Abstract
DNA sequences corresponding to the aerolysin gene (aer) of Aeromonas hydrophila AH2 DNA were identified by screening a cosmid gene library for hemolytic and cytotoxic activities. A plasmid containing a 5.8-kilobase EcoRI fragment of A. hydrophila DNA was required for full expression of the hemolytic and cytotoxic phenotype in Escherichia coli K-12. Deletion analysis and transposon mutagenesis allowed us to localize the gene product to 1.4 kilobases of Aeromonas DNA and define flanking DNA regions affecting aerolysin production. The reduced hemolytic activity with plasmids lacking these flanking regions is associated with a temporal delay in the appearance of hemolytic activity and is not a result of a loss of transport functions. The aerolysin gene product was detected as a 54,000-dalton protein in E. coli maxicells harboring aer plasmids and by immunoblotting E. coli whole cells carrying aer plasmids. We suggest that the gene coding aerolysin be designated aerA and that regions downstream and upstream of aerA which modulate its expression and activity be designated aerB and aerC, respectively.
Full text
PDF
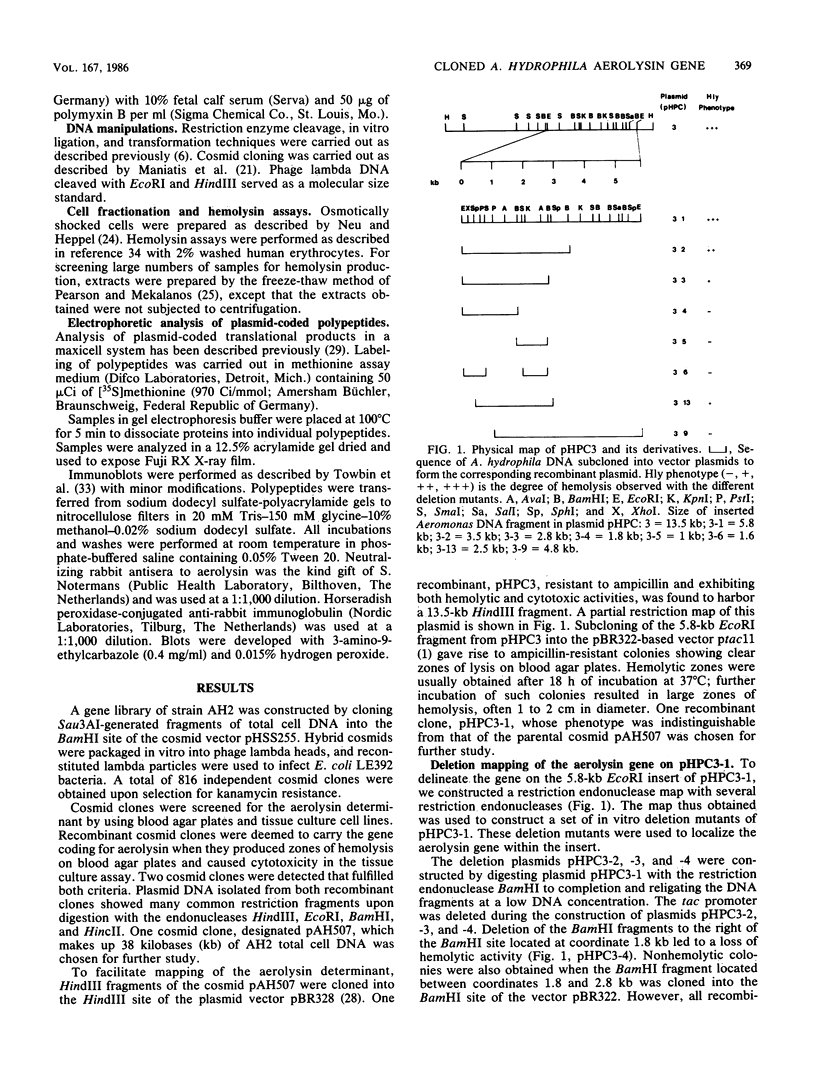

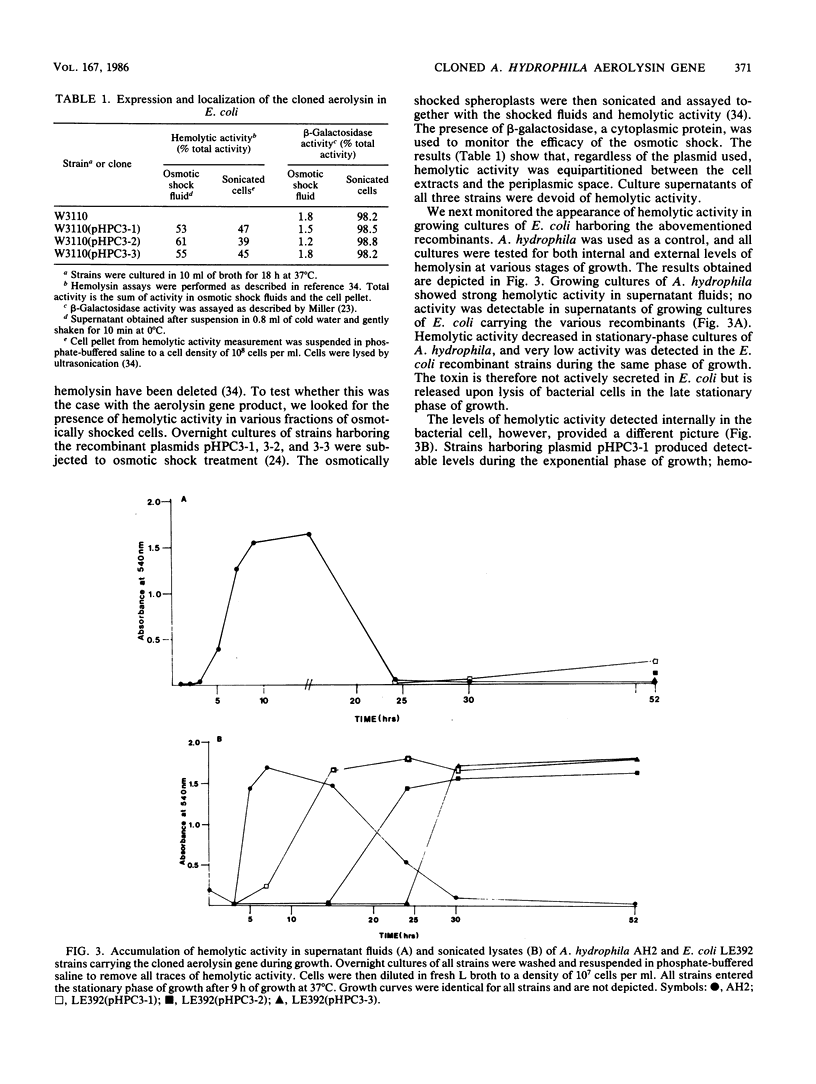
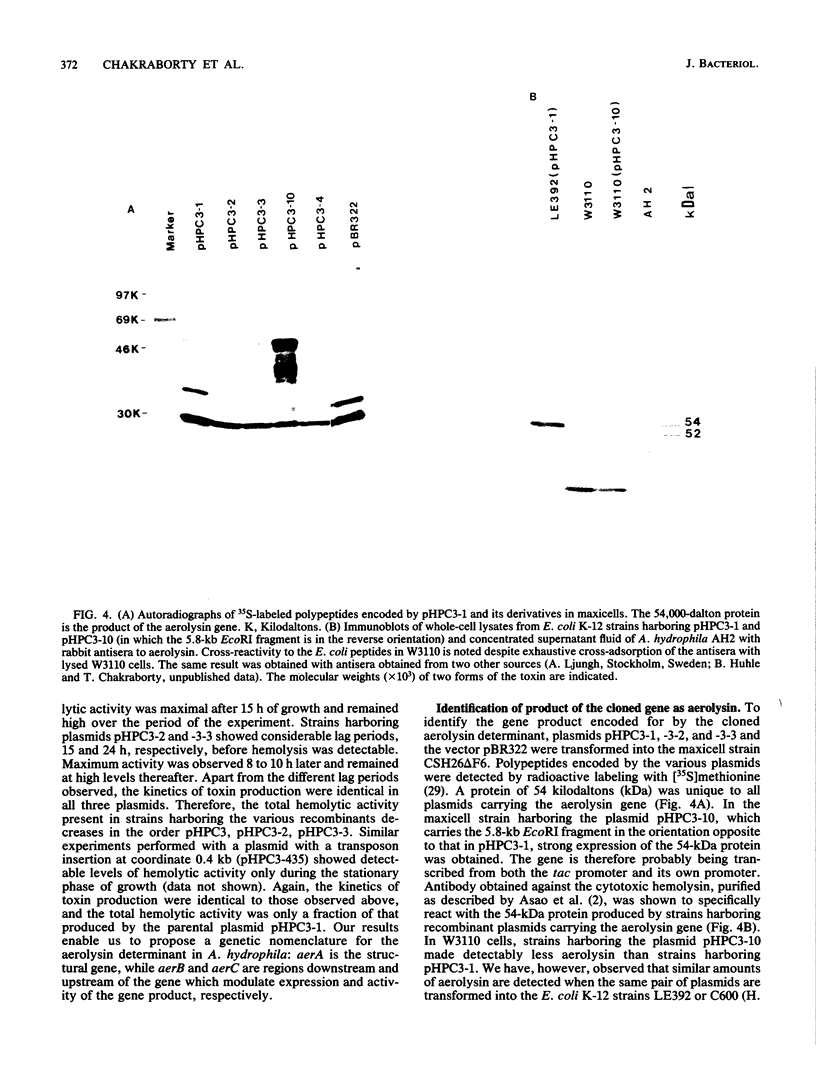


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Asao T., Kinoshita Y., Kozaki S., Uemura T., Sakaguchi G. Purification and some properties of Aeromonas hydrophila hemolysin. Infect Immun. 1984 Oct;46(1):122–127. doi: 10.1128/iai.46.1.122-127.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley J. T., Halasa L. N., Lund K. D., MacIntyre S. Purification and some properties of the hemolytic toxin aerolysin. Can J Biochem. 1981 Jun;59(6):430–435. doi: 10.1139/o81-059. [DOI] [PubMed] [Google Scholar]
- Burke V., Robinson J., Atkinson H. M., Gracey M. Biochemical characteristics of enterotoxigenic Aeromonas spp. J Clin Microbiol. 1982 Jan;15(1):48–52. doi: 10.1128/jcm.15.1.48-52.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke V., Robinson J., Beaman J., Gracey M., Lesmana M., Rockhill R., Echeverria P., Janda J. M. Correlation of enterotoxicity with biotype in Aeromonas spp. J Clin Microbiol. 1983 Nov;18(5):1196–1200. doi: 10.1128/jcm.18.5.1196-1200.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty T., Montenegro M. A., Sanyal S. C., Helmuth R., Bulling E., Timmis K. N. Cloning of enterotoxin gene from Aeromonas hydrophila provides conclusive evidence of production of a cytotonic enterotoxin. Infect Immun. 1984 Nov;46(2):435–441. doi: 10.1128/iai.46.2.435-441.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman K., Dougan G., Arbuthnott J. P. Cloning, and expression in Escherichia coli K-12, of the chromosomal hemolysin (phospholipase C) determinant of Pseudomonas aeruginosa. J Bacteriol. 1983 Feb;153(2):909–915. doi: 10.1128/jb.153.2.909-915.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily O. P., Joseph S. W., Coolbaugh J. C., Walker R. I., Merrell B. R., Rollins D. M., Seidler R. J., Colwell R. R., Lissner C. R. Association of Aeromonas sobria with human infection. J Clin Microbiol. 1981 Apr;13(4):769–777. doi: 10.1128/jcm.13.4.769-777.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donta S. T., Haddow A. D. Cytotoxic activity of Aeromonas hydrophila. Infect Immun. 1978 Sep;21(3):989–993. doi: 10.1128/iai.21.3.989-993.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracey M., Burke V., Robinson J. Aeromonas-associated gastroenteritis. Lancet. 1982 Dec 11;2(8311):1304–1306. doi: 10.1016/s0140-6736(82)91510-0. [DOI] [PubMed] [Google Scholar]
- Guyer M. S. The gamma delta sequence of F is an insertion sequence. J Mol Biol. 1978 Dec 15;126(3):347–365. doi: 10.1016/0022-2836(78)90045-1. [DOI] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Activation of the hole-forming toxin aerolysin by extracellular processing. J Bacteriol. 1985 Jul;163(1):336–340. doi: 10.1128/jb.163.1.336-340.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Membrane glycoprotein receptor and hole-forming properties of a cytolytic protein toxin. Biochemistry. 1982 Mar 30;21(7):1662–1667. doi: 10.1021/bi00536a029. [DOI] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Protein export by a gram-negative bacterium: production of aerolysin by Aeromonas hydrophila. J Bacteriol. 1985 Mar;161(3):1118–1124. doi: 10.1128/jb.161.3.1118-1124.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda J. M., Bottone E. J., Skinner C. V., Calcaterra D. Phenotypic markers associated with gastrointestinal Aeromonas hydrophila isolates from symptomatic children. J Clin Microbiol. 1983 Apr;17(4):588–591. doi: 10.1128/jcm.17.4.588-591.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda J. M., Reitano M., Bottone E. J. Biotyping of Aeromonas isolates as a correlate to delineating a species-associated disease spectrum. J Clin Microbiol. 1984 Jan;19(1):44–47. doi: 10.1128/jcm.19.1.44-47.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Lockman H., Colwell R. R., Joseph S. W. Aeromonas hydrophila: ecology and toxigenicity of isolates from an estuary. J Appl Bacteriol. 1981 Apr;50(2):359–377. doi: 10.1111/j.1365-2672.1981.tb00900.x. [DOI] [PubMed] [Google Scholar]
- Kehoe M., Duncan J., Foster T., Fairweather N., Dougan G. Cloning, expression, and mapping of the Staphylococcus aureus alpha-hemolysin determinant in Escherichia coli K-12. Infect Immun. 1983 Sep;41(3):1105–1111. doi: 10.1128/iai.41.3.1105-1111.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio A., Manning P. A. Cellular localization and export of the soluble haemolysin of Vibrio cholerae El Tor. Mol Gen Genet. 1985;200(3):472–475. doi: 10.1007/BF00425733. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Pearson G. D., Mekalanos J. J. Molecular cloning of Vibrio cholerae enterotoxin genes in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 May;79(9):2976–2980. doi: 10.1073/pnas.79.9.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitarangsi C., Echeverria P., Whitmire R., Tirapat C., Formal S., Dammin G. J., Tingtalapong M. Enteropathogenicity of Aeromonas hydrophila and Plesiomonas shigelloides: prevalence among individuals with and without diarrhea in Thailand. Infect Immun. 1982 Feb;35(2):666–673. doi: 10.1128/iai.35.2.666-673.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahim Z., Sanyal S. C., Aziz K. M., Huq M. I., Chowdhury A. A. Isolation of enterotoxigenic, hemolytic, and antibiotic-resistant Aeromonas hydrophila strains from infected fish in Bangladesh. Appl Environ Microbiol. 1984 Oct;48(4):865–867. doi: 10.1128/aem.48.4.865-867.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H., Ohta H., Ogawa M., Mizuguchi Y. Cloning and expression in Escherichia coli of Vibrio parahaemolyticus thermostable direct hemolysin and thermolabile hemolysin genes. J Bacteriol. 1985 May;162(2):510–515. doi: 10.1128/jb.162.2.510-515.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelestam M., Ljungh A. Membrane-damaging and cytotoxic effects on human fibroblasts of alpha- and beta-hemolysins from Aeromonas hydrophila. Infect Immun. 1981 Dec;34(3):949–956. doi: 10.1128/iai.34.3.949-956.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W., Vogel M., Goebel W. Transport of hemolysin across the outer membrane of Escherichia coli requires two functions. J Bacteriol. 1983 Apr;154(1):200–210. doi: 10.1128/jb.154.1.200-210.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock G. M., Berman M. L., Silhavy T. J. Chimeric genetics with beta-galactosidase. Gene Amplif Anal. 1983;3:27–64. [PubMed] [Google Scholar]