Abstract
Background
Hippocampal volumes previously determined in monkeys by magnetic resonance imaging are used to test the hypothesis that small hippocampi predict increased stress levels of adrenocorticotropic hormone (ACTH).
Methods
Plasma ACTH levels were measured after restraint stress in 19 male monkeys pretreated with saline or hydrocortisone. Monkeys were then randomized to an undisturbed control condition or intermittent social separations followed by new pair formations. After 17-months of exposure to the intermittent social manipulations, restraint stress tests were repeated to determine test/retest correlations.
Results
Individual differences in post-restraint stress ACTH levels over the 17-month test/retest interval were remarkably consistent for the saline (rs=0.82, P=0.0004) and hydrocortisone (rs=0.78, P=0.001) pretreatments. Social manipulations did not affect post-restraint stress ACTH levels, but monkeys with smaller hippocampal volumes responded to restraint after saline pretreatment with greater increases in ACTH levels with total brain volume variation controlled as a statistical covariate (β= −0.58, P=0.031). Monkeys with smaller hippocampal volumes also responded with diminished sensitivity to glucocorticoid feedback determined by greater post-restraint ACTH levels after pretreatment with hydrocortisone (β= −0.68, P=0.010).
Conclusions
These findings support clinical reports that small hippocampi may be a risk for impaired regulation of the hypothalamic-pituitary-adrenal axis in humans with stress-related psychiatric disorders.
Keywords: hippocampus, HPA axis, stress, ACTH, glucocorticoid feedback
INTRODUCTION
Hippocampal volumes are smaller in humans with stress-related psychiatric disorders compared to healthy controls (1, 2). Studies of humans (3–5) and animal models (6, 7) suggest that excessive stress levels of cortisol are a cause of hippocampal volume loss. Far less researched, but of equal importance, are indications that small hippocampi may also represent a risk factor for impaired regulation of the hypothalamic-pituitary-adrenal (HPA) axis response to stress (8–12).
Opportunities to study the causes and consequences of hippocampal volume variation are limited in humans with stress-related psychiatric disorders. Therefore, we recently examined the effects of early experiences and inherited variation in squirrel monkey hippocampal volumes (13). Paternal half-siblings raised apart from one another by different mothers in the absence of fathers were randomized to three postnatal conditions at 10 weeks of age (see supplementary materials). After weaning, at 9 months of age, all monkeys were socially housed in identical laboratory conditions. Sexual maturity occurs at 2–3 years of age, and the average maximum lifespan for squirrel monkeys is 21 years (14). In early adulthood, at 5 years of age (range=3.7–6.0 years), hippocampal volumes were determined from T1-weighted brain images (Fig. 1A). Image acquisition and processing details are provided in the supplementary materials.
Figure 1.
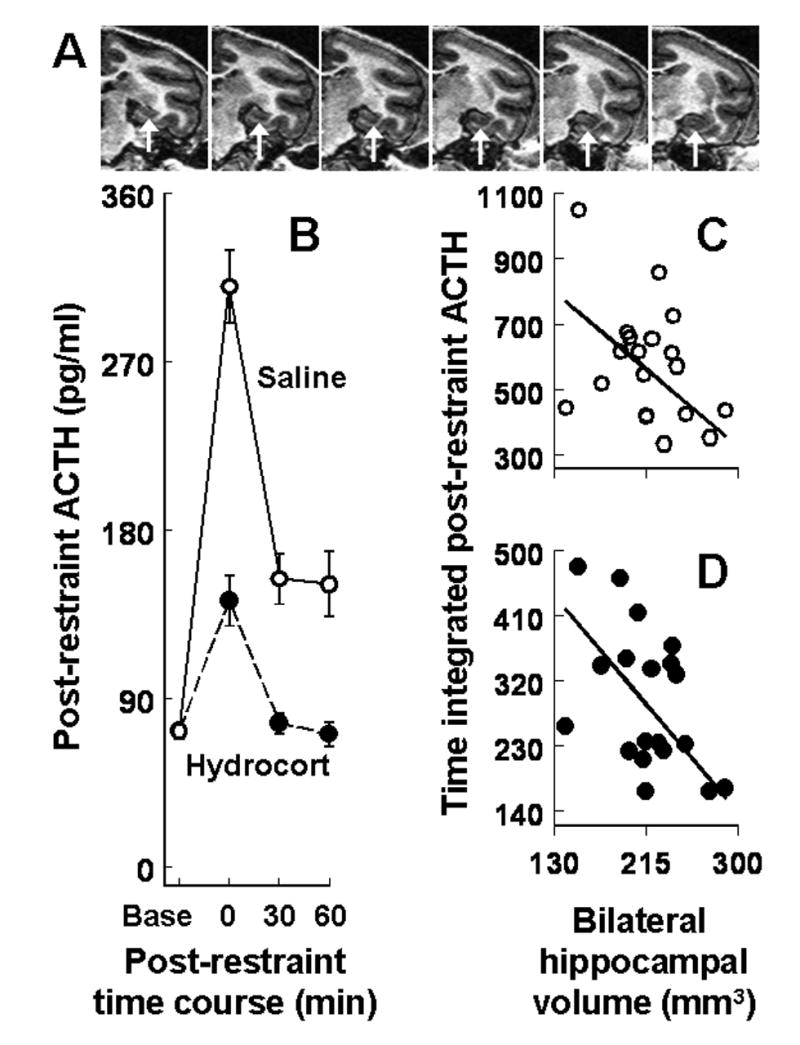
Hippocampal volume predicts post-restraint stress levels of ACTH. A. Representative magnetic resonance images of squirrel monkey hippocampus (arrows) at 1 mm intervals in the coronal plane. B. Post-restraint stress ACTH levels averaged across the 17-month test/retest interval for pretreatment with saline or hydrocortisone (mean ± SEM). Bilateral hippocampal volumes regressed on time integrated post-restraint ACTH levels in 19 adult male monkeys pretreated with (C) saline or (D) hydrocortisone.
Hippocampal volumes in the young adult monkeys did not differ significantly with respect to prior postnatal treatment-related differences in plasma cortisol levels at weaning (15). However, in keeping with studies of humans (16, 17), significant heritabilities were discerned in monkeys by paternal half-sibling analysis of left and right hippocampal volumes considered separately or combined (15). Here we investigate in the same monkeys whether these hippocampal measures predict subsequent post-stress levels of adrenocorticotropic hormone (ACTH) after pretreatment with saline or hydrocortisone. The dose of hydrocortisone used to assess glucocorticoid feedback is known to suppress stress-induced increases in squirrel monkey ACTH (18). Plasma ACTH levels are measured because endogenous cortisol cannot be distinguished from exogenous hydrocortisone. Only the males from our previous studies are examined because cyclical ovarian hormone effects on HPA-axis activity are difficult to control for in female monkeys.
METHODS
At 8.5 years of age (range=6.4–10.6 years), 19 pair-housed adult male squirrel monkeys were restrained for two separate 30-minute sessions in a standard primate chair. Restraint is a well-studied psychological stressor in animal biomedical research (19). An intramuscular saline injection was given 60 minutes before the first restraint test. Seven days later, an intramuscular injection of 2.5 mg/kg hydrocortisone sodium succinate was given 60 minute before the second and otherwise identical restraint test. All procedures were conducted in accordance with NIH guidelines, and were approved by Stanford University’s Panel on Laboratory Animal Care.
Immediately after each stress test, a blood sample was collected and monkeys were returned to the home cage. Subsequent samples were collected 30 and 60 minutes later to provide post-stress measures of recovery. Additional samples were also collected seven days before and seven days after the saline and hydrocortisone restraint stress tests to measure ACTH levels at baseline in home cage conditions. All samples were obtained as described elsewhere (supplementary materials) from manually restrained monkeys by femoral venipuncture between 13:30–14:30 hours to control for diurnal variation (20). Plasma ACTH levels were measured in duplicate with an established radioimmunoassay (21).
After the initial restraint stress tests, monkeys were randomized to the following adult treatment conditions. In one condition, 10 monkeys were exposed to six intermittent social separations that each lasted 3 weeks in duration. During each social separation session, monkeys were individually housed, and could see, hear, smell, but not touch other monkeys. After each intermittent separation, new pairs were formed and maintained for 9 weeks. New pair formations (22) and social separations (23) increase plasma cortisol levels in adult squirrel monkeys. In the undisturbed control condition, adult monkeys were housed with the same companion in stable same-sex pairs. Hippocampal volumes from 9 of 10 pair-housed control monkeys were available for analysis. Randomization to the adult conditions was stratified by prior postnatal condition to provide similar size samples in the factorial design (see supplementary materials). Ten weeks after the final separation, when all of the monkeys were housed in pairs, restraint stress tests were repeated to determine test/retest correlations.
Time integrated post-restraint ACTH levels were determined with the trapezoidal rule to estimate the area under each monkey’s saline and hydrocortisone curves. For each of these measures, Spearman correlations were used to evaluate the consistency of individual differences over the 17-month test/retest interval. The hypothesis that hippocampal volume predicts time integrated post-restraint ACTH levels after saline or hydrocortisone was assessed using linear least squares regressions with total brain volume variation controlled as a statistical covariate. Adult social manipulations and postnatal conditions were subsequently added to the analysis to statistically control for systematic experience-dependent effects. All test statistics were evaluated with two-tailed probabilities (P<0.05).
RESULTS
Individual differences in time integrated post-restraint ACTH levels were remarkably consistent over the 17-month test/retest interval for the saline (rs=0.82, P=0.0004) and hydrocortisone (rs=0.78, P=0.001) pretreatments. As expected from previous research (18), restraint stress after saline robustly increased ACTH levels and pretreatment with hydrocortisone suppressed post-restraint ACTH levels compared to pretreatment with saline (Fig 1B). Monkeys with greater time integrated post-restraint ACTH levels averaged across the test/retest interval for saline responded with greater time integrated post-restraint ACTH levels after hydrocortisone (rs=0.64, P=0.006).
Monkeys with smaller hippocampal volumes (left and right combined) responded to restraint after saline pretreatment with greater time integrated ACTH levels averaged across the test/retest interval with total brain volume variation controlled as a statistical covariate (β= −0.58, P=0.031; Figure 1C). Monkeys with smaller hippocampal volumes also responded with diminished sensitivity to glucocorticoid feedback determined by greater time integrated post-restraint ACTH levels after hydrocortisone (β= −0.68, P=0.010; Figure 1D). A significant hippocampal volume main effect (F(1,11)=5.85, P<0.034) was also discerned with the postnatal and adult social manipulations examined in a single mixed factor ANOVA with post-restraint ACTH levels after saline and hydrocortisone included as repeated measures. Adult social manipulations and postnatal effects were not significant in the omnibus ANOVA for all of the monkeys but ACTH levels were, on average, 24 percent greater during the follow-up retests compared to the initial test sessions (F(1,11)=5.26, P=0.043).
DISCUSSION
These findings suggest that naturally occurring hippocampal volume variation in monkeys predicts consistent individual differences in post-stress ACTH levels after saline or hydrocortisone pretreatment. Hippocampal lesions likewise increase the duration and peak levels of stress-induced HPA-axis activation and impair negative feedback determined by glucocorticoid administration in rodents (24–26). Impaired glucocorticoid feedback occurs in stress-related psychiatric disorders (27, 28), but evidence for hippocampal regulation of the HPA-axis in humans (29, 30) and monkeys (31) is limited to studies previously conducted at baseline in stress-free conditions.
Over the 17-month test/retest interval, post-stress ACTH levels were increased on average by 24 percent. Despite this normative age-related increase, the relative rank order of individual differences was consistent over time. These observations concur with reports that aging increases the HPA-axis response to stress in humans (32) and monkeys (18), and correspond with 1-year test/retest correlations of ~0.70 for human post-stress ACTH levels (33). Longitudinal evidence further suggests that 3.5- and 5-year test/retest correlations are ~0.90 for hippocampal volumes in humans between 14–83 years of age (34, 35).
The results of this study should be interpreted in the context of potential limitations. Our findings for males may or may not hold true for females. Small samples diminished the power to detect heritable variation and related interactions between hippocampal volumes and experience-dependent changes in post-stress ACTH levels. Saline and hydrocortisone pretreatments were not counterbalanced, but test-order acclimation is unlikely because several restraint sessions are needed for HPA-axis acclimation in monkeys (36, 37). Exposure to adult social stress did not enhance the restraint stress response in contrast to studies of rats (38). Paired-housing of monkeys after each separation may have blocked the expected stress facilitation effect.
In summary, this study of monkeys suggests that small hippocampi may be a risk for impaired regulation of the HPA-axis response to psychological stress. Similar studies of humans are needed because HPA-axis dysregulation is often a feature of stress-related psychiatric disorders (27, 28). Identification of risk factors for stress-related psychiatric disorders may help to define patient populations most likely to benefit from preventative interventions.
Supplementary Material
Supplementary material is provided for relevant methodological details
Acknowledgments
Supported by the Nancy Pritzker Network for the Study of Depression, and Public Health Service grants DA16902, MH50604, and MH47573.
Footnotes
FINANCIAL DISCLOSURES
The authors declare no conflicting financial or other competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–66. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 3.Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–26. [PMC free article] [PubMed] [Google Scholar]
- 4.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–43. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing’s disease. Biol Psychiatry. 1999;46:1595–602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- 6.McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54:200–7. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 7.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–35. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 8.Frodl T, Meisenzahl EM, Zetzsche T, Hohne T, Banac S, Schorr C, et al. Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. J Clin Psychiatry. 2004;65:492–9. doi: 10.4088/jcp.v65n0407. [DOI] [PubMed] [Google Scholar]
- 9.Sapolsky RM, Plotsky PM. Hypercortisolism and its possible neural bases. Biol Psychiatry. 1990;27:937–52. doi: 10.1016/0006-3223(90)90032-w. [DOI] [PubMed] [Google Scholar]
- 10.Schatzberg AF. Major depression: causes or effects? Am J Psychiatry. 2002;159:1077–1079. [Google Scholar]
- 11.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–7. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wignall EL, Dickson JM, Vaughan P, Farrow TF, Wilkinson ID, Hunter MD, et al. Smaller hippocampal volume in patients with recent-onset posttraumatic stress disorder. Biol Psychiatry. 2004;56:832–6. doi: 10.1016/j.biopsych.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Lyons DM. Stress, depression, and inherited variation in primate hippocampal and prefrontal brain development. Psychopharmacol Bull. 2002;36:27–43. [PubMed] [Google Scholar]
- 14.Brady AG. Research techniques for the squirrel monkey (Saimiri) ILAR J. 2000;41:10–18. doi: 10.1093/ilar.41.1.10. [DOI] [PubMed] [Google Scholar]
- 15.Lyons DM, Yang C, Sawyer-Glover AM, Moseley ME, Schatzberg AF. Early life stress and inherited variation in monkey hippocampal volumes. Arch Gen Psychiatry. 2001;58:1145–51. doi: 10.1001/archpsyc.58.12.1145. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan EV, Pfefferbaum A, Swan GE, Carmelli D. Heritability of hippocampal size in elderly twin men: equivalent influence from genes and environment. Hippocampus. 2001;11:754–62. doi: 10.1002/hipo.1091. [DOI] [PubMed] [Google Scholar]
- 17.van Erp TG, Saleh PA, Huttunen M, Lonnqvist J, Kaprio J, Salonen O, et al. Hippocampal volumes in schizophrenic twins. Arch Gen Psychiatry. 2004;61:346–53. doi: 10.1001/archpsyc.61.4.346. [DOI] [PubMed] [Google Scholar]
- 18.Lyons DM, Yang C, Eliez S, Reiss AL, Schatzberg AF. Cognitive correlates of white matter growth and stress hormones in female squirrel monkey adults. J Neurosci. 2004;24:3655–62. doi: 10.1523/JNEUROSCI.0324-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glavin GB, Pare WP, Sandbak T, Bakke HK, Murison R. Restraint stress in biomedical research: an update. Neurosci Biobehav Rev. 1994;18:223–49. doi: 10.1016/0149-7634(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 20.Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23:3555–60. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyons DM, Ha CM, Levine S. Social effects and circadian rhythms in squirrel monkey pituitary-adrenal activity. Horm Behav. 1995;29:177–90. doi: 10.1006/hbeh.1995.1013. [DOI] [PubMed] [Google Scholar]
- 22.Coe CL, Franklin D, Smith ER, Levine S. Hormonal responses accompanying fear and agitation in the squirrel monkey. Physiol Behav. 1982;29:1051–7. doi: 10.1016/0031-9384(82)90297-9. [DOI] [PubMed] [Google Scholar]
- 23.Lyons DM, Wang OJ, Lindley SE, Levine S, Kalin NH, Schatzberg AF. Separation induced changes in squirrel monkey hypothalamic-pituitary-adrenal physiology resemble aspects of hypercortisolism in humans. Psychoneuroendocrinology. 1999;24:131–42. doi: 10.1016/s0306-4530(98)00065-1. [DOI] [PubMed] [Google Scholar]
- 24.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–80. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Dallman MF, Akana SF, Levin N, Walker CD, Bradbury MJ, Suemaru S, et al. Corticosteroids and the control of function in the hypothalamo-pituitary-adrenal (HPA) axis. Ann N Y Acad Sci. 1994;746:22–31. doi: 10.1111/j.1749-6632.1994.tb39206.x. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–34. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 27.Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res. 1994;28:341–56. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 28.de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res. 2006;40:550–67. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Buchanan TW, Kern S, Allen JS, Tranel D, Kirschbaum C. Circadian regulation of cortisol after hippocampal damage in humans. Biol Psychiatry. 2004;56:651–6. doi: 10.1016/j.biopsych.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Wolf OT, Convit A, de Leon MJ, Caraos C, Qadri SF. Basal hypothalamo-pituitary-adrenal axis activity and corticotropin feedback in young and older men: relationships to magnetic resonance imaging-derived hippocampus and cingulate gyrus volumes. Neuroendocrinology. 2002;75:241–9. doi: 10.1159/000054715. [DOI] [PubMed] [Google Scholar]
- 31.Sapolsky RM, Zola-Morgan S, Squire LR. Inhibition of glucocorticoid secretion by the hippocampal formation in the primate. J Neurosci. 1991;11:3695–704. doi: 10.1523/JNEUROSCI.11-12-03695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veldhuis JD, Keenan DM, Roelfsema F, Iranmanesh A. Aging-related adaptations in the corticotropic axis: modulation by gender. Endocrinol Metab Clin North Am. 2005;34:993–1014. doi: 10.1016/j.ecl.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Burleson MH, Poehlmann KM, Hawkley LC, Ernst JM, Berntson GG, Malarkey WB, et al. Neuroendocrine and cardiovascular reactivity to stress in mid-aged and older women: long-term temporal consistency of individual differences. Psychophysiology. 2003;40:358–69. doi: 10.1111/1469-8986.00039. [DOI] [PubMed] [Google Scholar]
- 34.Liu RS, Lemieux L, Bell GS, Sisodiya SM, Shorvon SD, Sander JW, et al. A longitudinal study of brain morphometrics using quantitative magnetic resonance imaging and difference image analysis. Neuroimage. 2003;20:22–33. doi: 10.1016/s1053-8119(03)00219-2. [DOI] [PubMed] [Google Scholar]
- 35.Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional Brain Changes in Aging Healthy Adults: General Trends, Individual Differences and Modifiers. Cereb Cortex. 2005 doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 36.Golub MS, Anderson JH. Adaptation of pregnant rhesus monkeys to short-term chair restraint. Lab Anim Sci. 1986;36:507–11. [PubMed] [Google Scholar]
- 37.Ruys JD, Mendoza SP, Capitanio JP, Mason WA. Behavioral and physiological adaptation to repeated chair restraint in rhesus macaques. Physiol Behav. 2004;82:205–13. doi: 10.1016/j.physbeh.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 38.Bhatnagar S, Vining C. Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Horm Behav. 2003;43:158–65. doi: 10.1016/s0018-506x(02)00011-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is provided for relevant methodological details


