Abstract
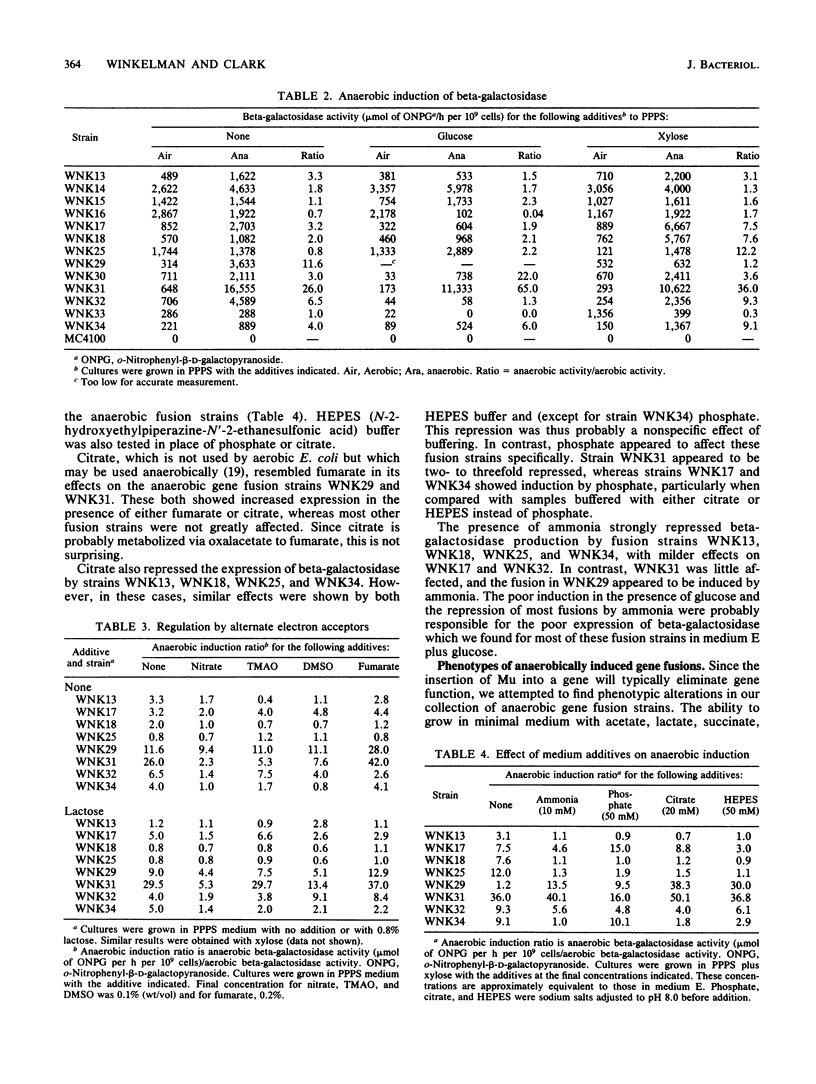
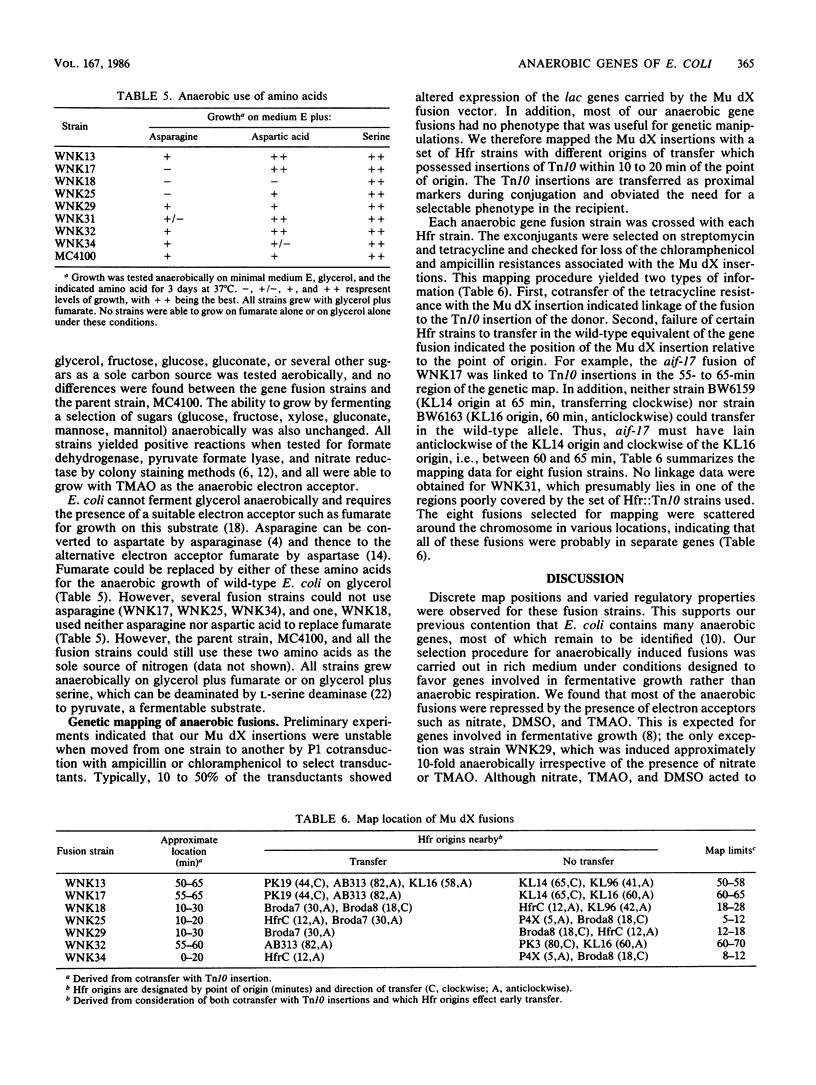
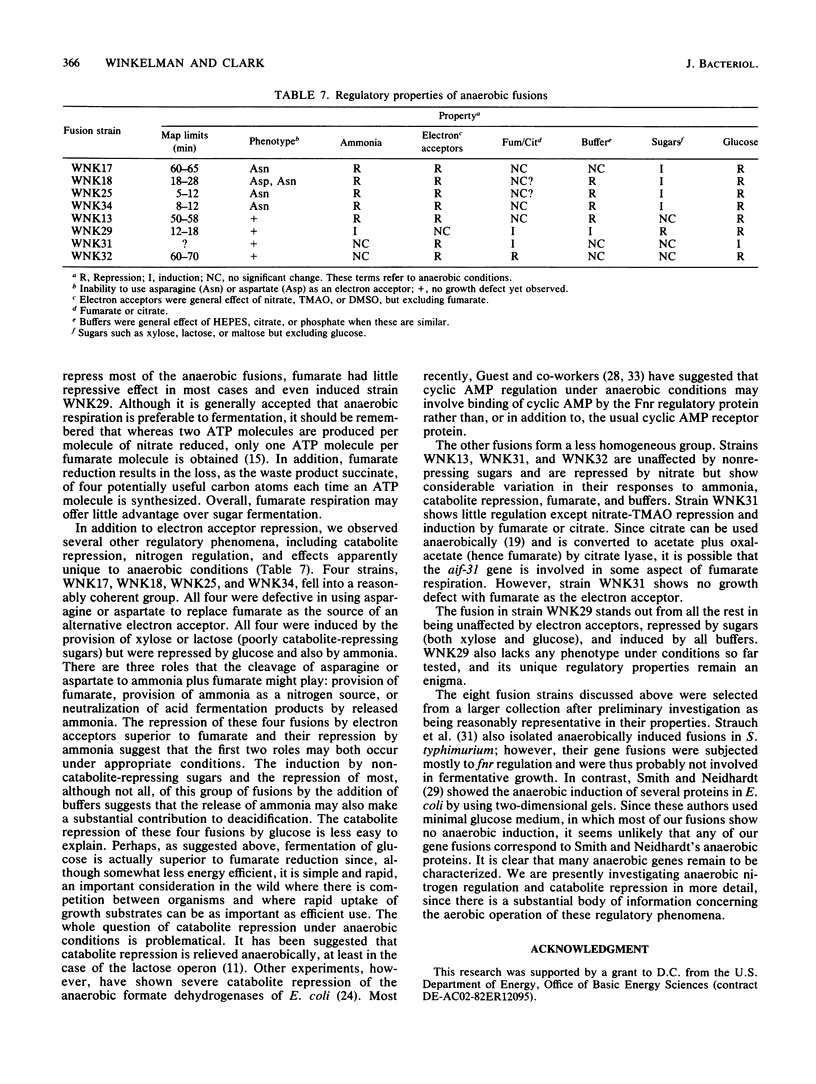
A collection of anaerobically induced gene fusions were isolated, and representative isolates were characterized with respect to their regulatory properties, phenotypes, and approximate map locations. Four fusion strains that had defects in the anaerobic metabolism of asparagine or aspartate were found. These fusions were all repressed by alternate electron acceptors, ammonia, and glucose but were induced by other sugars. Several other fusion strains which demonstrated no observable phenotype showed diverse regulatory responses. The anaerobically induced fusions were scattered around the Escherichia coli chromosome more or less at random, suggesting that all the isolates examined were in separate genes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. A., Howe M. M., Gross C. A. Mu dX, a derivative of Mu d1 (lac Apr) which makes stable lacZ fusions at high temperature. J Bacteriol. 1983 Nov;156(2):970–974. doi: 10.1128/jb.156.2.970-974.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilous P. T., Weiner J. H. Proton translocation coupled to dimethyl sulfoxide reduction in anaerobically grown Escherichia coli HB101. J Bacteriol. 1985 Jul;163(1):369–375. doi: 10.1128/jb.163.1.369-375.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. Production of L-asparaginase II by Escherichia coli. J Bacteriol. 1968 Dec;96(6):2043–2048. doi: 10.1128/jb.96.6.2043-2048.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. M., Lin E. C. Dual control of a common L-1,2-propanediol oxidoreductase by L-fucose and L-rhamnose in Escherichia coli. J Bacteriol. 1984 Mar;157(3):828–832. doi: 10.1128/jb.157.3.828-832.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux M., Casse F., Pascal M. C. Isolation and phenotypes of mutants from Salmonella typhimurium defective in formate hydrogenlyase activity. J Bacteriol. 1972 May;110(2):766–768. doi: 10.1128/jb.110.2.766-768.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux M., Giudici D., Abou-Jaoudé A., Casse F., Pascal M. C. Laboratoire de Chimie Bactérienne C.N.R.S., Marsielle, France. Mol Gen Genet. 1978 Apr 6;160(2):225–229. doi: 10.1007/BF00267485. [DOI] [PubMed] [Google Scholar]
- Clark D. P., Cronan J. E., Jr Acetaldehyde coenzyme A dehydrogenase of Escherichia coli. J Bacteriol. 1980 Oct;144(1):179–184. doi: 10.1128/jb.144.1.179-184.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D., Cronan J. E., Jr Escherichia coli mutants with altered control of alcohol dehydrogenase and nitrate reductase. J Bacteriol. 1980 Jan;141(1):177–183. doi: 10.1128/jb.141.1.177-183.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrogosz W. J. Altered end-product patterns and catabolite repression in Escherichia coli. J Bacteriol. 1966 Jun;91(6):2263–2269. doi: 10.1128/jb.91.6.2263-2269.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser J. H., DeMoss J. A. Phenotypic restoration by molybdate of nitrate reductase activity in chlD mutants of Escherichia coli. J Bacteriol. 1971 Nov;108(2):854–860. doi: 10.1128/jb.108.2.854-860.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J. R. Anaerobic growth of Escherichia coli K12 with fumarate as terminal electron acceptor. Genetic studies with menaquinone and fluoroacetate-resistant mutants. J Gen Microbiol. 1979 Dec;115(2):259–271. doi: 10.1099/00221287-115-2-259. [DOI] [PubMed] [Google Scholar]
- Guest J. R., Roberts R. E., Wilde R. J. Cloning of the aspartase gene (aspA) of Escherichia coli. J Gen Microbiol. 1984 May;130(5):1271–1278. doi: 10.1099/00221287-130-5-1271. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J., Poole R. K. The respiratory chains of Escherichia coli. Microbiol Rev. 1984 Sep;48(3):222–271. doi: 10.1128/mr.48.3.222-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D. J., Higgins C. F. Anaerobic and leucine-dependent expression of a peptide transport gene in Salmonella typhimurium. J Bacteriol. 1984 Oct;160(1):131–136. doi: 10.1128/jb.160.1.131-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. M., Gunsalus R. P. Transcription of the Escherichia coli fumarate reductase genes (frdABCD) and their coordinate regulation by oxygen, nitrate, and fumarate. J Bacteriol. 1985 Dec;164(3):1100–1109. doi: 10.1128/jb.164.3.1100-1109.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuritzkes D. R., Zhang X. Y., Lin E. C. Use of phi(glp-lac) in studies of respiratory regulation of the Escherichia coli anaerobic sn-glycerol-3-phosphate dehydrogenase genes (glpAB). J Bacteriol. 1984 Feb;157(2):591–598. doi: 10.1128/jb.157.2.591-598.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütgens M., Gottschalk G. Why a co-substrate is required for anaerobic growth of Escherichia coli on citrate. J Gen Microbiol. 1980 Jul;119(1):63–70. doi: 10.1099/00221287-119-1-63. [DOI] [PubMed] [Google Scholar]
- Mason T. G., Richardson G. Escherichia coli and the human gut: some ecological considerations. J Appl Bacteriol. 1981 Aug;51(1):1–16. doi: 10.1111/j.1365-2672.1981.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Newman E. B., Walker C. L-serine degradation in Escherichia coli K-12: a combination of L-serine, glycine, and leucine used as a source of carbon. J Bacteriol. 1982 Aug;151(2):777–782. doi: 10.1128/jb.151.2.777-782.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal M. C., Burini J. F., Chippaux M. Regulation of the trimethylamine N-oxide (TMAO) reductase in Escherichia coli: analysis of tor::Mud1 operon fusion. Mol Gen Genet. 1984;195(1-2):351–355. doi: 10.1007/BF00332770. [DOI] [PubMed] [Google Scholar]
- Patrick J. M., Dobrogosz W. J. The effect of cyclic AMP on anaerobic growth of Escherichia coli. Biochem Biophys Res Commun. 1973 Sep 18;54(2):555–561. doi: 10.1016/0006-291x(73)91458-7. [DOI] [PubMed] [Google Scholar]
- Pecher A., Blaschkowski H. P., Knappe K., Böck A. Expression of pyruvate formate-lyase of Escherichia coli from the cloned structural gene. Arch Microbiol. 1982 Oct;132(4):365–371. doi: 10.1007/BF00413390. [DOI] [PubMed] [Google Scholar]
- Ruch F. E., Kuritzkes D. R., Lin E. C. Use of lac operon fusions to isolate Escherichia coli mutants with altered expression of the fumarate reductase system in response to substrate and respiratory controls. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1365–1370. doi: 10.1016/0006-291x(79)91217-8. [DOI] [PubMed] [Google Scholar]
- Shaw D. J., Guest J. R. Amplification and product identification of the fnr gene of Escherichia coli. J Gen Microbiol. 1982 Oct;128(10):2221–2228. doi: 10.1099/00221287-128-10-2221. [DOI] [PubMed] [Google Scholar]
- Shaw D. J., Rice D. W., Guest J. R. Homology between CAP and Fnr, a regulator of anaerobic respiration in Escherichia coli. J Mol Biol. 1983 May 15;166(2):241–247. doi: 10.1016/s0022-2836(83)80011-4. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by anaerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):336–343. doi: 10.1128/jb.154.1.336-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V. Requirement of Fnr and NarL functions for nitrate reductase expression in Escherichia coli K-12. J Bacteriol. 1982 Sep;151(3):1320–1325. doi: 10.1128/jb.151.3.1320-1325.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Lenk J. B., Gamble B. L., Miller C. G. Oxygen regulation in Salmonella typhimurium. J Bacteriol. 1985 Feb;161(2):673–680. doi: 10.1128/jb.161.2.673-680.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi M., Tsuchiya T., Ishimoto M. Proton translocation coupled to trimethylamine N-oxide reduction in anaerobically grown Escherichia coli. J Bacteriol. 1981 Dec;148(3):762–768. doi: 10.1128/jb.148.3.762-768.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unden G., Guest J. R. Cyclic AMP and anaerobic gene expression in E. coli. FEBS Lett. 1984 May 21;170(2):321–325. doi: 10.1016/0014-5793(84)81336-8. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


