Abstract
In the present study, we evaluated the effects of memantine in a delayed radial arm maze rat task, consisting of an acquisition phase followed 18 h later by a win-shift retrieval test. When administered 20 min before acquisition, memantine elicited an inverted U-shape dose-response relationship, with low doses (0.3 and 0.56 mg/kg) reducing the number of errors committed during the retrieval test, while high doses (3 and 10 mg/kg) disrupted maze running. Memantine given immediately after acquisition or 20 min before retrieval failed to affect performance. Co-administration of subthreshold doses of memantine with either the CB1 receptor antagonist rimonabant or the acetylcholine esterase inhibitor donepezil failed to enhance performance. Thus, low doses of memantine enhance acquisition processes that lead to prolonged spatial memory.
Keywords: Radial-arm maze, Spatial Memory, Memantine, Rimonabant (SR141716), Donepezil
1. Introduction
Memantine, a low to moderate uncompetitive NMDA receptor antagonist that has been approved for treatment of Alzheimer’s disease in the United States, is hypothesized to offer neuroprotection and enhance memory without eliciting behavioral side effects typically produced by NMDA antagonists, such as memory and locomotor disturbances (Danysz and Parsons, 2003). Memantine has cognitive benefits in patients diagnosed as having mild to moderate Alzheimer’s disease (AD) symptoms in clinical trials (Bullock, 2006; Schmitt et al., 2006; Tariot et al., 2004). In several preclinical studies, memantine enhanced performance in various animal models of AD, such as transgenic mice carrying mutated human APP or PS1 (App/PS1) genes (Minkeviciene et al., 2004), the APP23 model (Van Dam and De Deyn, 2006), intracerebral ventricular infusion of β amyloid protein (Yamada et al., 2005), and rats with entorinal cortex lesions (Zajaczkowski et al., 1996). In naïve animals, memantine failed to enhance (Minkeviciene et al., 2004; Woodruff-Pak et al., 2006; Zajaczkowski et al., 1997) or disrupted (Creeley et al., 2006) memory performance in some studies, but was reported to improve memory retention in healthy rats evaluated in a Morris water maze task (Zoladz et al., 2006). Given the observation that disturbances in spatial orientation are a hallmark symptom of AD and radial arm maze tasks require appropriate visuospatial and working memory processing, a primary objective of the present study was to evaluate whether memantine would enhance memory in rats assessed in a delayed radial arm maze memory task. Additionally, memantine was administered either immediately after the acquisition phase or 20 min before retrieval testing to assess the impact of this compound on consolidation and retrieval processes.
There is a growing interest in using combination pharmacology to treat AD. Accordingly, we have previously reported that co-administration of subthreshold doses of the cannabinoid (CB1) receptor antagonist rimonabant, which has been found to improve performance in several rodent behavioral tasks, and donepezil, an acetylcholine esterase inhibitor approved to treat dementia related to AD, prolonged spatial memory in a delayed radial arm maze task (Wise et al., 2007). Given these findings, the final objective of the present study was to evaluate whether memantine administered in combination with donepezil or rimonabant would decrease errors of re-entry in the test phase 18 h after acquisition.
2. Methods
2.1. Subjects
All experiments were performed in Sprague Dawley (Harlan, IN) male rats aged 12-16 months that were individually housed in a temperature-controlled (20–22°C) environment, with a 12-h light/dark cycle served as subjects. The subjects were used in a previous study investigating interactions between rimonabant and donepezil in the radial arm maze task (Wise et al., 2007). Subjects were maintained on a food-restricted diet in order to sustain body weights between 320 and 350 g, approximately 85% of their free-feeding weight and water was available ad libitum. All animal protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee and were in concordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
2.2. Drugs
Memantine (0.1, 0.3, 0.56, 1.0, 3.0, and 10 mg/kg; Tocris, Ellisville, MO) was dissolved distilled water. Rimonabant (0.3 mg/kg; National Institute on Drug Abuse, Rockville, MD) and donepezil (0.1 mg/kg; gift from Pfizer Inc, Groton, CT) were dissolved in a 1:1 mixture of absolute ethanol and alkamuls-620 (Rhone-Poulenc, Princeton, NJ), and diluted with saline in a final ratio of 1:1:18 (ethanol/alkamuls/saline). Injections were given through the i.p. route of administration in a volume of 1 ml/kg.
2.3. Radial arm maze procedure
The initial training and repeated acquisition radial arm maze training procedure for these rats has already been described (Wise et al., 2007). Each session incorporated acquisition and retrieval test phases. During the acquisition phase, one of the arms was randomly selected and a Plexiglas barricade blocked its entryway. Each of the remaining seven arms was baited with a food pellet prior to the subject’s placement in the maze. After the subject entered the seven available arms and consumed each of the available food pellets, it was removed from the maze and returned to its home cage. During the test phase, all arms were available; however, only the previously blocked arm was baited with a food pellet. The number of entries and the duration of time required for each subject to enter the baited arm(s) and consume available food pellets were recorded for each phase. An 18 h delay between the acquisition and test phases was used, which we previously have shown to reduce choice accuracy to chance performance during the test phase (Wise et al., 2007).
In the first set of experiments, we evaluated the dose-response relationship of memantine (0.1 to 10 mg/kg; n = 13 rats/condition) given 20 min before acquisition. The next set of experiments evaluated an effective dose of memantine (0.3 mg/kg) compared to vehicle (n = 11 rats/condition) administered either immediately after acquisition or 20 min before the retrieval test. In the third set of experiments (see Table 1 for details), we evaluated a subthreshold dose of memantine (0.1 mg/kg) given in combination with a subthreshold dose of donepezil (0.1 mg/kg) or rimonabant (0.3 mg/kg) 20 min before acquisition (n = 11-13 rats/condition). The subthreshold dose of memantine was empirically derived and the subthreshold doses of donepezil and rimonabant were based on our previous report (Wise et al., 2007) and confirmed here. Given the drug history and the age of the rats, a final experiment evaluated the effects of vehicle (n = 7 rats) as well as effective doses of donepezil (0.3 mg/kg; n = 4 rats) and memantine (0.3 mg/kg; n = 7 rats) given 20 min before acquisition. Treatments in each experiment were counter-balanced to control for any order effects. Subjects were given a maximum of two tests/week, with at least 48 h between test sessions.
Table 1.
Experimental design of combination studies of the uncompetitive NMDA receptor antagonist memantine with either the CB1 receptor antagonist rimonabant or the cholinesterase inhibitor donepezil. The doses of rimonabant and donepezil were chosen based on our previous delayed radial arm maze study (Wise et al., 2007). Treatments in each experiment were counterbalanced to control for any order effects. Subjects were given a maximum of two tests/week, with at least 48 h between test sessions.
| Experiment | Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 |
|---|---|---|---|---|
| 3A: Subthreshold doses | ||||
| Memantine | 0 | 0.1 mg/kg | 0 | 0.1 mg/kg |
| Rimonabant | 0 | 0 | 0.3 mg/kg | 0.3 mg/kg |
| 3B: Subthreshold doses | ||||
| Memantine | 0 | 0.1 mg/kg | 0 | 0.1 mg/kg |
| Donepezil | 0 | 0 | 0.1 mg/kg | 0.1 mg/kg |
2.4. Data Analyses
A within subject analysis of variance (ANOVA) was used to analyze the dose effect curve of memantine. A between subjects ANOVA was used to analyze each dependent measure in the combination studies, as not every subject received every drug condition. Dunnett‘s post hoc test was to analyze differences between vehicle and each drug or drug combinations. T-tests were used to analyze the effect of administering memantine or vehicle after the acquisition phase or before the retention phase, as these experiments included two groups. Differences were considered significant at the P < 0.05 level.
3. Results
Memantine administered 20 min before the acquisition phase led to an inverted U-shaped dose-response relationship in reducing the number of errors committed during the retrieval test, F (4, 48) = 10.1, P < 0.01 (Fig. 1A). Both the 0.3 (P < 0.01) and 0.56 (P < 0.05) mg/kg doses of memantine reduced the number of errors compared to the vehicle condition. In contrast, memantine doses greater than 1.0 mg/kg led to profound performance deficits during the acquisition phase that precluded retrieval testing. Specifically, all of the rats failed to enter any arms when treated with 10 mg/kg memantine (n = 5). When evaluated with 3 mg/kg memantine (n=5), only one rat entered all eight arms, one rat entered four arms, and the other three rats failed to any arm.
Fig. 1.
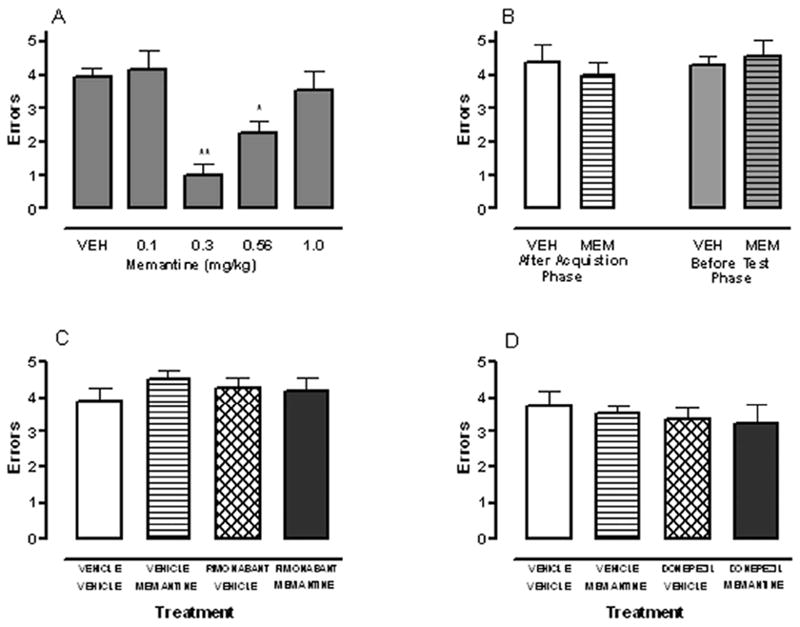
(A) Memantine administered 20 min before the acquisition phase decreased the number of errors committed during the test phase. (B) Memantine (0.3 mg/kg) given either immediately after the acquisition phase (post-training administration) or 20 min before the retrieval test (pre-test administration) failed to enhance choice accuracy. (C) Co-administration of subthreshold doses of memantine (0.1 mg/kg) and rimonabant (0.3 mg/kg) given 20min before acquisition failed to decrease the number of errors committed in the test phase. (D) Co-administration of subthreshold doses of memantine (0.1 mg/kg) and donepezil (0.1 mg/kg) given 20 min before acquisition failed to decrease the number of errors committed in the test phase.* P < 0.05 and ** P< 0.01 for each group vs. vehicle treatment. Results are shown as mean ± S.E.M.; n = 11-13 rats/group.
With the exception of the 3 and 10 mg/kg memantine doses, choice accuracy was virtually perfect during the acquisition phase in all experiments, as subjects entered each baited arm, ate all available pellets, and rarely made any errors of reentry. Additionally, the rate of arm entry in these other conditions was approximately 15 s/arm and was unaffected by any drug treatments. Accordingly, neither of these measures is presented.
In the second experiment, an effective dose of memantine (0.3 mg/kg) failed to affect performance when given either immediately after acquisition, P = 0.53, (Fig. 1B, left) or 20 min before the retrieval test, P = 0.70 (Fig. 1B, right). In the third experiment, we examined whether combined administration of a subthreshold dose of memantine (0.1 mg/kg) given with a subthreshold dose of either rimonabant (0.3 mg/kg) or donepezil (0.1 mg/kg) would enhance memory (see Table 1). Co-administration of memantine and rimonabant, P = 0.39 (Fig. 1C) or memantine and donepezil (0.1 mg/kg), P = 0.89 (Fig. 1D), did not decrease the number of errors in the test phase. The final experiment evaluated whether the previously observed memory enhancing effects of 0.3 mg/kg donepezil and 0.3 mg/kg memantine, as well as the initial effect of vehicle, on performance could still be achieved. Importantly, the performance (mean ± S.E.M. errors) of these rats after donepezil (0.9 ± 0.3), memantine (0.8 ± 0.5), and vehicle (3.8 ± 0.4) treatment was nearly identical to that initially observed.
4. Discussion
In the present study, we report that low doses of the uncompetitive NMDA receptor antagonist memantine decreased the number of re-entry errors committed during the test phase in a delayed radial arm maze task. Inserting an 18 h delay between the acquisition and retrieval test phases enabled us to distinguish between acquisition and retrieval processes by administering an effective dose of memantine before the acquisition phase, immediately after the acquisition phase, or before the retrieval test phase. Memantine enhanced memory when administered before the acquisition phase, but not when administered immediately after the acquisition phase or 20 min before the test phase. Importantly, these findings suggest that this compound acted on memory acquisition/consolidation, rather than retrieval, processes. The failure of memantine administered immediately after the acquisition phase to enhance retrieval tends to argue that it does not affect consolidation processes. However, the time course of consolidation is unknown. Accordingly, it is unknown whether memantine reached its site before the consolidation period was complete. Thus, the possibility that this compound affects consolidation cannot be ruled out. Nonetheless, memantine has recently been reported to enhance attentional processes and not memory in adults diagnosed with age-associated memory impairment (Ferris et al., 2007).
A recent report also found that memantine enhanced performance in healthy rats (Zoladz et al., 2006). Specifically, they found that memantine administered before three training sessions in the Morris water maze improved performance in a test given 24 h later. The results of Zoladz et al. (2006) and the present study are unique because memantine improved memory in the absence of pharmacologic, genetic, or neurosurgical induction of impairment. However, the effects of memantine on radial arm maze performance in our study occurred at 0.3 and 0.56 mg/kg, which are considerably lower than the 5 and 7.5 mg/kg doses employed by Zoladz et al. (2006). In contrast, we found that 3 and 10 mg/kg resulted in severe performance deficits during phase 1. Similarly, (Creeley et al., 2006) reported that 5 and 10 mg/kg of memantine disrupted memory in female rats evaluated in a hole board task. The discrepancy in the effective dose range may reflect different response requirements of these tasks (i.e., running versus swimming). Also, the fact that the radial arm maze and hole board tasks are appetitively motivated, while the water maze is motivated by aversive stimuli, may have contributed to the differences.
In contrast to other NMDA receptor antagonists that are well known to disrupt learning and memory as well as to disrupt long term potentiation, memantine is an uncompetitive channel blocker with a rapid off- rate that ameliorates excessive NMDA receptor activation (Lipton, 2006). One of memantine’s greatest benefits in AD, as well as in other neurodegenerative diseases, is believed to be its neuroprotective effects (Lipton, 2006; Parsons et al., 1999; Rogawski, 2004; Van Dam and De Deyn, 2006). This hypothesis is supported in recent clinical trials in which memantine delayed the progression of symptoms in AD patients (Bullock, 2006) as well as in animal studies demonstrating that repeated administration of memantine prevents the development of amyloid β-induced memory impairments (Yamada et al., 2005) and age-dependent cognitive decline in the APP23 model of AD (Van Dam and De Deyn, 2006).
Memantine has actions other than NMDA antagonism that may also account for the effects observed here, such as antagonist effects at 5-hydroxytryptamine receptors (Rammes et al., 2001) and α7 nicotinic receptor channels (Aracava et al., 2005). In addition, memantine has been reported to increase brain-deprived neurotrophic factor (BDNF) mRNA (Marvanova et al., 2001). Of consequence, BDNF enhances the induction of long term potentiation in the hippocampus (Figurov et al., 1996). Moreover, an interaction between BDNF/trkB signaling and NMDA receptors has been found to have an important role in the development of spatial memory in the hippocampus (Mizuno et al., 2003).
There is considerable interest in combination pharmacotherapy to treat cognitive deficits in neurodegenerative diseases. In a randomized, double-blind, placebo-controlled clinical trial in AD patients, combination therapy of donepezil and memantine was more efficacious than monotherapy of donepezil alone (Schmitt et al., 2006; Tariot et al., 2004). While there is evidence demonstrating that memantine, rimonabant, and donepezil enhance performance in rodent models of learning and memory, few preclinical studies have examined whether co-administration of these drugs would enhance memory. We have previously found that subthreshold doses of donepezil and rimonabant given in combination increased memory duration (Wise et al., 2007). However, here we show that memantine given in combination with either donepezil or rimonabant failed to facilitate radial arm maze performance. While speculative, it is possible that increases in acetylcholine concentrations may account for the effect in the radial arm maze found with co-administration of rimonabant and donepezil, whereas co-administration with memantine, which has been shown to block α7 nicotinic receptor channels (Aracava et al., 2005; Maskell et al., 2003), would have had an opposite effect.
One concern of the present study is that the previous drug history of the rats may have adversely affected performance. However, the results of our final experiment suggest that previous drug history was not a relevant factor. Specifically, 0.3 mg/kg donepezil, which we previously found to prolong memory in the radial arm maze (Wise et al., 2007), and 0.3 mg/kg memantine continued to produce significant decreases in errors committed during the retrieval test. Additionally, the vehicle-treated animals committed the same number of errors during the retrieval test at the beginning of the study as at the end of the study. Combined treatment of a subthreshold dose of memantine and an effective dose of donepezil also failed to improve learning in a rabbit eye blink classical conditioning paradigm (Woodruff-Pak et al., 2006). It should be noted that in our experiments, all drugs were given acutely and were conducted in healthy animals. Thus, it is possible that chronic combination of these drugs would enhance choice accuracy in the radial arm maze task. In an amyloid β-induced memory impairment rat model, chronic infusion of memantine or repeated oral administration of donepezil enhanced performance in a delay-matched-to-position task (Yamada et al., 2005). However, combination dosing with these drugs failed to improve performance more than either drug administered alone (Yamada et al., 2005). Because an active dose of each drug was given, any potential potentiation was obscured by ceiling effects.
In conclusion, the present study demonstrates that the low to moderate uncompetitive NMDA receptor antagonist, memantine, decreases the number of re-entry errors in the test phase in a rat delay radial arm maze task when administered before the acquisition phase, but not after the acquisition phase or before the test phase. Co-administration of a subthreshold dose of memantine with a subthreshold dose of either rimonabant or donepezil before the acquisition phase did not enhance memory performance. These results suggest that low doses of memantine may facilitate acquisition rather than retrieval processes.
Acknowledgments
This research was supported by NIDA grants T23DA07027 and DA015683.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aracava Y, Pereira EF, Maelicke A, Albuquerque EX. Memantine blocks alpha7* nicotinic acetylcholine receptors more potently than n-methyl-D-aspartate receptors in rat hippocampal neurons. J Pharmacol Exp Ther. 2005;312:1195–1205. doi: 10.1124/jpet.104.077172. [DOI] [PubMed] [Google Scholar]
- Bullock R. Efficacy and safety of memantine in moderate-to-severe Alzheimer disease: the evidence to date. Alzheimer Dis Assoc Disord. 2006;20:23–29. doi: 10.1097/01.wad.0000201847.29836.a5. [DOI] [PubMed] [Google Scholar]
- Creeley C, Wozniak DF, Labruyere J, Taylor GT, Olney JW. Low doses of memantine disrupt memory in adult rats. J Neurosci. 2006;26:3923–3932. doi: 10.1523/JNEUROSCI.4883-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danysz W, Parsons CG. The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer’s disease: preclinical evidence. Int J Geriatr Psychiatry. 2003;18:S23–32. doi: 10.1002/gps.938. [DOI] [PubMed] [Google Scholar]
- Ferris S, Schneider L, Farmer M, Kay G, Crook T. A double-blind, placebo-controlled trial of memantine in age-associated memory impairment (memantine in AAMI) Int J Geriatr Psychiatry. 2007;22:448–455. doi: 10.1002/gps.1711. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- Marvanova M, Lakso M, Pirhonen J, Nawa H, Wong G, Castren E. The neuroprotective agent memantine induces brain-derived neurotrophic factor and trkB receptor expression in rat brain. Mol Cell Neurosci. 2001;18:247–258. doi: 10.1006/mcne.2001.1027. [DOI] [PubMed] [Google Scholar]
- Maskell PD, Speder P, Newberry NR, Bermudez I. Inhibition of human alpha 7 nicotinic acetylcholine receptors by open channel blockers of N-methyl-D-aspartate receptors. Br J Pharmacol. 2003;140:1313–1319. doi: 10.1038/sj.bjp.0705559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkeviciene R, Banerjee P, Tanila H. Memantine improves spatial learning in a transgenic mouse model of Alzheimer’s disease. J Pharmacol Exp Ther. 2004;311:677–682. doi: 10.1124/jpet.104.071027. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, He J, Nakajima A, Nabeshima T. Involvement of BDNF receptor TrkB in spatial memory formation. Learn Mem. 2003;10:108–115. doi: 10.1101/lm.56003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999;38:735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Rammes G, Rupprecht R, Ferrari U, Zieglgansberger W, Parsons CG. The N-methyl-D-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner. Neurosci Lett. 2001;306:81–84. doi: 10.1016/s0304-3940(01)01872-9. [DOI] [PubMed] [Google Scholar]
- Rogawski MA. What is the rationale for new treatment strategies in Alzheimer’s disease? CNS Spectr. 2004;9:6–12. doi: 10.1017/s1092852900024743. [DOI] [PubMed] [Google Scholar]
- Schmitt FA, van Dyck CH, Wichems CH, Olin JT. Cognitive response to memantine in moderate to severe Alzheimer disease patients already receiving donepezil: an exploratory reanalysis. Alzheimer Dis Assoc Disord. 2006;20:255–262. doi: 10.1097/01.wad.0000213860.35355.d4. [DOI] [PubMed] [Google Scholar]
- Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291:317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- Van Dam D, De Deyn PP. Cognitive evaluation of disease-modifying efficacy of galantamine and memantine in the APP23 model. Eur Neuropsychopharmacol. 2006;16:59–69. doi: 10.1016/j.euroneuro.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Wise LE, Iredale PA, Stokes RJ, Lichtman AH. Combination of Rimonabant and Donepezil Prolongs Spatial Memory Duration. Neuropsychopharmacology. 2007;32:1805–1812. doi: 10.1038/sj.npp.1301297. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Tobia MJ, Jiao X, Beck KD, Servatius RJ. Preclinical Investigation of the Functional Effects of Memantine and Memantine Combined with Galantamine or Donepezil. Neuropsychopharmacology. 2006;32:1284–1294. doi: 10.1038/sj.npp.1301259. [DOI] [PubMed] [Google Scholar]
- Yamada K, Takayanagi M, Kamei H, Nagai T, Dohniwa M, Kobayashi K, Yoshida S, Ohhara T, Takauma K, Nabeshima T. Effects of memantine and donepezil on amyloid beta-induced memory impairment in a delayed-matching to position task in rats. Behav Brain Res. 2005;162:191–199. doi: 10.1016/j.bbr.2005.02.036. [DOI] [PubMed] [Google Scholar]
- Zajaczkowski W, Frankiewicz T, Parsons CG, Danysz W. Uncompetitive NMDA receptor antagonists attenuate NMDA-induced impairment of passive avoidance learning and LTP. Neuropharmacology. 1997;36:961–971. doi: 10.1016/s0028-3908(97)00070-1. [DOI] [PubMed] [Google Scholar]
- Zajaczkowski W, Quack G, Danysz W. Infusion of (+) -MK-801 and memantine -- contrasting effects on radial maze learning in rats with entorhinal cortex lesion. Eur J Pharmacol. 1996;296:239–246. doi: 10.1016/0014-2999(95)00716-4. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Campbell AM, Park CR, Schaefer D, Danysz W, Diamond DM. Enhancement of long-term spatial memory in adult rats by the noncompetitive NMDA receptor antagonists, memantine and neramexane. Pharmacol Biochem Behav. 2006;85:298–306. doi: 10.1016/j.pbb.2006.08.011. [DOI] [PubMed] [Google Scholar]


