Abstract
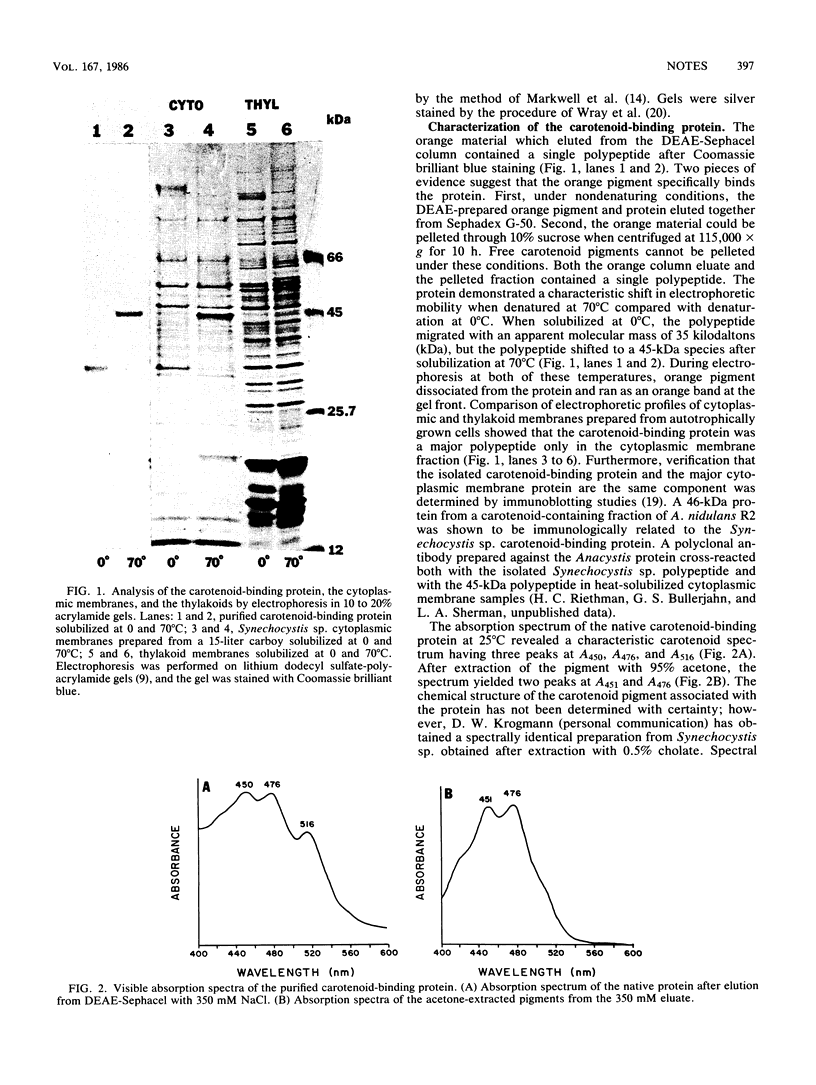
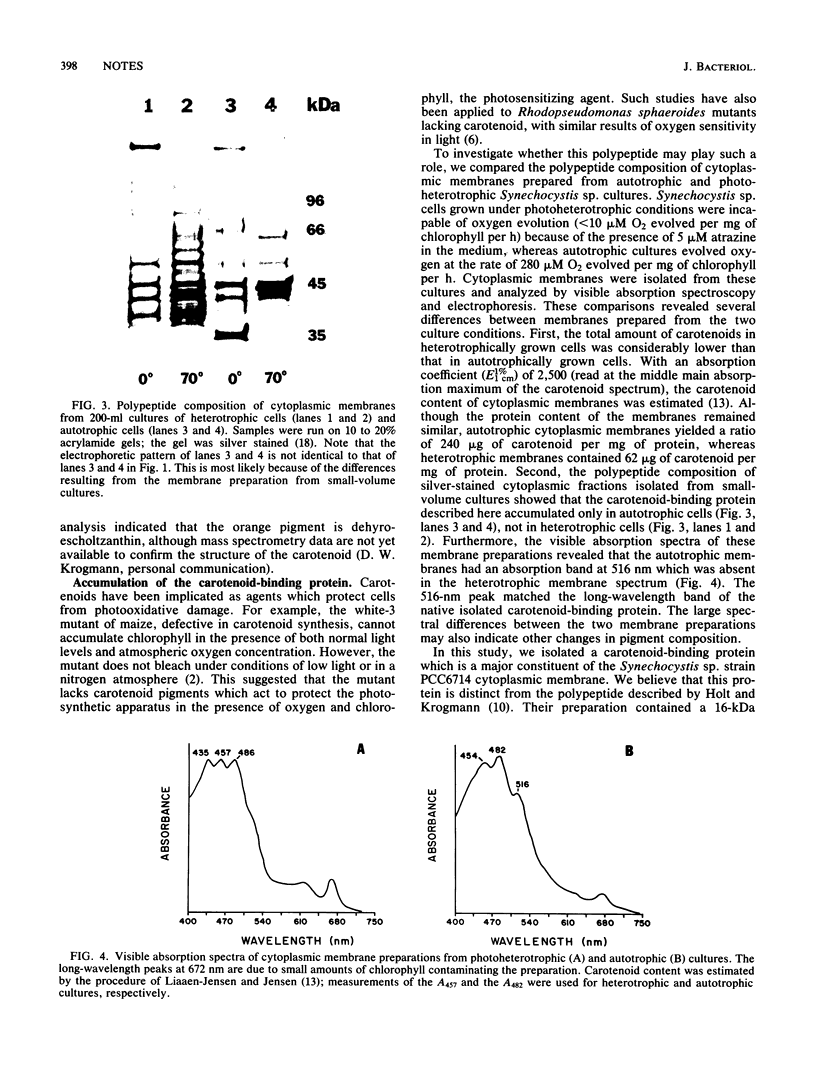
We isolated a carotenoid-binding protein from the cytoplasmic membrane of the cyanobacterium Synechocystis sp. strain PCC6714. The polypeptide demonstrated a characteristic mobility shift when electrophoresed in lithium dodecyl sulfate-polyacrylamide gels. The protein migrated with an apparent molecular mass of 35 kilodaltons when solubilized at 0 degrees C, but after solubilization at 70 degrees C, the protein migrated as a 45-kilodalton species. The carotenoid-binding protein accumulated only in autotrophically grown cells; cytoplasmic membranes prepared from photoheterotrophically grown cells lacked this component.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. C., Robertson D. S. Role of Carotenoids in Protecting Chlorophyll From Photodestruction. Plant Physiol. 1960 Jul;35(4):531–534. doi: 10.1104/pp.35.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullerjahn G. S., Riethman H. C., Sherman L. A. Organization of the thylakoid membrane from the heterotrophic cyanobacterium, Aphanocapsa 6714. Biochim Biophys Acta. 1985 Nov 27;810(2):148–157. doi: 10.1016/0005-2728(85)90130-6. [DOI] [PubMed] [Google Scholar]
- DWORKIN M. Endogenous photosensitization in a carotenoidless mutant of Rhodopseudomonas speroides. J Gen Physiol. 1958 Jul 20;41(6):1099–1112. doi: 10.1085/jgp.41.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Vartanian M., Joset-Espardellier F., Astier C. Contributions of Respiratory and Photosynthetic Pathways during Growth of a Facultative Photoautotrophic Cyanobacterium, Aphanocapsa 6714. Plant Physiol. 1981 Oct;68(4):974–978. doi: 10.1104/pp.68.4.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Metronidazole and the isolation of temperature-sensitive photosynthetic mutants in cyanobacteria. J Bioenerg Biomembr. 1980 Aug;12(3-4):277–295. doi: 10.1007/BF00744689. [DOI] [PubMed] [Google Scholar]
- Jürgens U. J., Weckesser J. Carotenoid-containing outer membrane of Synechocystis sp. strain PCC6714. J Bacteriol. 1985 Oct;164(1):384–389. doi: 10.1128/jb.164.1.384-389.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]