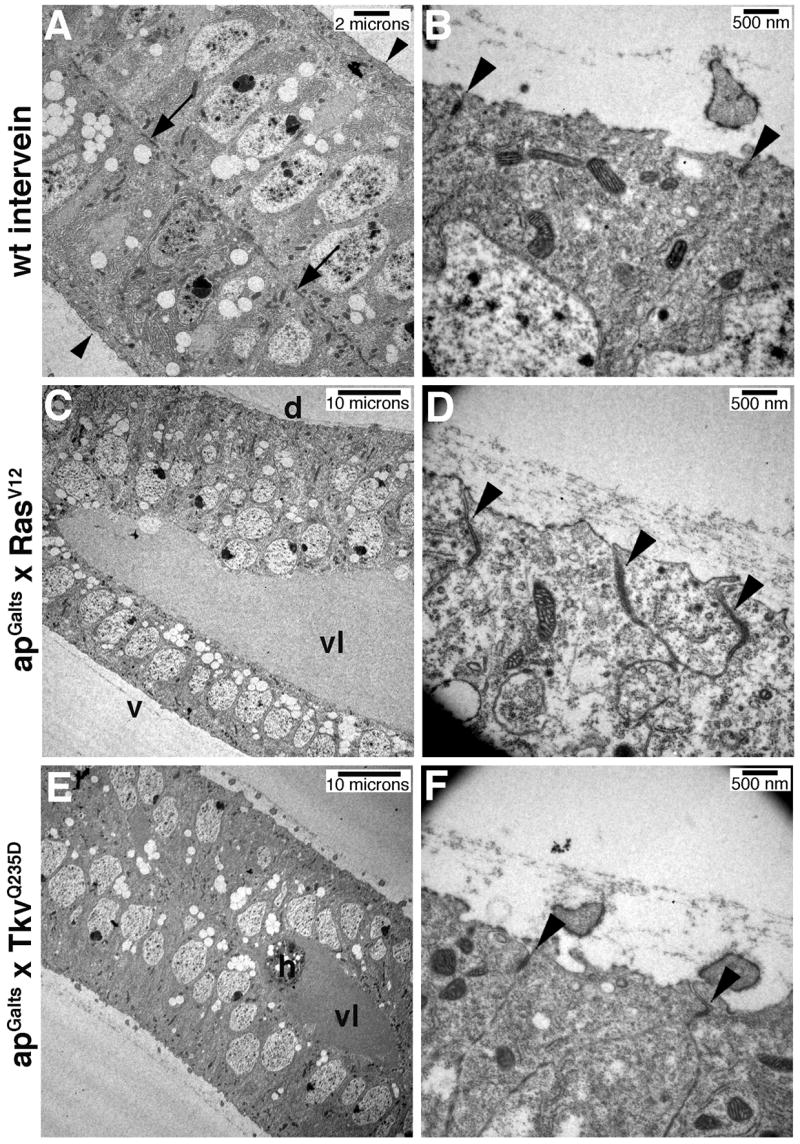
The apGalts system was used to express GFP (A,B), RasV12 (C,D), or TkvQ235D (E,F) from 0–36 hours APF. Sections though 36 hour APF pupal wings imaged by TEM are shown. B,D,F are high magnification views of the apical, dorsal cell surface. (A) Arrows point to basal adhesion junctions between the dorsal and ventral wing epithelia. Arrowheads indicate the apical surfaces. (B) Arrowheads indicate adherens junctions between intervein cells. (C,D) RasV12 expression causes apico-basal extension of dorsal epithelial cells, wing blistering and enlarged apical adherens junctions (arrowheads D). (E,F) In contrast, TkvQ235D expression does not affect apical adherens junction morphology (arrowheads F). Vein lumen (vl), dorsal (d), ventral (v), haemocyte (h).
Altering Shg levels affects wing vein differentiation
Having established that shg expression and protein localization are controlled by Ras signaling in the developing wing, we wanted to investigate the role shg plays in vein morphogenesis. To address this question, we initially generated shg loss-of-function clones (using the null allele shgR69) and examined the adult wing vein pattern. Clones were induced at 72 hours AED, and P35 was expressed via 1096-Gal4 to increase mutant cell survival. Compared to control wings (Fig. S3A), loss of shg in clones of cells often resulted in ectopic vein material (Fig. S3B). However, by the adult stage, shg mutant cells (marked with pawn) had been eliminated, indicating a non-autonomous effect on wing vein patterning. In addition, we generated shg gain-of-function clones and observed a similar effect on wing morphology: ectopic wing veins and margin defects (Fig. S3C–E). Examining newly eclosed adults revealed that ectopic vein branches were always associated with shg-expressing clones of cells (Fig. S3E). While these results indicate that Shg-mediated adhesion between cells is required for proper wing vein formation, it is not clear whether these phenotypes are related to the Egfr/Ras-dependent effects on Shg localization we have described during pupal stages.
To more effectively investigate the role Shg plays during pupal wing vein morphogenesis, we used a shg inverse repeat transgene (UAS-shg-IR), together with the apGalts system, to deplete shg at this developmental stage. When shg-IR was expressed from 0–36 hours APF, the pupal wing epithelium remained intact, as measured by Dlg localization (Fig. S4C), but Shg levels (Fig. 8D) and epithelial adherens junctions (Fig. 8I,K) were severely reduced. Using this system, loss of Shg after puparium formation inhibited the morphogenesis of veins. In both wildtype and GFP-expressing control animals, vein and intervein cells were morphologically distinct by 36 hours APF. Dorsal and ventral vein cells did not adhere to one another at this stage (in contrast to intervein cells), and constricted along their apical/basal axis to form a vein lumen (Fig. 8A–C, (Fristrom et al., 1993)). Depletion of Shg from 0–36 hours APF, however, prevented formation of the vein lumen. shg-IR expressing pupal vein and intervein cells were morphologically indistinguishable (Fig. 8D–F), even though DSRF localization revealed the presence of both cell types (Fig. 8G). This indicates that Shg-mediated adhesion between cells is important during pupal stages for the cell shape changes necessary for veins to morphologically differentiate. These animals did not survive till adulthood, however, precluding analysis of the adult wing vein structure.
Fig. 8. Loss of shg inhibits vein morphogenesis.
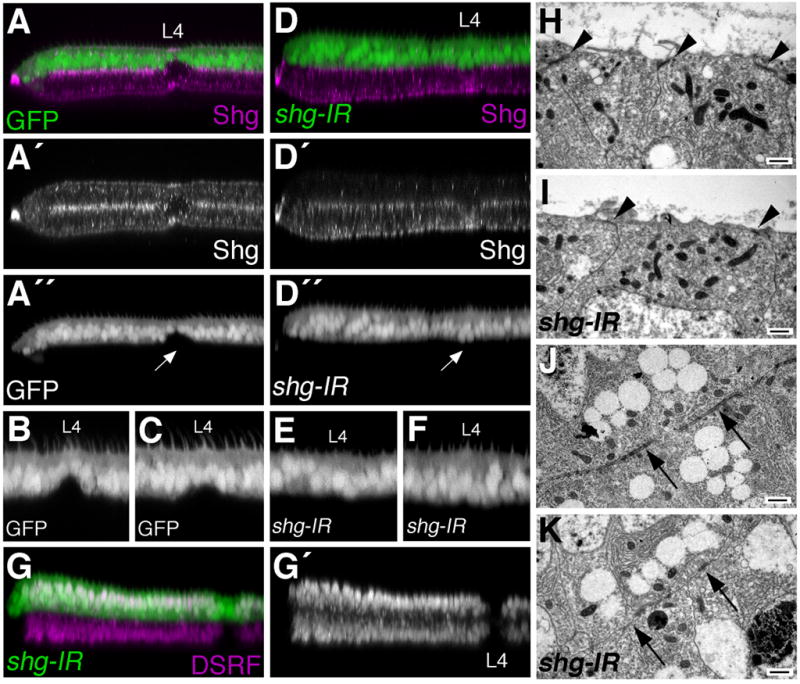
apGal4 was used to express either GFP (A–C,G,I), or GFP together with a UAS-shg-IR transgene (D–F,H,J) in dorsal wing cells from 0–36 hours APF. (A–F) Optical cross-sections through vein L4 and the posterior margin of 36 hour APF wings are shown. Eliminating shg expression (compare A′ to D′) impairs the ability of vein cells to form a vein lumen (compare A,B,C to D,E,F). Arrow in A″ points to the L4 vein cells which have constricted along the apical/basal axis to form the dorsal portion of a vein lumen, while shg-ir expressing L4 vein cells do not contrict (arrow D″). Additional examples of dorsal L4 cells expressing GFP (B,C), or shg-IR (E,F) are shown. (G) Depletion of shg expression does not affect the pattern of vein/intervein identity, as measured by DSRF expression. (H–K) TEM analysis indicates that shg-IR expression affects adherens junction morphology. Apical adherens junctions found in control wings (arrowheads H) are nearly eliminated in shg-IR expressing cells (arrowheads I). Basal adherens junctions (arrows J) are similarly reduced (arrows K). Scale bars indicate 500nm.
To ask whether other epithelial cell junctions play a similar role during wing vein morphogenesis, we disrupted septate junctions and analyzed vein cell shape. Septate junctions are located more basally than adherens junctions, and Dlg, a membrane-associated guanylate kinase homolog, is necessary for their formation (Woods et al., 1996). In 36 hour APF pupal wings, Dlg was localized near the apical surface of both vein and intervein cell types (Fig. S4A). Using a UAS-dlg-IR transgene together with apGalts, Dlg was reduced to undetectable levels in the dorsal pupal wing. Despite the absence of visible septate junctions in these cells, vein morphology was undisturbed and the vein lumen formed normally (Fig. S4B). Therefore, not every type of cell-cell contact is required for the morphological differentiation of wing veins.
Experiments in which Shg levels were depleted during pupal stages also indicated that Shg is not required for apposition of the dorsal and ventral wing surfaces. Based on the timing of Shg relocalization toward the basal surface, we had hypothesized that Shg was involved (along with integrins) in mediating adhesion between intervein cells of the dorsal and ventral wing epithelia (Brower and Jaffe, 1989). However, while the expression of shg-IR in pupal wings eliminated the accumulation of basal Shg in intervein cells and disrupted basal adherens junction complexes between the dorsal and ventral wing surfaces (Fig. 8K), it did not result in wing blistering (Fig. 8D,K). Therefore, Shg is not essential for adhesion between the dorsal and ventral wing epithelia.
Discussion
A complex array of genetic factors that pattern the developing Drosophila wing field and specify cell identities within the initially uniform epithelium has been well described. It is less clear, however, how these signals are translated into epithelial cell shape changes necessary for formation of the adult wing structure. The pupal wing blade, examined 36 hours APF, consists primarily of two morphologically distinct cell types: vein and intervein. Signaling through the Egf/Ras/MAPK pathway is a critical determinant of vein cell identity, and yet the downstream effectors that control vein cytoarchitecure remain undescribed. Here we demonstrate that Ras signaling regulates adhesion between cells by altering both the levels of DE-cadherin/shg expression, and the sub-cellular localization of Shg protein. In turn, Shg-mediated adhesion is necessary for the morphogenesis of veins.
Egfr/Ras signaling regulates Shg-mediated cell adhesion in developing wing vein cells
We have demonstrated that presumptive vein cells in the larval wing imaginal disc express elevated levels of DE-cadherin/shotgun. We found that rho, vn and ras are normally required to maintain high levels of shg expression in these cells. Furthermore, we found that ectopic Ras activity is sufficient to induce shg expression in the developing wing, resulting in increased levels of adherens junction-associated proteins at the sub-apical cell surface. However, we subsequently determined that uniform expression of shg throughout the imaginal disc (via a tubulin-shg transgene) is sufficient for normal wing vein patterning, and thus we conclude that Ras-dependent effects on shg expression are not required during the larval stage of development.
While differences in shg expression are not required to determine the spatial pattern of vein and intervein cell identities in the wing imaginal disc, we found that Shg-mediated adhesion is necessary for morphogenesis of the wing vein structure later in development. Using a temperature sensitive Gal4 system in combination with a UAS-shg-RNAi transgene, we depleted Shg levels during pupal stages when vein and intervein cells were becoming morphologically distinct. Depleting shg from 0–36 hours APF did not interfere with vein/intervein cell identities, or disrupt integrin-mediated adhesion between dorsal and ventral intervein cells, or disturb epithelial integrity of the wing blade. However, wing vein morphology was compromised. By 36 hours APF, wildtype vein cells are constricted along their apical/basal axis (compared to intervein cells), thereby forming a wing vein lumen. Vein cells lacking Shg, and therefore the adherens junction complex, failed to form a lumen and were morphologically indistinguishable from the adjacent intervein cells.
Ras regulates vein cell identity and Shg localization via independent mechanisms
The basolateral distribution of Shg is dynamically regulated during pupal wing differentiation. From larval stages until 24 hours APF, Shg was concentrated sub-apically (where adherens junctions are typically found) in both presumptive vein and intervein cells. By 36 hours APF, however, the majority of Shg in intervein cells was found near the basal cell surface, while Shg remained sub-apical in vein cells. RasV12 expression during pupal development created ectopic vein cells that had high levels of sub-apically localized Shg, as is normally observed in developing veins. TEM analysis indicated that adherens junctions in RasV12 expressing cells were greatly enlarged. By inhibiting Dpp signaling (via Dad) in RasV12 expressing cells, we were able to generate RasV12 expressing cells with intervein identity. Shg levels in these cells were elevated and sub-apcially localized as in vein cells. Conversely, by expressing Egfr-IR together with TkvQ235D, we generated cells with vein identity that lacked Egfr signaling. In these cells, Shg localization was primarily basal, resembling the intervein pattern. These results indicate that Ras, in addition to controlling vein cell identity, independently regulates Shg localization to control vein cell morphology (Fig. 9).
Fig. 9. Model of Egfr/Ras pathway function in early pupal vein cells.

Rhomboid expression in presumptive vein cells activates Egfr ligands, leading to signaling through the Egfr/Ras pathway. Ras signaling directs vein cell identity through Dpp, and affects shg expression and protein localization (apical concentration) to alter vein cell shape in a Dpp-independent fashion.
Our results indicate that Ras signaling post-translationally affects both the level and basolateral distribution of Shg and the adherens junction complex of proteins. This is in agreement with other studies describing post-translational effects of Egfr signaling on Shg levels in the Drosophila eye and trachea (Brown et al., 2006; Cela and Llimargas, 2006), although mechanistically it is unclear how Ras affects Shg. As a first step toward answering this question, we examined effector-loop mutants of Ras that prevent activation of specific downstream effectors. For example, RasV12,S35 specifically activates Raf/MAPK signaling (Kinashi et al., 2000; Pacold et al., 2000; Prober and Edgar, 2002; Rodriguez-Viciana et al., 1997), whereas RasV12,G37 activates PI3-Kinase and RalGDS, but not Raf/MAPK signaling (Rodriguez-Viciana et al., 1997). While the specificities of the activated Ras isoforms are not absolute (Rodriguez-Viciana et al., 2004), we found that the Raf/MAPK-specific, activated form of Ras (RasV12,S35) promoted vein cell identity and the sub-apical localization of Shg. Further down the pathway, Raf-DN eliminated vein cells, while an activated version of MAPK caused ectopic veins to form. Finally, MKP3, an inhibitor of MAPK, suppressed the ability of RasV12 to inhibit DSRF expression and drive apical Shg localization. While these data support the idea that Shg is controlled via the canonical Egfr/Ras/Raf/MAPK pathway, inconsistencies exist. Most strikingly, an activated version of Raf inhibited vein cell identity. Why Raf-DN and Raf-GOF had similar effects on vein/intervein specification remains unresolved. What is clear, however, is that a split in the pathway (one fork affecting vein cell fate, and the other Shg localization) has not been found. Elucidating the pathway of control between Ras and the adherens junction complex, therefore, is an interesting challenge for the future.
Egf and Dpp signaling affect different aspects of vein morphology
In the pupal wing, Dpp is a principle effector of Egfr/Ras signaling, and Dpp activity is required for maintenance of vein cell identity. However, the phenotypic differences between RasV12 and TkvQ235D expression in the pupal wing indicate that Ras has Dpp-independent effects in pupal vein cells as they differentiate. Using apGalts, both RasV12 and TkvQ235D transgenes transformed dorsal intervein cells into vein cells. However, RasV12 expressing cells expanded along their apical/basal axis, stacking on top of one another, while TkvQ235D expressing cells did not. In addition, while Shg levels were upregulated in TkvQ235D expressing cells, Shg was not sub-apically concentrated as in RasV12 cells. TEM images did not detect a significant difference in adherens junction morphology between wildtype intervein cells and TkvQ235D expressing cells. While Dpp acts downstream of Ras in the specification of vein cell identity (downregulation of DSRF expression), it is clear that Ras and Dpp signaling control different aspects of vein cell shape.
These results underscore the importance of a cell’s developmental history in achieving a particular differentiated state. While the ultimate determinant of vein cell identity in the pupal wing is activation of the Dpp signaling pathway, our results demonstrate that without previous receipt of an Egf signal, the entire repertoire of vein cell characteristics (i.e., apical Shg localization) cannot be achieved. Egf and Dpp signaling may sequentially activate or repress different target genes, or the two signaling pathways may synergistically regulate genes that neither affects alone, in a feed-forward manner. This latter type of feed-forward loop (Mangan et al., 2003), has been recently described in neuronal subtypes that differentiate in the Drosophila ventral nerve cord. Sequentially activated transcription factors (collier, apterous and dimmed) together activate a target gene (Neuropeptide like precurson protein 1) that none of these transcription factors can effectively activate on their own (Baumgardt et al., 2007). Hence, it would be interesting to compare, on a genomic level, the transcriptional response pupal wing cells have to activation of the Egfr/Ras and Dpp signaling pathways.
Supplementary Material
Acknowledgments
We would like to thank Seth Blair, Pernille Rorth, Ernst Hafen, Jose de Celis, Marta Llimargas, Celeste Berg, Hannele Ruohola-Baker, The Developmental Studies Hybridoma Bank, and the Bloomington Drosophila Stock Center for reagents. We are particularly grateful to Ryu Ueda and his colleagues at the National Institute of Genetics in Japan for generation of the UAS-RNAi transgenes used in this study. Thanks to the University of Puget Sound and Dr. Scott Weatherwax for supporting the UPS Weatherwax Electron microscopy Lab. Thanks to Julio Vazquez, Dave McDonald and Adrian Quintanilla for help with confocal microscopy. Finally, thanks to Leslie Saucedo, Steve “Jahman” Lee and the entire Edgar lab for advice and entertainment. This work was supported by NIH U56 CA096288 to Beti Thompson, NIH R01 GM51186 to B.A.E., and an American Cancer Society Postdoctoral Fellowship to D.D.O.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson S, Saeboe-Larssen S, Lambertsson A, Merriam J, Jacobs-Lorena M. A Drosophila third chromosome Minute locus encodes a ribosomal protein. Genetics. 1994;137:513–20. doi: 10.1093/genetics/137.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgardt M, Miguel-Aliaga I, Karlsson D, Ekman H, Thor S. Specification of neuronal identities by feedforward combinatorial coding. PLoS Biol. 2007;5:e37. doi: 10.1371/journal.pbio.0050037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bier E. Localized activation of RTK/MAPK pathways during Drosophila development. Bioessays. 1998;20:189–94. doi: 10.1002/(SICI)1521-1878(199803)20:3<189::AID-BIES1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Brower DL, Jaffe SM. Requirement for integrins during Drosophila wing development. Nature. 1989;342:285–287. doi: 10.1038/342285a0. [DOI] [PubMed] [Google Scholar]
- Brown KE, Baonza A, Freeman M. Epithelial cell adhesion in the developing Drosophila retina is regulated by Atonal and the EGF receptor pathway. Dev Biol. 2006 doi: 10.1016/j.ydbio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Brunner D, Oellers N, Szabad J, Biggs WH, III, Zipursky SL, Hafen E. A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell. 1994;76:875–888. doi: 10.1016/0092-8674(94)90362-x. [DOI] [PubMed] [Google Scholar]
- Calleja M, Moreno E, Pelaz S, Morata G. Visualization of gene expression in living adult Drosophila. Science. 1996;274:252–255. doi: 10.1126/science.274.5285.252. [DOI] [PubMed] [Google Scholar]
- Cela C, Llimargas M. Egfr is essential for maintaining epithelial integrity during tracheal remodelling in Drosophila. Development. 2006;133:3115–25. doi: 10.1242/dev.02482. [DOI] [PubMed] [Google Scholar]
- Clifford RJ, Schupbach T. Coordinately and differentially mutable activities of torpedo, the Drosophila melanogaster homolog of the vertebrate EGF receptor gene. Genetics. 1989;123:771–87. doi: 10.1093/genetics/123.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozatier M, Glise B, Vincent A. Patterns in evolution: veins of the Drosophila wing. Trends Genet. 2004;20:498–505. doi: 10.1016/j.tig.2004.07.013. [DOI] [PubMed] [Google Scholar]
- D’Souza-Schorey C. Disassembling adherens junctions: breaking up is hard to do. Trends Cell Biol. 2005;15:19–26. doi: 10.1016/j.tcb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Dahmann C, Basler K. Opposing transcriptional outputs of Hedgehog signaling and Engrailed control compartmental cell sorting at the Drosophila A/P boundary. Cell. 2000;100:411–22. doi: 10.1016/s0092-8674(00)80677-7. [DOI] [PubMed] [Google Scholar]
- de Celis JF. Expression and function of decapentaplegic and thick veins during the differentiation of the veins in the Drosophila wing. Development. 1997;124:1007–18. doi: 10.1242/dev.124.5.1007. [DOI] [PubMed] [Google Scholar]
- De Celis JF. Pattern formation in the Drosophila wing: The development of the veins. Bioessays. 2003;25:443–51. doi: 10.1002/bies.10258. [DOI] [PubMed] [Google Scholar]
- De Celis JF, Diaz-Benjumea FJ. Developmental basis for vein pattern variations in insect wings. Int J Dev Biol. 2003;47:653–63. [PubMed] [Google Scholar]
- Diaz-Benjumea FJ, Hafen E. The sevenless signalling cassette mediates Drosophila EGF receptor function during epidermal development. Development. 1994;120:569–78. doi: 10.1242/dev.120.3.569. [DOI] [PubMed] [Google Scholar]
- Dumstrei K, Wang F, Shy D, Tepass U, Hartenstein V. Interaction between EGFR signaling and DE-cadherin during nervous system morphogenesis. Development. 2002;129:3983–94. doi: 10.1242/dev.129.17.3983. [DOI] [PubMed] [Google Scholar]
- Freeman M, Kimmel BE, Rubin GM. Identifying targets of the rough homeobox gene of Drosophila: evidence that rhomboid functions in eye development. Development. 1992;116:335–46. doi: 10.1242/dev.116.2.335. [DOI] [PubMed] [Google Scholar]
- Fristrom D, Wilcox M, Fristrom J. The distribution of PS integrins, laminin A and F-actin during key stages in Drosophila wing development. Development. 1993;117:509–23. doi: 10.1242/dev.117.2.509. [DOI] [PubMed] [Google Scholar]
- Gabay L, Seger R, Shilo BZ. In situ activation pattern of Drosophila EGF receptor pathway during development. Science. 1997;277:1103–6. doi: 10.1126/science.277.5329.1103. [DOI] [PubMed] [Google Scholar]
- Gomez AR, Lopez-Varea A, Molnar C, de la Calle-Mustienes E, Ruiz-Gomez M, Gomez-Skarmeta JL, de Celis JF. Conserved cross-interactions in Drosophila and Xenopus between Ras/MAPK signaling and the dual-specificity phosphatase MKP3. Dev Dyn. 2005;232:695–708. doi: 10.1002/dvdy.20227. [DOI] [PubMed] [Google Scholar]
- Guichard A, Biehs B, Sturtevant MA, Wickline L, Chacko J, Howard K, Bier E. rhomboid and Star interact synergistically to promote EGFR/MAPK signaling during Drosophila wing vein development. Development. 1999;126:2663–76. doi: 10.1242/dev.126.12.2663. [DOI] [PubMed] [Google Scholar]
- Hay BA, Wolff T, Rubin GM. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120:2121–9. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–58. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Jaiswal M, Agrawal N, Sinha P. Fat and Wingless signaling oppositely regulate epithelial cell-cell adhesion and distal wing development in Drosophila. Development. 2006;133:925–35. doi: 10.1242/dev.02243. [DOI] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–22. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarov A, Williams KP, Ling LE, Koteliansky VE, Duband JL, Fournier-Thibault C. A dual role for Sonic hedgehog in regulating adhesion and differentiation of neuroepithelial cells. Dev Biol. 2003;261:520–36. doi: 10.1016/s0012-1606(03)00351-8. [DOI] [PubMed] [Google Scholar]
- Karim FD, Rubin GM. Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development. 1998;125:1–9. doi: 10.1242/dev.125.1.1. [DOI] [PubMed] [Google Scholar]
- Kinashi T, Katagiri K, Watanabe S, Vanhaesebroeck B, Downward J, Takatsu K. Distinct mechanisms of alpha 5beta 1 integrin activation by Ha-Ras and R-Ras. J Biol Chem. 2000;275:22590–6. doi: 10.1074/jbc.M000633200. [DOI] [PubMed] [Google Scholar]
- Lecuit T. Adhesion remodeling underlying tissue morphogenesis. Trends Cell Biol. 2005;15:34–42. doi: 10.1016/j.tcb.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Lee JR, Urban S, Garvey CF, Freeman M. Regulated intracellular ligand transport and proteolysis control EGF signal activation in Drosophila. Cell. 2001;107:161–71. doi: 10.1016/s0092-8674(01)00526-8. [DOI] [PubMed] [Google Scholar]
- Mangan S, Zaslaver A, Alon U. The coherent feedforward loop serves as a sign-sensitive delay element in transcription networks. J Mol Biol. 2003;334:197–204. doi: 10.1016/j.jmb.2003.09.049. [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E, Roch F, Noll E, Baonza A, Duffy JB, Perrimon N. A temporal switch in DER signaling controls the specification and differentiation of veins and interveins in the Drosophila wing. Development. 1999;126:5739–47. doi: 10.1242/dev.126.24.5739. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–8. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Mirkovic I, Mlodzik M. Cooperative activities of drosophila DE-cadherin and DN-cadherin regulate the cell motility process of ommatidial rotation. Development. 2006;133:3283–93. doi: 10.1242/dev.02468. [DOI] [PubMed] [Google Scholar]
- Montagne J, Groppe J, Guillemin K, Krasnow MA, Gehring WJ, Affolter M. The Drosophila Serum Response Factor gene is required for the formation of intervein tissue of the wing and is allelic to blistered. Development. 1996;122:2589–97. doi: 10.1242/dev.122.9.2589. [DOI] [PubMed] [Google Scholar]
- Muda M, Theodosiou A, Rodrigues N, Boschert U, Camps M, Gillieron C, Davies K, Ashworth A, Arkinstall S. The dual specificity phosphatases M3/6 and MKP-3 are highly selective for inactivation of distinct mitogen-activated protein kinases. J Biol Chem. 1996;271:27205–8. doi: 10.1074/jbc.271.44.27205. [DOI] [PubMed] [Google Scholar]
- Nellen D, Burke R, Struhl G, Basler K. Direct and long-range action of a DPP morphogen gradient. Cell. 1996;85:357–368. doi: 10.1016/s0092-8674(00)81114-9. [DOI] [PubMed] [Google Scholar]
- Neufeld TP, de la Cruz AF, Johnston LA, Edgar BA. Coordination of growth and cell division in the Drosophila wing. Cell. 1998;93:1183–93. doi: 10.1016/s0092-8674(00)81462-2. [DOI] [PubMed] [Google Scholar]
- Nose A, Nagafuchi A, Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988;54:993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- Oda H, Uemura T, Harada Y, Iwai Y, Takeichi M. A Drosophila homolog of cadherin associated with armadillo and essential for embryonic cell-cell adhesion. Dev Biol. 1994;165:716–26. doi: 10.1006/dbio.1994.1287. [DOI] [PubMed] [Google Scholar]
- Pacold ME, Suire S, Perisic O, Lara-Gonzalez S, Davis CT, Walker EH, Hawkins PT, Stephens L, Eccleston JF, Williams RL. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase gamma. Cell. 2000;103:931–43. doi: 10.1016/s0092-8674(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Pacquelet A, Lin L, Rorth P. Binding site for p120/delta-catenin is not required for Drosophila E-cadherin function in vivo. J Cell Biol. 2003;160:313–9. doi: 10.1083/jcb.200207160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M. The product of the Drosophila segment polarity gene armadillo is part of a multi-protein complex resembling the vertebrate adherens junction. J Cell Sci. 1993;105(Pt 4):993–1000. doi: 10.1242/jcs.105.4.993. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Zipursky SL. Induction of Drosophila eye development by decapentaplegic. Development. 1997;124:271–8. doi: 10.1242/dev.124.2.271. [DOI] [PubMed] [Google Scholar]
- Price SR, De Marco Garcia NV, Ranscht B, Jessell TM. Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell. 2002;109:205–16. doi: 10.1016/s0092-8674(02)00695-5. [DOI] [PubMed] [Google Scholar]
- Prober DA, Edgar BA. Ras1 promotes cellular growth in the Drosophila wing. Cell. 2000;100:435–46. doi: 10.1016/s0092-8674(00)80679-0. [DOI] [PubMed] [Google Scholar]
- Prober DA, Edgar BA. Interactions between Ras1, dMyc, and dPI3K signaling in the developing Drosophila wing. Genes Dev. 2002;16:2286–99. doi: 10.1101/gad.991102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queenan AM, Ghabrial A, Schupbach T. Ectopic activation of torpedo/Egfr, a Drosophila receptor tyrosine kinase, dorsalizes both the eggshell and the embryo. Development. 1997;124:3871–80. doi: 10.1242/dev.124.19.3871. [DOI] [PubMed] [Google Scholar]
- Rintelen F, Hafen E, Nairz K. The Drosophila dual-specificity ERK phosphatase DMKP3 cooperates with the ERK tyrosin phosphatase PTP-ER. Development. 2003;130:3479–90. doi: 10.1242/dev.00568. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Sabatier C, McCormick F. Signaling Specificity by Ras Family GTPases Is Determined by the Full Spectrum of Effectors They Regulate. Mol Cell Biol. 2004;24:4943–54. doi: 10.1128/MCB.24.11.4943-4954.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–67. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- Sanson B, White P, Vincent JP. Uncoupling cadherin-based adhesion from wingless signalling in Drosophila. Nature. 1996;383:627–30. doi: 10.1038/383627a0. [DOI] [PubMed] [Google Scholar]
- Schnorr JD, Berg CA. Differential activity of Ras1 during patterning of the Drosophila dorsoventral axis. Genetics. 1996;144:1545–57. doi: 10.1093/genetics/144.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillos S, De Celis JF. Interactions between the Notch, EGFR, and decapentaplegic signaling pathways regulate vein differentiation during Drosophila pupal wing development. Dev Dyn. 2005;232:738–52. doi: 10.1002/dvdy.20270. [DOI] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Sturtevant MA, Bier E. Analysis of the genetic hierarchy guiding wing vein development in Drosophila. Development. 1995;121:785–801. doi: 10.1242/dev.121.3.785. [DOI] [PubMed] [Google Scholar]
- Sturtevant MA, Roark M, Bier E. The Drosophila rhomboid gene mediates the localized formation of wing veins and interacts genetically with components of the EGF-R signaling pathway. Genes Dev. 1993;7:961–73. doi: 10.1101/gad.7.6.961. [DOI] [PubMed] [Google Scholar]
- Tanaka-Matakatsu M, Uemura T, Oda H, Takeichi M, Hayashi S. Cadherin-mediated cell adhesion and cell motility in Drosophila trachea regulated by the transcription factor Escargot. Development. 1996;122:3697–705. doi: 10.1242/dev.122.12.3697. [DOI] [PubMed] [Google Scholar]
- Tepass U, Gruszynski-DeFeo E, Haag TA, Omatyar L, Torok T, Hartenstein V. shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes Dev. 1996;10:672–85. doi: 10.1101/gad.10.6.672. [DOI] [PubMed] [Google Scholar]
- Tsuneizumi K, Nakayama T, Kamoshida Y, Kornberg TB, Christian JL, Tabata T. Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nature. 1997;389:627–31. doi: 10.1038/39362. [DOI] [PubMed] [Google Scholar]
- Ulrich F, Krieg M, Schotz EM, Link V, Castanon I, Schnabel V, Taubenberger A, Mueller D, Puech PH, Heisenberg CP. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell. 2005;9:555–64. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Urban S, Lee JR, Freeman M. Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases. Cell. 2001;107:173–82. doi: 10.1016/s0092-8674(01)00525-6. [DOI] [PubMed] [Google Scholar]
- Urban S, Lee JR, Freeman M. A family of Rhomboid intramembrane proteases activates all Drosophila membrane-tethered EGF ligands. Embo J. 2002;21:4277–86. doi: 10.1093/emboj/cdf434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DF, Hough C, Peel D, Callaini G, Bryant PJ. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J Cell Biol. 1996;134:1469–82. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–37. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


