SUMMARY
While the motor and attentional roles of the frontal eye field (FEF) are well documented, the relationship between them is unknown. We exploited the known influence of visual motion on the apparent position of targets, and measured how this illusion affects saccadic eye movements during FEF microstimulation. Without microstimulation, saccades to a moving grating are biased in the direction of motion, consistent with the apparent position illusion. Here we show that microstimulation of spatially-aligned FEF representations increases the influence of this illusion on saccades. Rather than simply impose a fixed-vector signal, subthreshold stimulation directed saccades away from the FEF movement field, and instead more strongly in the direction of visual motion. These results demonstrate that the attentional effects of FEF stimulation govern visually-guided saccades, and suggest that the two roles of the FEF work together to select both the features of a target and the appropriate movement to foveate it.
INTRODUCTION
A crucial component of visually-guided behavior is the accurate positioning of visual targets onto the two foveae via saccadic eye movements. The transformation of visual target features into saccade commands takes place several times per second, and the accuracy of foveal placement largely determines the speed at which the details within the visual environment can be processed. For example, the landing position of saccades made to single words during reading strongly influences the likelihood that words will be comprehended or will need to be re-fixated (Vitu et al., 1990). The contribution of saccades to visual discrimination and perception has long been appreciated (Dodge, 1900; Yarbus, 1967; Kowler and Steinman, 1977; He and Kowler, 1992), but to date surprisingly little is known about the neural mechanisms that transform visual information into specific saccade plans. Perhaps this lack of knowledge stems from the fact that studies of structures involved in this transformation have typically considered either saccade production or visual attention, but not both.
The involvement of the frontal eye field (FEF) and superior colliculus (SC) in saccade production has long been recognized, due in part to the fact that saccades can be evoked by electrical microstimulation of either region (Robinson and Fuchs, 1969; Robinson, 1972). A number of past studies have examined the role of the FEF and SC in saccade production by investigating the interaction of stimulation-evoked saccade signals and endogenous saccade plans. When microstimulation of either structure is delivered concurrently with the execution of a voluntary, visually-guided saccade, the resulting saccade vector is a weighted average of the voluntary vector, determined by the visual target, and the electrically-evoked vector (Schiller and Sandell, 1983; Sparks and Mays, 1983). Saccade vector averaging also results from microstimulation delivered during the preparation of a voluntary saccade (Kustov and Robinson, 1996; Gold and Shadlen, 2000; Barborica and Ferrera, 2004; Juan et al., 2004). Even low-frequency microstimulation of the SC, which is not sufficient to evoke a saccade, biases the direction and amplitude of spontaneous and visually-guided eye movements toward the center of the movement field (MF) of the stimulation site (Glimcher and Sparks, 1993).
In addition to their known roles in the production of saccades, the FEF and the SC have more recently been implicated in the control of visual spatial attention. Subthreshold electrical microstimulation of the FEF (Moore and Fallah, 2001; Moore and Fallah, 2004) or of the intermediate layers of the SC (Cavanaugh and Wurtz, 2004; Muller et al., 2005; Cavanaugh et al., 2006) facilitates performance on spatial attention tasks in monkeys. Reversible inactivation of either structure also impairs performance on visual search tasks (McPeek and Keller, 2004; Wardak et al., 2006), and microstimulation of the FEF results in an attention-like modulation of responses in visual cortex (Moore and Armstrong, 2003; Armstrong et al., 2006). Furthermore, recent evidence has shown that FEF microstimulation increases the ability of V4 neurons to discriminate visual stimuli (Armstrong and Moore, 2007), as does voluntary spatial attention (McAdams and Maunsell, 1999). Taken together, this evidence suggests that spatial attention is driven at least in part by these structures. Yet, it remains unclear how the role of the FEF and the SC in visual attention coexists with their known role in saccade production.
In contrast to previous studies which investigated either saccade production or visual attention, but not both, we examined the relationship between these two functions using microstimulation of the FEF. Specifically, we studied the mechanism controlling placement of the foveae onto visual targets during voluntary saccades. We exploited the known influence of visual motion on the apparent positions of visual targets using a saccade task. Motion contained within a stationary aperture distorts the perception of visual space, shifting the subjective location of nearby objects and of the aperture itself (Ramachandran and Anstis, 1990; De Valois and De Valois, 1991; Nishida and Johnston, 1999; Whitney and Cavanagh, 2000; Whitney, 2002). Furthermore, it is known that spatial attention augments both the perceived contrast (Carrasco et al., 2004) and perceived speed (Turatto et al., 2007) of moving gratings. For example, Turatto et al. (2007) showed that a spatial cue presented near the location of one of two drifting gratings, and immediately before the grating onset, makes subjects perceive the motion as faster than that of the non-cued grating. These findings are consistent with the report that attention influences the apparent position illusion itself (Whitney, 2006). We found that the vectors of voluntary saccades targeting a drifting grating deviated away from the center of the grating, and were biased in the direction of motion, consistent with previous observations (Moore et al., 2001; Gross et al., 2004; Xiao et al., 2006) and with the apparent position illusion. We used this directional deviation to measure the “motion-induced bias” (MIB) of saccades. We found that subthreshold (low-frequency) stimulation of the FEF during the planning and execution of these saccades did not result in the averaging of visually-guided and stimulated vectors, as would be expected if microstimulation injected a dominant, fixed-vector motor signal. Instead, microstimulation increased the influence of the apparent position illusion on the MIB, causing saccades to deviate away from the center of the FEF MF, and more strongly in the direction of visual motion. These results indicate that the attentional effects of microstimulation determine the metrics of concurrently-planned saccades, causing them to be more strongly influenced by the visual target features. Therefore, although the saccadic and visual attention roles of the FEF can be experimentally dissociated (Juan et al., 2004; Murthy et al., 2001; Chambers and Mattingley, 2005), as can the contributions of different FEF neuronal subpopulations to these two functions (Thompson et al., 2005), our results suggest that the saccadic role depends on the attentional role to select the features of the visual target and the best movement to foveate it.
RESULTS
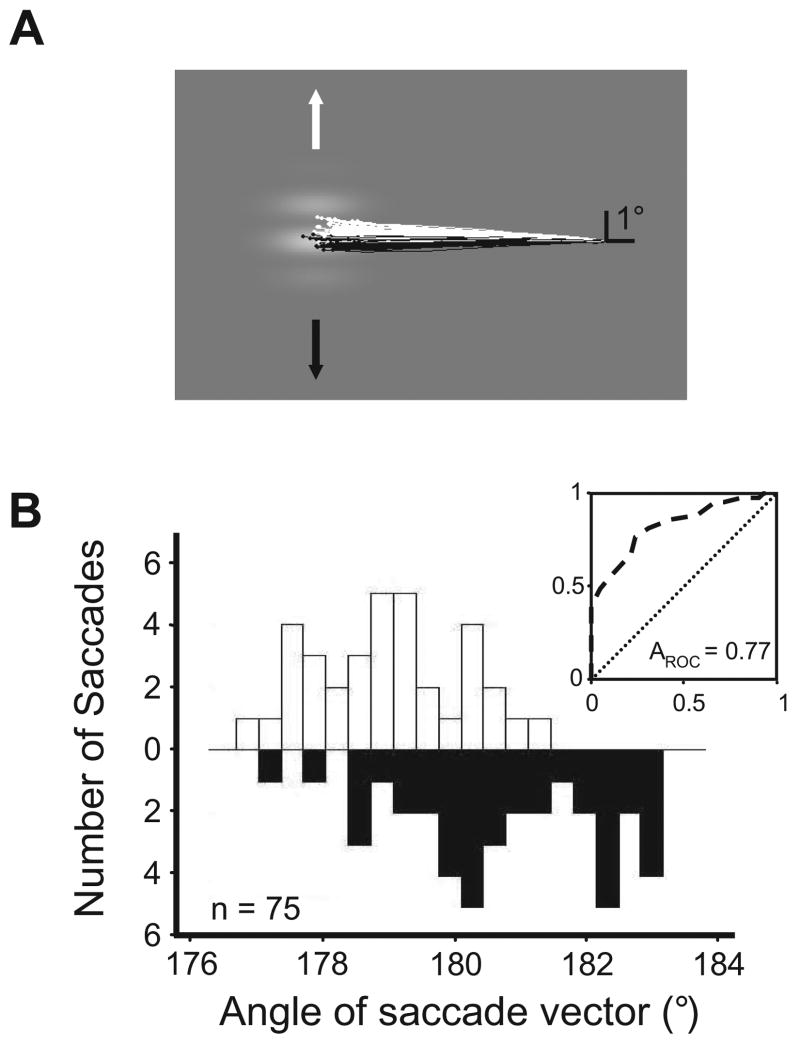
To examine the effect of FEF stimulation on the MIB of saccades, we took advantage of the known influence of visual motion on the perceived position of targets using a saccade task in monkeys. We first quantified the impact of grating motion contained within a stationary aperture on saccades targeting the aperture. The grating drifted in one of the two directions orthogonal to the saccade needed to foveate the aperture (Figure 1A). Thus, for example, an aperture directly left of fixation would contain a grating that drifted either upward or downward on a given trial. We examined the influence of grating motion on the distribution of angles of saccades made to the aperture. We compared the distributions of saccade angles made to gratings drifting in opposing directions using a receiver-operating characteristic (ROC) analysis (Green and Swets, 1966) (Figure 1B). We chose this analysis in order to provide an index of an ideal observer’s performance at judging the direction of grating motion using only the saccades. Saccades made to target apertures were influenced by grating motion, such that the direction of motion could be inferred from the saccade angle with performance greater than that expected by chance. This influence of visual motion on the angles of saccades did not depend on the behavioral relevance of grating motion, as the monkey was rewarded regardless of where a saccade landed within the stationary aperture.
Figure 1. Measuring the motion-induced bias (MIB) of saccadic eye movements.
(A) Directional bias of saccades to a drifting grating. Eye position traces show voluntary saccades to a sinusoidal grating that drifted either upward (white traces and arrows) or downward (black traces and arrows). Monkeys were rewarded for saccades landing anywhere within the target grating.
(B) Distributions of saccade vector angles and ROC analysis. Data are from a different experiment than that shown in (A). Distributions of saccade angles to gratings drifting in opposite directions (white and black) were used to generate an ROC curve (inset), the area under which (AROC) determines the amount of MIB.
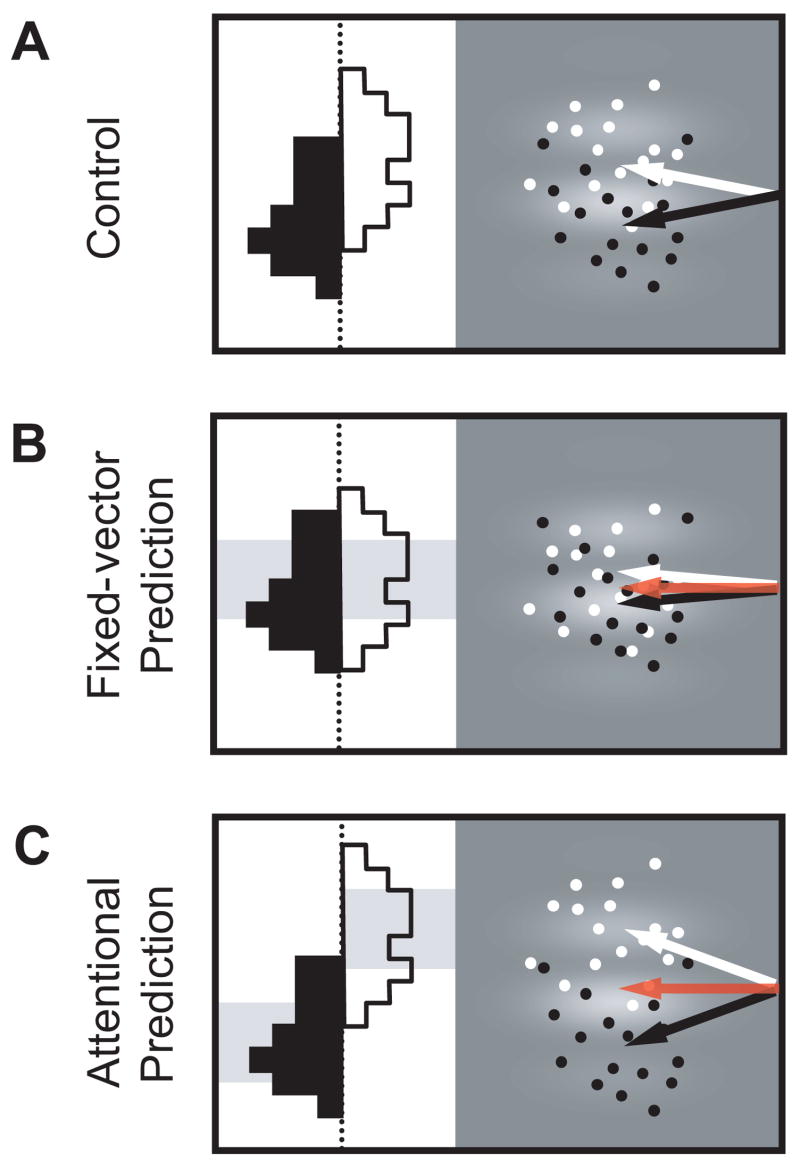
We studied the effects of subthreshold (low-frequency) microstimulation on the MIB of saccades at 35 FEF sites in two monkeys. We considered two possible effects of stimulation on voluntary saccades to the grating stimuli. First, the motor effects of microstimulation might dominate the attentional ones, imposing a fixed-vector signal on the MIB, independent of the direction of drift of the grating (Figure 2A,B). This signal would take the form of a constant saccade plan that would average with the endogenous plan. According to this fixed-vector prediction, the MIB would be decreased as the monkey’s saccade would be driven toward the center of the FEF MF equally on all trials. Alternatively, the attentional effects of FEF stimulation might dominate and increase the MIB for saccades to MF targets (Figure 2C). According to this attentional prediction, saccade plans would be specified by the attentional effects of microstimulation rather than the motor effects, and thus the stimulation-driven bias in the monkey’s saccade plans would depend on the direction of grating motion. As a result, the saccades should deviate away from the MF center.
Figure 2. Possible effects of FEF stimulation on the MIB.
(A) MIB on control trials. Without microstimulation, saccade vectors are moderately influenced by grating motion. Points indicate hypothetical endpoints of saccades on trials with upward (white) and downward (black) grating motion; arrows indicate the mean saccade vectors. Histograms depict the distributions of saccade angles.
(B) Fixed-vector prediction. Red arrow indicates the representative evoked saccade vector at the FEF site using suprathreshold (high-frequency) stimulation. Gray shaded region behind the histograms illustrates the fixed-vector bias of subthreshold (low-frequency) microstimulation, which is constant regardless of the direction of grating motion. If the motor effects of stimulation dominate, then the mean saccade vectors on trials with upward (white arrows) and downward (black arrows) grating motion are driven toward the electrically-evoked vector, and are thus more similar than in (A), leading to a decrease in the MIB.
(C) Attentional prediction. Shaded regions again show the bias of microstimulation on saccade endpoints, this time away from the location of the evoked vector (red arrow) and dependent on the direction of grating motion. Mean saccade vectors (white and black arrows) deviate away from the electrically-evoked vector, leading to an increase in the MIB compared to (A).
To investigate these possible effects of microstimulation, we trained monkeys to make a saccade to one of two drifting gratings to receive a juice reward (Figure 3A). Since we expected microstimulation to increase the probability of saccades to targets aligned with the FEF MF (Schiller and Tehovnik, 2001), we used two simultaneous targets to allow us to measure that choice effect. We could therefore use the change in the fraction of saccades to the MF target both to confirm and to measure the efficacy of stimulation on the FEF site. Furthermore, since previous studies of FEF stimulation have shown that the presence of visual distracters is necessary for obtaining effects on attention and its correlates in visual cortex (Moore & Fallah, 2004; Moore & Armstrong, 2003), using two simultaneous targets also provided each choice trial with a non-chosen, distracter stimulus. To encourage the monkey to distribute saccades between the two targets, the occurrence of the reward following each saccade was determined by a variable schedule in which the probability of receiving a reward for choosing one target decreased as it was chosen more often, while the probability of receiving a reward for choosing the opposite target increased (see Experimental Procedures).
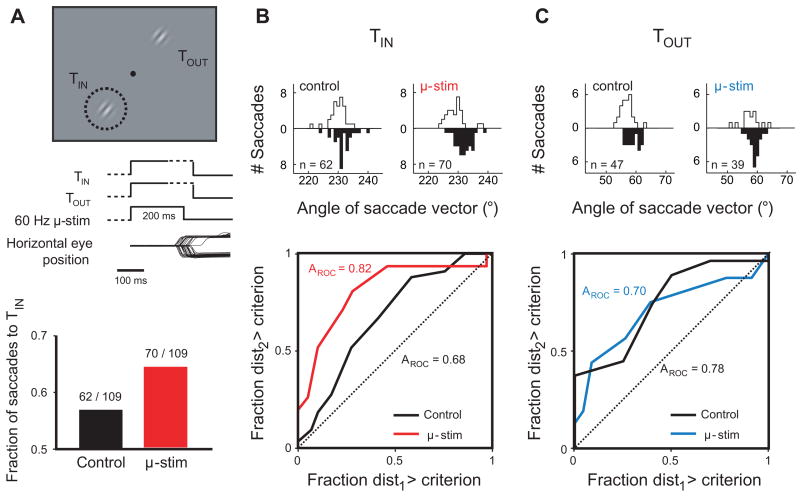
Figure 3. Example experiment.
(A) Task design and effect of subthreshold (low-frequency) FEF stimulation on the choice of saccade target. The dashed circle circumscribes the MF of the stimulation site, mapped prior to the experiment using suprathreshold (high-frequency) stimulation. TIN and TOUT refer to the visual targets placed at the center of, and directly opposite, the MF, respectively. Event plots indicate the sequence of appearance and disappearance of the visual targets and the duration of microstimulation; dashed lines denote variable time intervals. Horizontal eye position traces are from a subset of trials from this experiment, and show choice saccades to both TIN (downward deflecting traces) and TOUT (upward deflecting traces). Bar graph at bottom shows the effect of microstimulation on the fraction of saccades to TIN. Numbers above each bar are the number of TIN choices over the number of trials during each condition.
(B) Effect of subthreshold microstimulation on the MIB of TIN saccades. At top, distributions of angles of saccade vectors (as in Figure 1B) to TIN during control (left) and microstimulation (right) trials. At bottom, ROC curves and areas (AROC) resulting from these distributions.
(C) Effect of subthreshold microstimulation on the MIB of TOUT saccades.
Figure 3 shows the results of a representative experiment. Prior to the experiment, suprathreshold, high-frequency (200 Hz) stimulation was used to map the saccades evoked from this FEF site. The mean evoked vector had an amplitude of 10.8° (visual angle) and direction of 235° (θ), which shifted the monkey’s gaze to a point in the lower quadrant of the contralateral visual field. We defined this point as the center of the FEF site’s MF, and placed one of the two target apertures, TIN, at this location (Figure 3A, top). Subthreshold (low-frequency) microstimulation pulses delivered simultaneously with the onset of the targets slightly increased the number of saccades to the MF (“TIN choices”) from 62 of 109 control trials with no stimulation to 70 of 109 trials with stimulation, resulting in a 7.3% increase in saccades to TIN (Figure 3A, bottom).
Consistent with the apparent position illusion, ROC analysis of saccades made to TIN gratings during control trials revealed a significant influence of grating motion on saccade angle (AROC = 0.68, AROC > 0.5: p < 0.02) (Figure 3B). Distributions of angles of TIN saccades to up-and-leftward and down-and-rightward moving gratings had means that differed by 1.8° θ (up-left = 229.8 +/− 0.5°, down-left = 231.6 +/− 0.6°) during these trials. When saccades were made to TIN gratings during stimulation they were more strongly influenced by the direction of grating motion, yielding a greater ROC area (AROC = 0.82, AROC > 0.5: p < 10−6). During stimulation trials, the distributions of angles of TIN saccades to up-and-leftward and down-and-rightward moving gratings had means that differed by 3.0° θ (up-left = 229.0 +/− 0.6°, down-right = 232.0 +/− 0.4°). However, despite the increase in motion-dependent difference, there was no systematic angular deviation with microstimulation: the grand distribution of all TIN saccade angles on stimulation trials (i.e., all saccades to both directions of grating drift) did not differ significantly from the grand distribution of all angles on control trials (AROC = 0.50, AROC > 0.5: p = 0.93). Furthermore, saccades to TIN from control and stimulation trials did not differ in latency (control = 199.1 +/− 2.4 ms, stim = 194.9 +/− 2.1 ms, Student’s t-test, p = 0.19).
Similar to the TIN saccade analysis, analysis of the saccades to TOUT gratings during control trials revealed a significant influence of grating motion on saccade angle (AROC = 0.78, AROC > 0.5: p < 10−3) (Figure 3C). The distributions of angles of TOUT saccades to up-and-leftward and down-and-rightward moving gratings had means that differed by 2.1° θ (up-left = 59.0 +/− 0.5°, down-right = 56.9 +/− 0.4°). Unlike saccades to TIN, however, saccades to TOUT during microstimulation were not more strongly influenced by the direction of grating motion (AROC = 0.70 with stimulation, AROC > 0.5: p < 0.05). With FEF stimulation, the means of the distributions of angles to up-and-leftward and down-and-rightward moving TOUT gratings were more similar than on control trials, differing only by 1.5° θ (up-left = 59.1 +/− 0.4°, down-right = 57.6 +/− 0.8°). Thus, at this FEF site, microstimulation selectively enhanced the MIB of saccades to the MF. Importantly, this enhancement took the form of a directional deviation of the saccade angles away from the FEF site’s characteristic evoked vector.
Population analysis
For analysis of FEF stimulation on visually-guided saccades at the population level, we included experimental blocks in which the average latencies of control and stimulation saccades to TIN were statistically equal. Across the population of experiments there was a small but significant “speed-accuracy tradeoff” for saccades to the drifting gratings, such that longer latencies (and thus longer target viewing times) were correlated with greater angular deviations in the direction of grating motion (mean slope of deviation-versus-latency lines of best fit = 0.0056 +/− 0.0019 °/ms, Student’s t-test, p < 0.005) (Supplemental Figure 1). Therefore, matching TIN latencies ensured that the target viewing time on stimulation trials was similar to that of control trials. In total, 47 experiments (29 from monkey W and 18 from monkey B) from 27 FEF sites satisfied this latency criterion, including the experiment shown in Figure 3. FEF MF locations spanned the left visual hemifields of each monkey, ranging in eccentricity from 6.4° to 14.8° visual angle (mean 9.2°), and in θ from 114° to 245° (mean 211°).
The mean latency of saccades to TIN on stimulation trials, normalized by the mean control latency, was 0.993 +/− 0.005, which was not significantly different from unity (Student’s t-test, p = 0.14, Supplemental Figure 2). As expected, in these 47 experiments microstimulation influenced the fraction of saccades to TIN, the target placed at the FEF MF. In 40 of 47 experiments (85%), TIN was chosen more often on microstimulation trials than on control trials. On average, microstimulation resulted in a 10.0 +/− 1.9% increase in the fraction of saccades to TIN (Student’s t-test, p < 10−5) (Figure 4A). This increase was not dependent on the absolute stimulation current used (range of currents: 9 μA to 50 μA, R2 = 0.02, linear regression, p = 0.35), but larger increases in the fraction of saccades to TIN were observed during experiments in which the monkey allocated fewer of its control trial choices to TIN (R2 = 0.14, linear regression, p < 0.01).
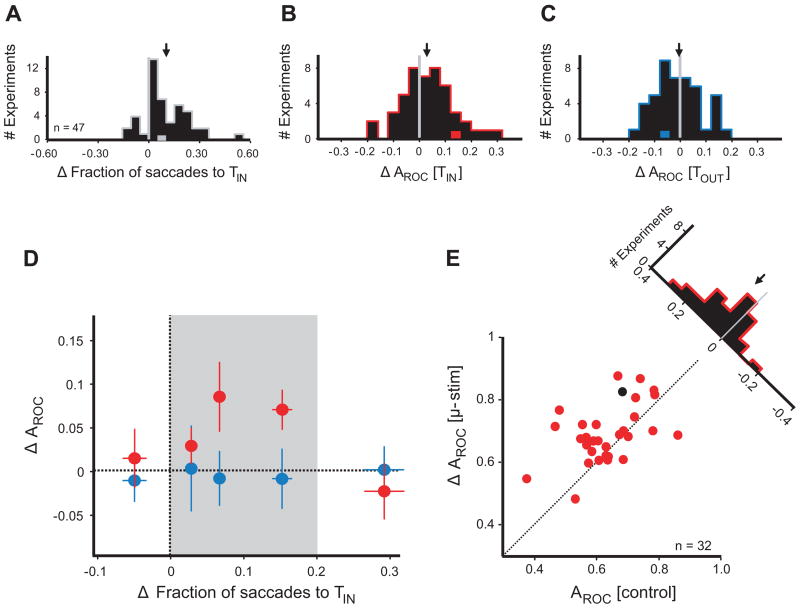
Figure 4. Population analysis.
(A) Effect of microstimulation on target choice. Histogram shows the change in the fraction of saccades to TIN with microstimulation. Arrow indicates the mean change. Gray data point represents the example experiment from Figure 3.
(B) Effect of microstimulation on the MIB of TIN saccades. Histogram shows the difference in AROC of TIN saccade angle distributions between microstimulation and control trials. Arrow indicates the mean change. Red data point represents the experiment from Figure 3.
(C) Effect of microstimulation on the MIB of TOUT saccades. As in b. Blue data point represents experiment from Figure 3.
(D) Relationship between the MIB and target choice effects. The stimulation effect on the MIB is plotted against its effect on the fraction of saccades to TIN. Red circles indicate TIN MIB effects, blue circles indicate TOUT effects. Each circle is the mean of 9 or 10 experiments: the leftmost and rightmost circles of each color comprise 10. Error bars indicate standard error of the mean for both axes. Mean absolute current amplitudes used in the experiments represented by each of the five data points, from left to right, were 24.9 μA, 28.1 μA, 24.5 μA, 23.5 μA, and 23.7 μA. The shaded area (“effective zone”) highlights the range of target choice effects in which FEF stimulation increases the MIB.
(E) MIB within the effective zone. For experiments falling within the microstimulation effective zone, AROC of TIN saccades with microstimulation is plotted against AROC of TIN control saccades. Each circle represents one experimental block. Black circle indicates the experiment from Figure 3. The histogram shows the difference in AROC due to microstimulation.
In addition to the effect on the fraction of saccades to TIN, microstimulation also increased the MIB of TIN saccades (Figure 4B). The average AROC of TIN saccades on control trials was 0.656 +/− 0.015, and was increased to 0.690 +/− 0.012 with microstimulation (permutation test for paired samples, p < 0.01). Thus, microstimulation increased the TIN AROC by an average of 0.034, and these effects were statistically indistinguishable in the two monkeys (an increase of 0.035 in monkey W and 0.033 in monkey B, two-sample t-test, p = 0.93). The change in AROC with microstimulation corresponded to an increase in the angular deviation between the means of distributions of saccade angles to opposing directions of grating motion (control = 1.83 +/− 0.26° θ, stim = 2.38 +/− 0.24° θ). In contrast, TIN saccades on microstimulation trials did not differ in amplitude (mean of 1.005 +/− 0.005 normalized to control trials, Student’s t-test, p = 0.34) or peak velocity (1.014 +/− 0.016, Student’s t-test, p = 0.37). Microstimulation also had no effect on the amount of scatter in saccade angles to a single direction of grating motion (1.011 +/− 0.032, Student’s t-test, p = 0.73) (Supplemental Figure 2). The absence of a microstimulation-induced change in scatter indicates that the increase in AROC caused by FEF stimulation was due primarily to an increase in the difference of the means of the two distributions of TIN saccade angles, not a sharpening of each distribution. In contrast to its effect on TIN saccades, FEF stimulation did not increase the MIB of saccades to TOUT, as there was no difference between AROC with stimulation (0.673 +/− 0.017) and AROC on control trials (0.679 +/− 0.016, ΔAROC = −0.006, permutation test for paired samples, p = 0.70) (Figure 4C). The absence of any effect of microstimulation on AROC of TOUT saccades was similar in the two monkeys (monkey W = −0.008, monkey B = −0.002, two-sample t-test, p = 0.82). Similarly, stimulation did not increase the difference between the mean angular deviations of saccades to opposing directions of grating motion (control = 2.21 +/− 0.25° θ, stim = 2.18 +/− 0.22° θ). The lack of improvement was apparent despite the fact that TOUT saccades on stimulation trials had slightly, but significantly, greater latencies than on control trials (1.020 +/− 0.005 normalized to control trials, Student’s t-test, p < 10−3) (Supplemental Figure 2), permitting slightly longer viewing times of the grating motion preceding TOUT choices (Supplemental Figure 1).
We considered the possibility that stimulation-induced increases in the MIB were a result of changes in smooth pursuit, which have been observed previously with FEF stimulation. Gardner and Lisberger (2002) reported enhanced pursuit gain in conjunction with saccades evoked to MF targets by suprathreshold (high-frequency), but not subthreshold (low-frequency), microstimulation of the FEF. Saccades evoked to one of two moving targets by stimulation were automatically chosen for pursuit. Thus, it is possible that, despite our use of subthreshold (low-frequency) stimulation parameters, our microstimulation could have driven the pursuit of the grating motion of the MF target, thereby altering the saccade angle and the MIB without actually changing the saccade command. However, in agreement with Gardner and Lisberger (2002), we found no effect of subthreshold microstimulation on smooth pursuit. We examined pursuit by measuring the component of eye velocity in the direction of grating motion both pre- and postsaccadically. (As it is known that the frequency response of pursuit is less than 15 Hz (Goldreich et al., 1992), and thus slower than the duration of a saccade, it would not be possible for stimulation to alter the pursuit during the saccade without being measurable pre- or postsaccadically.) For the presaccadic analysis, we found that stimulation had no effect on eye velocity in the direction of grating motion during the 30 ms window before saccades to TIN (control = 0.004 +/− 0.056°/s, stim = 0.070 +/− 0.060°/s, paired t-test, p = 0.37) or to TOUT (control = 0.008 +/− 0.047°/s, stim = 0.002 +/− 0.054°/s, paired t-test, p = 0.89). For the postsaccadic analysis, we measured pursuit velocity using the change in eye position from 1 to 30 ms, as well as from 31 to 60 ms after the end of the saccade, i.e. during the remainder of the open-loop phase of pursuit (Krauzlis & Lisberger, 1994; Lisberger, 1998; Gardner & Lisberger, 2001; Gardner & Lisberger, 2002). Stimulation did not affect postsaccadic pursuit velocity in the direction of grating motion for saccades to TIN (control, 1–30 ms postsaccadic: 0.02 +/− 0.33 °/s, stimulation: 0.12 +/− 0.32 °/s, paired t-test, p = 0.81; control, 31–60 ms postsaccadic: 0.13 +/− 0.24 °/s, stimulation: 0.19 +/− 0.19 °/s, paired t-test, p = 0.29) or to TOUT (control, 1–30 ms postsaccadic: 0.01 +/− 0.24 °/s, stimulation: 0.05 +/− 0.25 °/s, paired t-test, p = 0.87; control, 31–60 ms postsaccadic: 0.05 +/− 0.15 °/s, stimulation: 0.09 +/− 0.14 °/s, paired t-test, p = 0.48). Therefore, the effects of stimulation on the MIB were entirely saccadic.
Stimulation of an FEF site with sufficient, suprathreshold current can elicit saccades to a fixed retinotopic location, regardless of the presence of a visual stimulus. Therefore, even with low-frequency stimulation, we considered that particularly potent microstimulation (in terms of its effects on target choice) might result in a degradation of the MIB, rather than an enhancement. In addition, we expected that ineffective microstimulation would have no detectable effect on the MIB. Thus, we expected the facilitation of the MIB to be limited to an intermediate range of stimulation potencies. To address this hypothesis, we used the effect of microstimulation on fraction of saccades to TIN as a measure of the potency of FEF stimulation, and looked at the relationship between that measure and the change in the MIB. Consistent with our expectations, the microstimulation-induced increase in AROC of TIN saccades varied with the change in the fraction of saccades to TIN (Figure 4D). When microstimulation increased the fraction of saccades to TIN by more than 20%, or failed to increase the fraction of saccades to TIN at all, it had no effect on the MIB of TIN saccades (n = 15 experiments, mean ΔAROC = −0.011, Student’s t-test, p = 0.63). However, within an intermediate range of TIN choiceincreases (the “effective zone”), FEF stimulation resulted in a large increase in AROC (Figure 4E). Of the 32 experiments that fell within this range, the mean increase in AROC was 0.055 +/− 0.017 (permutation test for paired samples, p < 0.002). For these experimental blocks, FEF stimulation resulted in saccades that conveyed more information about the direction of grating motion by driving them away from the center of each FEF site’s MF.
Effect of target luminance on target choice and the MIB
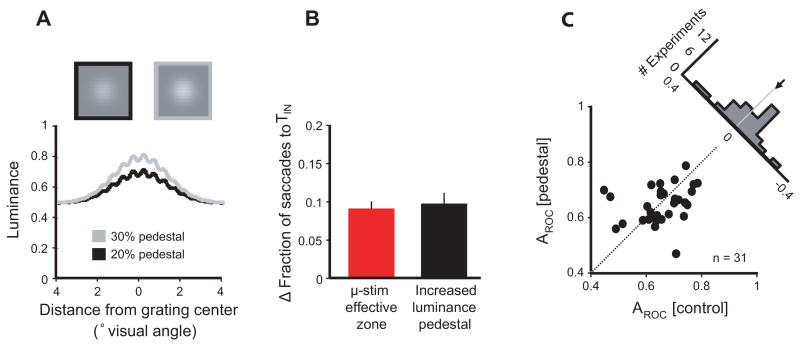
While one explanation for the observed effects of FEF stimulation on the MIB is a direct increase in the salience of the TIN stimulus which subsequently increases the influence of motion on the saccade, it is nonetheless possible that microstimulation acted on the MIB indirectly. In particular, FEF stimulation could have induced a visual percept (“phosphene”) that increased both the MIB and fraction of saccades to TIN by acting as an exogenous attention cue (Posner, 1980). Although human subjects do not typically report visual percepts during stimulation of the FEF via electrodes (Penfield and Rasmussen, 1950; Blanke et al., 2000) or transcranial magnetic stimulation (Silvanto et al., 2006), the fact that many FEF neurons are visually responsive warrants consideration of this possibility (Murphey and Maunsell, 2007). Two recent studies of the effects of SC stimulation on attention explored this alternative by substituting microstimulation with simulated phosphenes (Muller et al., 2005; Cavanaugh et al., 2006). In a similar manner, we tested this possibility in 31 behavioral experiments by substituting microstimulation with an increase in luminance of one target (still called TIN for comparison to the microstimulation task). On half the trials, TIN contained a grating displayed at the same contrast, but on a brighter luminance pedestal (Figure 5A). As observed with microstimulation within the effective zone, the increased luminance pedestal resulted in a greater fraction of saccades to TIN (increase of 9.4 +/− 1.0%, compared to 10.0 +/− 1.4% with microstimulation) (Figure 5B). However, unlike microstimulation, the increased luminance pedestal did not increase the influence of grating motion on TIN saccades. Instead, there was no significant difference in AROC observed with the luminance pedestal (ΔAROC = −0.009 +/− 0.019, Student’s t-test, p = 0.64) (Figure 5C). Therefore, although the luminance pedestal did increase the fraction of saccades to TIN to the same extent as microstimulation, that increase was not accompanied by an increase in MIB. Thus, consistent with the effects of simulated phosphenes observed in SC stimulation studies, it is unlikely that the increases in MIB were indirectly caused by stimulation-driven visual percepts.
Figure 5. Increased target salience effects on choice and the MIB.
(A) Normal grating and increased luminance pedestal grating. At top, a grating combined with a normal (20%) luminance pedestal (black border) is shown alongside a grating combined with a brighter (30%) luminance pedestal (gray border). For detail, both targets are shown at a higher spatial frequency than those used in the experiments. Below, a cross section through the center of each target is plotted in luminance space.
(B) Comparison of the increases in saccades to TIN caused by microstimulation and the increased luminance pedestal. Only microstimulation experiments falling within the effective zone are included. Error bars indicate standard error of the mean.
(C) Effect of target salience on the MIB. AROC of saccades to the high luminance pedestal target is plotted against AROC of saccades to the same target without the increased pedestal. Each circle represents one experimental block. The histogram shows the difference in AROC attributed to the increased luminance pedestal.
DISCUSSION
When saccades were directed toward a drifting grating within a stationary aperture, they were biased in the direction of grating motion. This directional bias could reflect the influence of visual motion on the perceived positions of the apertures, consistent with motion-induced apparent position effects observed in human subjects (Ramachandran and Anstis, 1990; De Valois and De Valois, 1991; Nishida and Johnston, 1999; Whitney and Cavanagh, 2000; Whitney, 2002). By using ROC analysis to compare the saccade vector angles resulting from opposing directions of grating drift, we measured the extent to which the apparent position illusion influenced the saccade vectors.
The central finding of this study is that subthreshold FEF stimulation increases the influence of the apparent position illusion on saccades, driving them away from, rather than toward, the center of the FEF MF. This result directly contradicts the fixed-vector hypothesis, which predicts that the motor effects of microstimulation should dominate the attentional effects, and that all saccade vectors should be driven toward the electrically-evoked vector, rather than in the direction of motion. These results might seem surprising given the abundance of studies in the FEF and SC demonstrating vector averaging of stimulation- and visually-driven saccades (Schiller and Sandell, 1983; Sparks and Mays, 1983; Kustov and Robinson, 1996; Gold and Shadlen, 2000; Barborica and Ferrera, 2004; Juan et al., 2004). However, these studies have invariably involved visual targets that were not aligned with the MF, and therefore could not test the interaction of the attentional and motor effects of microstimulation. On the other hand, studies that have involved the alignment of a visual stimulus with the FEF MF deliberately excluded saccades from the behavioral paradigm in order to examine the effects of microstimulation on visual attention (Moore and Fallah, 2001; Moore and Fallah, 2004). By using a saccade paradigm with MF-aligned visual targets, we tested the effect of FEF stimulation on saccade preparation and visual attention simultaneously, and found evidence that the metrics of visually-guided saccades are driven by the attentional effects, not the motor effects, of stimulation.
We found that the increase in the MIB covaried with the ability of microstimulation to influence the monkey’s choice behavior. Increases in the fraction of saccades to TIN of 0–20% were associated with robust increases in the MIB, whereas decreases in the fraction of saccades to TIN and increases of greater than 20% were associated with no change. This relationship could mean that increasing the fraction of saccades to TIN within a certain range necessarily affects the MIB of saccades. Alternatively, it may be that the relationship between stimulation’s effects on choice behavior and on the MIB is a function of the type of FEF neurons most affected by stimulation at a particular site. For example, stimulation predominantly of visual or visuomovement neurons might be expected to produce attentional effects more readily than stimulation of neurons with movement responses alone. Stimulation of sites with predominantly movement neurons would be expected to result in greater increases the fraction of saccades to TIN and lower current thresholds, but merely an imposition of a fixed saccade vector independent of the visual stimulus (Bruce et al., 1985; Stanton et al., 1989; Thompson et al., 2005). However, it appeared that the composition of FEF neurons stimulated was similar across the entire range of target choice effects, as current thresholds were comparable (Figure 4D, caption).
The present study examines the interaction of the known attentional and saccadic roles of the FEF. Recent work has reported that these two roles of the FEF can be experimentally dissociated (Juan et al., 2004; Murthy et al., 2001; Chambers and Mattingley, 2005). Furthermore, recent work has shown that separate populations of FEF neurons contribute to visual attention and saccade preparation (Thompson et al., 2005). Thus, it might be expected that FEF stimulation would independently affect these two functions, and that the effects on attention should not be incorporated into a concurrently planned saccade. However, the results of this study demonstrate that the attentional effects of microstimulation not only influence the saccade plan, but dominate movement effects in the specification of saccade metrics. This result is consistent with a model in which the selection of visual target parameters and saccade metrics are interdependent (Deubel and Schneider, 1996; Moore et al., 2003). For example, subthreshold microstimulation of the FEF could act through connections with visual cortex to enhance the representation of target motion, perhaps by increasing the effective contrast of the grating or influencing competition between directionally-selective visual cortical neurons, consistent with its influence on visual cortical response discriminability (Armstrong and Moore, 2007). The motion’s salience in turn could influence the apparent position of the target, which would further specify the appropriate saccade. In this scheme, it is the plan specified by way of visual cortex that determines the resulting saccade rather than the plan imposed by direct FEF stimulation.
Recent studies in a variety of brain areas have demonstrated that the effects of microstimulation are not limited to purely sensory or motor effects (Bisley et al., 2001; Cooke and Graziano, 2003; Williams and Eskandar, 2006; Hanks et al., 2006; Histed and Miller, 2006). In line with these studies, our results indicate that the effects of microstimulation on saccade preparation are not purely motor, but seem to involve the integration of visual and motor representations. FEF neurons represent a continuum of visual and motor functions (Bruce, 1990), and their relative roles in vision and movement have long been debated (Bizzi, 1967; Bruce and Goldberg, 1985). Recent work has established the role of the FEF in visual spatial attention (Moore, 2006) in addition to its previously known role in the preparation and triggering of saccades, thus raising the question of how the two functions interrelate (Awh et al., 2006). We propose that these seemingly disparate functions coexist interactively during visually-guided behavior, and that the attentional role plays an integral part in guiding the production of accurate saccades.
EXPERIMENTAL PROCEDURES
Two male monkeys (Macaca mulatta) weighing 6 kg (monkey W) and 11 kg (monkey B) were used as subjects in these experiments. All surgical and behavioral procedures were approved by the Stanford University Administrative Panel on Laboratory Animal Care and the consultant veterinarian, and were in accordance with National Institutes of Health and Society for Neuroscience guidelines.
Visual stimuli
Each saccade target was a sinusoidal grating that drifted within a stationary Gaussian aperture spanning 8° of visual angle. Gratings had spatial frequencies of 0.5 cyc/°, and varied from 2% – 8% Michelson contrast. In trials with more than one target, gratings were of identical contrast, and contrast was held constant throughout an experimental block. Every grating was added to a “pedestal”, or Gaussian background of 20% or 30% higher luminance (at its center) than the background. Gratings drifted at 5 °/sec within their stationary apertures. Drift was present during the entirety of the target presentation, and was directed perpendicular to the saccade required to acquire the target. The direction of grating motion was chosen randomly on each trial.
Quantifying the motion-induced bias
Throughout all experiments, eye position was monitored and stored at 500 Hz using the scleral search coil method (Fuchs and Robinson, 1966; Judge et al., 1980). Control of the display, electrical stimulation, and data storage was maintained by way of the CORTEX data acquisition system.
Voluntary saccades to the target described above were analyzed to determine the extent to which the motion of the target grating influenced the vector of the saccade. Saccades were detected in the eye position data using a combination of a velocity threshold (10 °/sec) and a “moving boxcar” technique which detected deflections in eye position (Armstrong et al., 2006). For each saccade detected, the eye position prior to the start of the saccade was subtracted from the position of the saccade endpoint, and the resulting vector was converted to polar coordinates. Saccade amplitude was recorded in degrees of visual angle, and saccade angle was in the interval [0°, 360°) where 0° was directly right of the fixation point and 90° was directly above it. Saccade analyses excluded smooth pursuit eye movements. Post-saccadic smooth pursuit eye velocity was calculated separately, as the eye position 60 ms after the saccade endpoint minus the endpoint position itself, divided by 60 ms.
Because grating motion was always perpendicular to the saccade vector required to attain the grating, the MIB was measured by comparing angular deviation of the saccade vectors caused by the motion. Specifically, two distributions were compared: the angles of saccades made to a target with drift in one direction (e.g. upward drift) and the angles of saccades made to the same target with drift in the opposite direction (e.g. downward drift). Saccade amplitude was not used in the MIB calculation.
To quantify the difference between these two distributions, a receiver operating characteristic (ROC) analysis was applied (Green and Swets, 1966). A criterion was successively set to every angle value in the combined range of the two distributions. For each criterion value the fraction of saccades in one distribution that exceeded the criterion was plotted against the fraction of saccades in the other distribution that exceeded the criterion. The quantity used to describe the difference in distributions of saccade angles was the ROC area, or AROC, which is the area under the curve comprising the points produced at each criterion position. Note that an AROC of 0.5 is consistent with two distributions of saccades that are not affected by target motion and are thus impossible to discriminate, whereas an AROC of 1.0 means that the two distributions of angles do not overlap at all and report with perfect certainty which direction of drift was present when each saccade was planned and executed. For testing whether individual AROC values were greater than 0.5, standard error was calculated as in Hanley and McNeil (Hanley and McNeil, 1982). A permutation test for paired samples was used to test the significance of differences between control and stimulation AROC values. For one million iterations, the labels (“control” or “stimulation”) within each pair of AROC values were randomly assigned, and the mean difference (stimulation minus control) of all pairs was recorded. The significance level of a veridical mean difference was calculated as the fraction of the means from these random assignments that exceeded it.
Choice task
Monkeys were trained to direct their gaze to a central fixation spot and await the appearance of two peripheral visual targets, and to execute a saccade to either target. Fixation was held for a variable time between 200 and 600 ms before target appearance. The two targets appeared simultaneously, at equal eccentricities and directly opposite each other with respect to the fixation spot. Within an experimental block of trials (~240 saccades) the location of each target remained constant. Additionally, contrast, spatial frequency, and orientation of the grating within each target aperture was constant across all trials within an experiment. The only difference in target appearance across trials was the direction of grating motion: each target grating could drift in one of two directions within its stationary aperture, and the direction of motion of each grating on a given trial was chosen randomly and independently of the direction of the opposite grating.
A juice reward was delivered on a variable schedule following any saccade that landed within an 8° square window around either target within 400 ms of target appearance. To encourage the monkey to distribute choices to both targets, a variant of a “matching shoulders” reward schedule (Abe and Takeuchi, 1993) was implemented such that choosing a target on one trial decremented its likelihood of yielding a reward on the subsequent trial, and incremented the likelihood of reward for a choice of the opposite target. Specifically, the probability of receiving a reward for choosing a target followed the sigmoid equations for TIN choices and for TOUT, where f is the fraction of the last 20 trials in which TIN was chosen, s determines the slope of the sigmoid, and a controls the experimentally-varied optimal allocation of choices to TIN. In all blocks of trials, s was set to 0.07, and a was equal to 0.3, 0.5 or 0.7 (monkey W) or 0.25, 0.5 or 0.75 (monkey B). When choices were allocated nearly optimally, rewards were delivered on approximately 80% of trials.
Electrical microstimulation
Electrical stimulation of an FEF site was delivered via tungsten electrodes (0.1–1.0 MΩ impedance, at 1 kHz) using a Grass stimulator (S88) and two Grass stimulation isolation units (PSIU-6). Current amplitude was measured via the voltage drop across a 1 kΩ resistor in series with the return lead of the current source. In each monkey, the FEF was localized on the basis of its surrounding physiological and anatomical landmarks and our ability to evoke fixed-vector, saccadic eye movements with stimulation using currents below 50 μA at a frequency of 200 Hz (0.3 ms pulse duration, 100 ms trains).
During each experimental session, we determined the saccade vector elicited at the cortical site under study, and the current threshold to evoke a saccade using a separate calibration paradigm (Moore and Fallah, 2004). The endpoints of saccades evoked from the central position were used to define the MF of the stimulation site. The direction and amplitude of the evoked saccade vector and the corresponding threshold were measured both at the beginning and at the end of the experimental session to ensure that neither had changed significantly throughout the session. Experimental blocks of trials were used in the analysis only if the MF did not change during the session. During the choice task, one of the two targets was positioned at the center of the MF. Subthreshold, low-frequency (60 Hz) microstimulation at threshold current (+/− 2 μA) was applied for 200 ms to the FEF site on half of the trials, randomly interleaved. During microstimulation trials, the stimulation train began simultaneously with the appearance of the targets.
Analysis of microstimulation and luminance pedestal effects
Experimental blocks included in the MIB and target choice analyses met two criteria. First, an experimental block was included only if the mean saccade vector angle of all TIN choices with stimulation differed from the mean angle of all TIN saccades on control trials by less than 1.5°. This criterion ensured that the target within the FEF MF was accurately placed, and that the electrode’s position within the FEF had not changed over the course of the experiment. Second, experimental blocks were only included if the latencies of TIN saccades with stimulation were statistically matched with the latencies of TIN saccades on control trials (Wilcoxon rank sum test, p > 0.01). Latency matching ensured that the viewing times of TIN gratings were the same with and without microstimulation.
For analysis of saccade metrics other than the MIB, namely latency, amplitude, peak velocity and scatter, values of each metric from stimulation trials were normalized to those of control trials. Scatter was defined as the variability in the angles of saccade vectors. For each target, the saccade vector angles composed two distributions: one of saccades to the target during one direction of grating motion, and another to the same target during the opposite direction of motion. For each of these two distributions, scatter was calculated as the mean positive difference of each vector angle from the mean angle of its distribution. The scatter for each of the two distributions was averaged to give a single value for all saccades to the given target.
Supplementary Material
Acknowledgments
We thank D. S. Aldrich for technical assistance, and M. M. Churchland for expert advice on pursuit analyses. This work was supported by NIH Grant EY14924, the Pew Charitable trusts, the Sloan Foundation, and an NDSEG fellowship to R.J.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe N, Takeuchi J. The “lob-pass” problem and an on-line learning model of rational choice. Proc 6th annual conference on computational learning theory; 1993. pp. 422–428. [Google Scholar]
- Armstrong KM, Fitzgerald JK, Moore T. Changes in visual receptive fields with microstimulation of frontal cortex. Neuron. 2006;50:791–798. doi: 10.1016/j.neuron.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Armstrong KM, Moore T. Rapid enhancement of visual cortical response discriminability by microstimulation of the frontal eye field. Proc Natl Acad Sci USA. 2007;104:9499–9504. doi: 10.1073/pnas.0701104104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Armstrong KM, Moore T. Visual and oculomotor selection: links, causes and implications for spatial attention. Trends Cogn Sci. 2006;10:124–130. doi: 10.1016/j.tics.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Barborica A, Ferrera VP. Modification of saccades evoked by stimulation of frontal eye field during invisible target tracking. J Neurosci. 2004;24:3260–3267. doi: 10.1523/JNEUROSCI.4702-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, Zaksas D, Pasternak T. Microstimulation of cortical area MT affects performance on a visual working memory task. J Neurophysiol. 2001;85:187–196. doi: 10.1152/jn.2001.85.1.187. [DOI] [PubMed] [Google Scholar]
- Bizzi E. Discharge of frontal eye field neurons during eye movements in unanesthetized monkeys. Science. 1967;157:1588–1590. doi: 10.1126/science.157.3796.1588. [DOI] [PubMed] [Google Scholar]
- Blanke O, Spinelli L, Thut T, Michel CM, Perrig S, Landis T, Seeck M. Location of the human frontal fields as defined by electrical stimulation: anatomical, functional and electrophysiological characteristics. Neuroreport. 2000;11:1907–1913. doi: 10.1097/00001756-200006260-00021. [DOI] [PubMed] [Google Scholar]
- Bruce CJ. Integration of sensory and motor signals for saccadic eye movements in the primate frontal eye fields. In: Edelman GM, Gall WE, Cowan WM, editors. Signal and Sense, Local and Global Order in Perceptual Maps. New York: Wiley-Liss; 1990. pp. 261–314. [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Ling S, Read S. Attention alters appearance. Nat Neurosci. 2004;7:308–313. doi: 10.1038/nn1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Alvarez BD, Wurtz RH. Enhanced performance with brain stimulation: attentional shift or visual cue? J Neurosci. 2006;26:11347–11358. doi: 10.1523/JNEUROSCI.2376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci. 2004;24:11236–11243. doi: 10.1523/JNEUROSCI.3724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CD, Mattingley JB. Neurodisruption of selective attention: insights and implications. Trends Cogn Sci. 2005;9:542–550. doi: 10.1016/j.tics.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Cooke DF, Graziano MS. Defensive movements evoked by air puff in monkeys. J Neurophysiol. 2003;90:3317–3339. doi: 10.1152/jn.00513.2003. [DOI] [PubMed] [Google Scholar]
- De Valois RL, De Valois KK. Vernier acuity with stationary moving Gabors. Vision Res. 1991;31:1619–1626. doi: 10.1016/0042-6989(91)90138-u. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- Nishida S, Johnston A. Influence of motion signals on the perceived position of spatial pattern. Nature. 1999;397:610–612. doi: 10.1038/17600. [DOI] [PubMed] [Google Scholar]
- Dodge R. Visual perception during eye movement. Psychol Rev. 1900;7:465. [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Gardner JL, Lisberger SG. Linked target selection for saccadic and smooth pursuit eye movements. J Neurosci. 2001;21:2075–2084. doi: 10.1523/JNEUROSCI.21-06-02075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JL, Lisberger SG. Serial linkage of target selection for orienting and tracking eye movements. Nat Neurosci. 2002;5:892–899. doi: 10.1038/nn897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher PW, Sparks DL. Effects of low-frequency stimulation of the superior colliculus on spontaneous and visually guided saccades. J Neurophysiol. 1993;69:953–964. doi: 10.1152/jn.1993.69.3.953. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. Representation of a perceptual decision in developing oculomotor commands. Nature. 2000;404:390–394. doi: 10.1038/35006062. [DOI] [PubMed] [Google Scholar]
- Goldreich D, Krauzlis RJ, Lisberger SG. Effect of changing feedback delay on spontaneous oscillations in smooth pursuit eye movements of monkeys. J Neurophysiol. 1992;67:625–638. doi: 10.1152/jn.1992.67.3.625. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory. New York: Wiley; 1966. [Google Scholar]
- Gross CG, Moore T, Rodman HR. Visually guided behavior after V1 lesions in young and adult monkeys and its relation to blindsight in humans. Prog Brain Res. 2004;144:279–294. doi: 10.1016/S0079-6123(03)14419-6. [DOI] [PubMed] [Google Scholar]
- Hanks TD, Ditterich J, Shadlen MN. Microstimulation of macaque area LIP affects decision-making in a motion discrimination task. Nat Neurosci. 2006;9:682–689. doi: 10.1038/nn1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- He P, Kowler E. The role of saccades in the perception of texture patterns. Vision Res. 1992;32:2151–2163. doi: 10.1016/0042-6989(92)90076-u. [DOI] [PubMed] [Google Scholar]
- Histed MH, Miller EK. Microstimulation of frontal cortex can reorder a remembered spatial sequence. PLoS Biol. 2006;4:826–835. doi: 10.1371/journal.pbio.0040134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan CH, Shorter-Jacobi SM, Schall JD. Dissociation of spatial attention and saccade preparation. Proc Natl Acad Sci USA. 2004;101:15541–15544. doi: 10.1073/pnas.0403507101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Kowler E, Steinman RM. The role of small saccades in counting. Vision Res. 1977;17:141–146. doi: 10.1016/0042-6989(77)90212-7. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Lisberger SG. Temporal properties of visual motion signals for the initiation of smooth pursuit eye movements in monkeys. J Neurophysiol. 1994;72:150–162. doi: 10.1152/jn.1994.72.1.150. [DOI] [PubMed] [Google Scholar]
- Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature. 1996;384:74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- Lisberger SG. Postsaccadic enhancement of initiation of smooth pursuit eye movements in monkeys. J Neurophysiol. 1998;79:1918–1930. doi: 10.1152/jn.1998.79.4.1918. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JH. Effects of attention on the reliability of individual neurons in monkey visual cortex. Neuron. 1999;23:765–773. doi: 10.1016/s0896-6273(01)80034-9. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci. 2004;7:757–763. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]
- Moore T. The neurobiology of visual attention: finding sources. Curr Opin Neurobiol. 2006;16:159–165. doi: 10.1016/j.conb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM, Fallah M. Visuomotor origins of covert spatial attention. Neuron. 2003;40:671–683. doi: 10.1016/s0896-6273(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. Control of eye movements and spatial attention. Proc Natl Acad Sci USA. 2001;98:1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. J Neurophysiol. 2004;91:152–162. doi: 10.1152/jn.00741.2002. [DOI] [PubMed] [Google Scholar]
- Moore T, Rodman HR, Gross CG. Recovery of Visual Function Following Damage to Striate Cortex in Monkeys. In: de Gelder B, DeHaan E, Heywood C, editors. Out of Mind: Varieties of unconscious processing. Oxford: Oxford Univeristy Press; 2001. pp. 35–51. [Google Scholar]
- Muller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc Natl Acad Sci USA. 2005;102:524–529. doi: 10.1073/pnas.0408311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey DK, Maunsell JH. Behavioral detection of electrical microstimulation in different cortical visual areas. Curr Biol. 2007;17:862–867. doi: 10.1016/j.cub.2007.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy A, Thompson KG, Schall JD. Dynamic dissociation of visual selection from saccade programming in frontal eye field. J Neurophysiol. 2001;86:2634–2637. doi: 10.1152/jn.2001.86.5.2634. [DOI] [PubMed] [Google Scholar]
- Penfield W, Rasmussen T. The Cerebral Cortex of Man: A Clinical Study of Localization of Function. New York: Macmillan; 1950. [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Anstis SM. Illusory displacement of equiluminous kinetic edges. Perception. 1990;19:611–616. doi: 10.1068/p190611. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res. 1972;12:1795–1808. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- Robinson DA, Fuchs AF. Eye movements evoked by stimulation of frontal eye fields. J Neurophysiol. 1969;32:637–648. doi: 10.1152/jn.1969.32.5.637. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Sandell JH. Interactions between visually and electrically elicited saccades before and after superior colliculus and frontal eye field ablations in the rhesus monkey. Exp Brain Res. 1983;49:381–392. doi: 10.1007/BF00238780. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ. Look and see: how the brain moves your eyes about. Prog Brain Res. 2001;134:127–142. doi: 10.1016/s0079-6123(01)34010-4. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Lavie N, Walsh V. Stimulation of the human frontal eye fields modulates sensitivity of extrastriate visual cortex. J Neurophysiol. 2006;96:941–945. doi: 10.1152/jn.00015.2006. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Mays LE. Spatial localization of saccade targets. I. Compensation for stimulation-induced perturbations in eye position. J Neurophysiol. 1983;49:45–63. doi: 10.1152/jn.1983.49.1.45. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Deng SY, Goldberg ME, McMullen NT. Cytoarchitectural characteristic of the frontal eye fields in macaque monkeys. J Comp Neurol. 1989;282:415–427. doi: 10.1002/cne.902820308. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J Neurosci. 2005;25:9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turatto M, Vescovi M, Valsecchi M. Attention makes moving objects be perceived to move faster. Vision Res. 2007;47:166–178. doi: 10.1016/j.visres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Vitu F, O’Regan JK, Mittau M. Optimal landing position in reading isolated words and continuous text. Percept Psychophys. 1990;47:583–600. doi: 10.3758/bf03203111. [DOI] [PubMed] [Google Scholar]
- Wardak C, Ibos G, Duhamel JR, Olivier E. Contribution of the monkey frontal eye field to covert visual attention. J Neurosci. 2006;26:4228–4235. doi: 10.1523/JNEUROSCI.3336-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney D. The influence of visual motion on perceived position. Trends Cogn Sci. 2002;6:211–216. doi: 10.1016/s1364-6613(02)01887-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney D. Contribution of bottom-up and top-down motion processes to perceived position. J Exp Psychol Hum Percept Perform. 2006;32:1380–1397. doi: 10.1037/0096-1523.32.6.1380. [DOI] [PubMed] [Google Scholar]
- Whitney D, Cavanagh P. Motion distorts visual space: shifting the perceived position of remote stationary objects. Nat Neurosci. 2000;3:954–959. doi: 10.1038/78878. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Eskandar EN. Selective enhancement of associative learning by microstimulation of the anterior caudate. Nat Neurosci. 2006;9:562–568. doi: 10.1038/nn1662. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Barborica A, Ferrera VP. Radial motion bias in macaque frontal eye field. Vis Neurosci. 2006;23:49–60. doi: 10.1017/S0952523806231055. [DOI] [PubMed] [Google Scholar]
- Yarbus AL. Eye Movements and Vision. New York: Plenum Press; 1967. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.