Abstract
Background
SSRIs effectively treat various anxiety disorders, although symptoms of anxiety are often exacerbated during early stages of treatment. We previously reported that acute treatment with the SSRI citalopram enhances the acquisition of auditory fear conditioning, which is consistent with the initial anxiogenic effects reported clinically. Here, we extend our findings by assessing the effects of acute SSRI treatment on the expression of previously acquired conditioned fear.
Methods
Rats underwent fear conditioning drug-free. Tone-evoked fear responses were tested after drug treatment the following day. This protocol more closely resembles the clinical setting than pre-conditioning treatment, since it evaluates effects of treatment on a pre-existing fear, rather than on the formation of a new fear memory.
Results
A single pre-testing injection of the SSRIs citalopram or fluoxetine significantly increased fear expression. There was no effect of the antidepressant tianeptine, or the norepinephrine reuptake inhibitor, tomoxetine, indicating that this effect is specific to SSRIs. The SSRI induced enhancement in fear expression was not blocked by tropisetron, a 5-HT3 receptor antagonist, but was blocked by SB 242084, a specific 5-HT2C receptor antagonist.
Conclusions
Enhanced activation of 5-HT2C receptors may be a mechanism for the anxiogenic effects of SSRIs observed initially during treatment.
Keywords: fear conditioning, citalopram, 5-HT2C receptor, amygdala, serotonin, 5-HT3 receptor
Introduction
Selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed to treat depression (Bondareff et al 2000; Stahl 2000) as well as a range of anxiety disorders, such as panic disorder, obsessive compulsive disorder, post-traumatic stress disorder, and social anxiety disorder (Kent et al 1998; Pollack and Doyle 2003; Stein and Stahl 2000). Typically, several weeks of treatment with SSRIs are necessary before patients experience the therapeutic effects (Feighner and Boyer 1991), and symptoms of anxiety or agitation are frequently exacerbated when treatment is first initiated (Mir 1997; Spigset 1999). To minimize this initial “anxiogenic” effect, drug dose is titrated (Gorman et al 1987) and benzodiazepines are often prescribed concomitantly (Bingefors and Isacson 1998; Gregor et al 1996). However, benzodiazepines can lead to adverse effects (O’Brien 2005; Verster and Volkerts 2004), and some evidence indicates they may decrease the therapeutic effects of SSRIs (Martin and Puech 1996). Thus, it is important to develop our understanding of the mechanisms underlying this anxiogenic effect, since advances could lead to alternative treatment options.
A number of animal studies using various tests of anxiety, such as the social interaction test, elevated-plus maze, and the two-compartment black and white box also report an anxiogenic-like effect of SSRIs following acute treatment (Dekeyne et al 2000; Griebel et al 1994; Matto et al 1996; Sanchez and Meier 1997). Also, in our previous study we found that acute SSRI treatment increases fear when administered prior to fear learning (Burghardt et al 2004).
The advantage of using auditory fear conditioning is that it is a model of fear for which the neural circuitry has been elucidated in detail (LeDoux 2000; Maren 2001). In this procedure, a neutral conditioned stimulus (CS), such as a tone, elicits defensive responses after being paired with an aversive unconditioned stimulus (US), typically a footshock. An extensive body of evidence indicates that the acquisition and expression of fear conditioning depends on the amygdala (LeDoux 2000; Maren 2001; Muller et al 1997), a brain region that has been implicated in a variety of anxiety disorders (Britton et al 2005; Cannistraro et al 2004; Milham et al 2005). Imaging and electrophysiological studies reveal that amygdala activity is modulated by the serotonin transporter gene (Canli et al 2005; Hariri et al 2002) and serotonin neurotransmission (Stutzmann et al 1998). Furthermore, a single systemic SSRI injection leads to an increase in amygdala extracellular serotonin (Bosker et al 2001), an increase in amygdala Fos-like immunoreactivity (Morelli et al 1999; Veening et al 1998), and changes in amygdala activity in healthy humans (Del-Ben et al 2005; McKie et al 2005). Together, these studies, as well as our previous fear conditioning study (Burghardt et al 2004), indicate that the amygdala may be an important site of action for the anxiogenic effects of acute SSRI treatment.
As a means of gaining further insight into how acute SSRI treatment alters amygdala-dependent fear, the present study extends our previous findings by assessing the effects of acute SSRI treatment on the expression of conditioned fear. Unlike the previous study, rats were trained to associate the CS and US drug-free, and were injected with drug the next day, prior to exposure to the fear provoking CS. Given that patients are typically treated with SSRIs for their anxiety symptoms after the disorder has already developed, the present focus on fear expression more closely resembles the clinical setting. We evaluated the effects of two SSRIs, citalopram and fluoxetine, on conditioned fear expression, and compared their effects to those of tianeptine, an effective antidepressant that is proposed to be a serotonin reuptake enhancer, and tomoxetine, a norepinephrine reuptake inhibitor.
In an effort to better understand the mechanisms through which SSRIs affect fear circuits, we also explored the role of specific serotonin receptor subtypes in mediating the effects of citalopram on conditioned fear expression. We focused on the 5-HT2C and 5-HT3 receptor subtypes because previous studies have shown that their presence in the amygdala influences its excitability (Stein et al 2000), and blocking them systemically with selective antagonists alters fear in several animal models (Costall 1991; Martin et al 2002), including fear conditioning (Hensman et al 1991; Yoshioka et al 1995). We therefore tested whether blocking these receptors with their respective antagonists, SB 242082 and tropisetron, alone or in combination with acute citalopram treatment, affects the expression of conditioned fear.
Materials and Methods
Subjects
Adult male Sprague-Dawley rats (Hilltop Laboratories, Scottdale, PA) weighing 350–400g were housed individually in clear plastic cages in a thermally controlled colony room. They were placed on a 12 hr light/dark cycle and food and water were provided ad libitum throughout the duration of the experiment. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by the New York University Animal Care and Use Committee.
Drugs
Citalopram hydrobromide, fluoxetine hydrochloride, tomoxetine, SB 242084, and tropisetron were purchased from Sigma-Aldrich Co. (St. Louis, MO). Tianeptine sodium salt was provided by Servier (Courbevoie, France). SB 242084 and tomoxetine were dissolved in pure water and all other drugs were dissolved in 0.9% sterile saline. Drugs were administered at the following doses, which were chosen based on previous work that found these drugs to be effective in a fear conditioning task and/or another anxiety-related tasks: Citalopram (10 mg/kg) (Burghardt et al 2004; Matto and Allikmets 1999); Fluoxetine (10 mg/kg) (Bodnoff et al 1989; Silva and Brandao 2000); Tianeptine (10 mg/kg) (Burghardt et al 2004; Conrad et al 1999); Tropisetron (0.1 mg/kg) (Yoshioka et al 1995); SB 242084 (0.2 mg/kg) (Bagdy et al 2001). Tomoxetine was administered at 1 mg/kg, which has been shown to effectively increase extracellular norepinephrine levels (Bymaster et al 2002). Previous studies indicate that tropisetron and SB 242084 selectively antagonize 5-HT3 and 5-HT2C receptors, respectively, in vivo at the doses used in this study (Higgins et al 1993; Kennett et al 1997). All drugs were injected intraperitoneally (i.p.) in a volume of 1 ml.
Apparatus
Rats were fear conditioned in a Plexiglas rodent conditioning chamber that was brightly lit with three white house lights, and contained a metal grid floor (ENV-001; Med Associates, Inc. Georgia, VT). During tone testing, the context of this chamber was altered with a fitted flat black Formica floor cover scented with peppermint soap and was dimly lit with two house lights covered with red lenses. Previous studies have shown that this testing environment is distinct enough to minimize generalization from the training environment (Nader and LeDoux 1999; Schafe et al 1999). Behavior during training and testing was videotaped with a camera mounted at the top of the chamber.
Habituation, Auditory Fear Conditioning, and Testing
Rats were habituated to the conditioning context for 20 minutes, as well as to handling. Fear conditioning occurred the next day, during which rats were trained in a single conditioning session involving two pairings of a 20-sec tone CS (10 kHz, 75 dB) that co-terminated with a footshock US (0.5 sec, 0.7 mA), with an inter-trial interval that varied randomly between 90 and 120 sec. After conditioning, rats were returned to their home cages and freezing was scored (see below) so that natural variability in acquisition could be counterbalanced across treatment groups the next day. This particular training protocol was selected because pilot studies determined that it produced approximately 50% of maximal freezing during the first tone presentation in vehicle-treated rats at test. This allowed for the detection of increases in freezing resulting from drug treatment, and was the protocol used in our previous study, which found that acute citalopram treatment increases fear when administered before conditioning (Burghardt et al 2004).
Twenty-four hours after conditioning, separate groups of rats were weighed and injected with citalopram, fluoxetine, tianeptine, tomoxetine, or their respective vehicle. Each drug treated group was tested with a separate vehicle group. In the experiment involving the 5-HT3 antagonist, rats received a single injection of citalopram, tropisetron, citalopram + tropisetron, or saline. In the experiment with the 5-HT2C antagonist, we followed a protocol similar to that used by Dekeyne et al (2000), and rats were given two injections. The first injection of SB 242084 or water was given 15 minutes before the second injection of citalopram or saline (Dekeyne et al 2000). All treatment began 60 minutes before testing.
During testing, rats were presented with ten 20-sec CS tones (10 kHz, 75 dB; inter-trial interval, 90–120 sec), without the US. The inter-trial interval and the properties of the tones presented during training and testing were identical. The experimenter, who was blind to the treatment group, measured fear expression from the videotape using a timer to count the number of seconds rats spent freezing during each 20 second tone. Freezing was expressed as a percentage of the total tone presentation time. Baseline freezing was measured by scoring freezing during the 20-sec interval prior to the first tone onset on the testing day. Freezing was defined as the absence of all movement, with the exception of breathing. Data were analyzed using Student’s t-test for independent samples, or a two- or three-way ANOVA followed by Tukey’s HSD post hoc test, and significance levels were set at p<0.05.
Results
Acute SSRI Treatment Enhances Conditioned Fear Expression
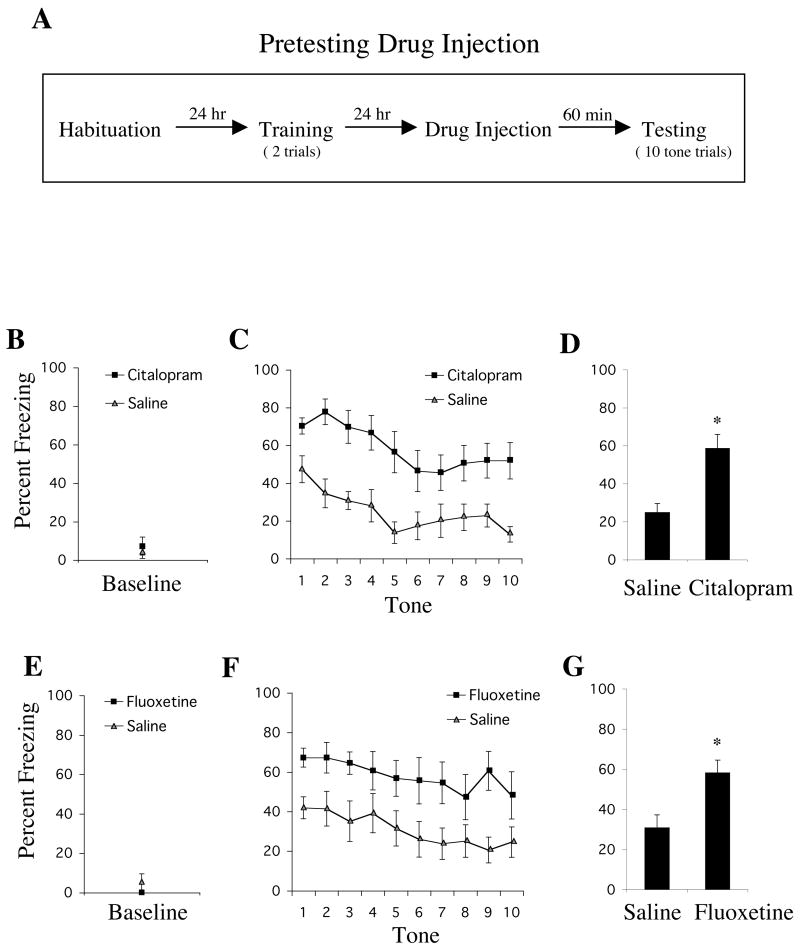
Animals given an injection of citalopram (10 mg/kg, i.p.) 60 minutes before testing showed enhanced fear expression (Figure 1). We performed a two-way ANOVA with factors: SSRI group (citalopram vs. saline) and tone trial (1–10; repeated measures). There was a significant main effect of group [F1,20=14.97, p<.01], indicating that citalopram-treated animals froze significantly more than control animals during testing. There was a significant effect of tone trial [F9,180=7.37, p<.01], indicating that fear responses extinguished over the course of 10 CS presentations. The group × tone trial interaction was not significant [F9,180=.96, p=.47], suggesting that citalopram treatment did not alter the rate of fear reduction over the trials. Rats showed virtually no freezing prior to the initial CS, and a t-test on baseline freezing indicated no significant difference between groups [t20=.46, p=.65].
Figure 1.
Animals given a pre-testing injection of an SSRI showed enhanced conditioned fear expression. (A) General behavioral procedures: 24 hours after habituation, rats were fear conditioned with two tone-shock pairings. The next day, animals were given an injection of drug or vehicle and tested 60 minutes later to ten presentations of the tone alone. (B) Mean ± SEM percent freezing of rats treated with citalopram (n=11) or saline (n=9) during the baseline period prior to the first tone trial. (C) Mean ± SEM percent freezing of citalopram-treated (10 mg/kg, i.p.) or saline-treated animals during each tone trial. (D) Mean ± SEM percent freezing of each group (citalopram vs. saline) averaged across all 10 tones. * p<.01 versus saline. (E) Mean ± SEM percent freezing of rats given a single injection of fluoxetine (n=9) or saline (n=11) during the baseline period prior to the first tone trial. (F) Mean ± SEM percent freezing of fluoxetine-treated (10 mg/kg, i.p.) or saline-treated animals during each tone trial. (G) Mean ± SEM percent freezing of each group (fluoxetine vs. saline) averaged across all 10 tones * p<.01 versus saline.
Animals treated acutely with fluoxetine (10 mg/kg, i.p.) showed a similar enhancement in conditioned fear expression (Figure 1). An ANOVA with factors: SSRI group (fluoxetine vs. saline) and tone trial (1–10; repeated measures) revealed a significant effect of group [F1,18=8.93, p<.01], a significant effect of tone trial [F9,162=162, p<.05], and no significant group × tone trial interaction [F9,162=.35, p=.96]. This indicated that acute fluoxetine treatment also significantly increased freezing compared with control animals across tones. There was no significant difference between groups in baseline freezing [t18=1.21, p=.24], indicating that the enhancement in freezing was specific to the tone. Together, these findings indicate that acute treatment with either citalopram or fluoxetine increases the expression of conditioned fear.
An Acute Injection of Tianeptine Does Not Affect the Expression of Conditioned Fear
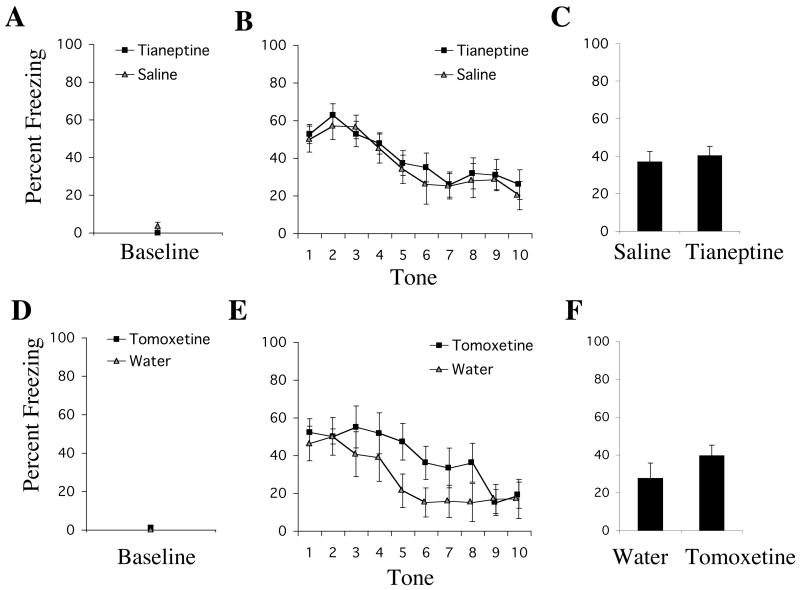
In contrast, animals that received a single pre-testing injection of tianeptine (10 mg/kg, i.p.) showed no significant difference from control animals in their levels of freezing during testing (Figure 2). The ANOVA with factors: drug group (tianeptine vs. saline) and tone trial (1–10; repeated measures) revealed no significant effect of group [F1,19=2.0, p=.66] and no significant group × tone trial interaction [F9,171=.20, p=.99]. A significant effect of tone trial [F9,171=12.1, p<.01] was observed, indicating that fear extinguished throughout the test session. A t-test revealed no significant difference between groups in baseline freezing [t19=1.74, p=.10].
Figure 2.
Using the same general behavioral procedures outlined in Figure 1, acute treatment with tianeptine (10 mg/kg, i.p.) or tomoxetine (1 mg/kg, i.p.) did not affect conditioned fear expression. (A) Mean ± SEM percent freezing of rats treated with tianeptine (n=11) or saline (n=10) during the baseline period prior to the first tone trial. (B) Mean ± SEM percent freezing of tianeptine-treated or saline-treated animals during each tone trial. (C) Mean ± SEM percent freezing of each group (tianeptine vs. saline) averaged across all 10 tones. (D) Mean ± SEM percent freezing of rats treated with tomoxetine (n=9) or water (n=8) during the baseline period prior to the first tone trial. (E) Mean ± SEM percent freezing of tomoxetine-treated or water-treated animals during each tone trial. (F) Mean ± SEM percent freezing of each group (tomoxetine vs. water) averaged across all 10 tones.
Acute Treatment with Tomoxetine Does Not Affect Conditioned Fear Expression
A pre-testing injection of the norepinephrine reuptake inhibitor, tomoxetine (1 mg/kg, i.p.), did not significantly affect conditioned fear expression (Figure 2). The ANOVA with factors: drug group (tomoxetine vs. water) and tone trial (1–10; repeated measures) revealed no significant main effect of group [F1,15=1.51, p=.24] and no significant group × tone trial interaction [F9,135=1.10, p=.37]. The significant effect of tone trial [F9,135=8.16, p<.01] indicated extinction in both groups during the test session. There was also no significant effect of tomoxetine on baseline freezing compared to controls [t15=1.42, p=.18].
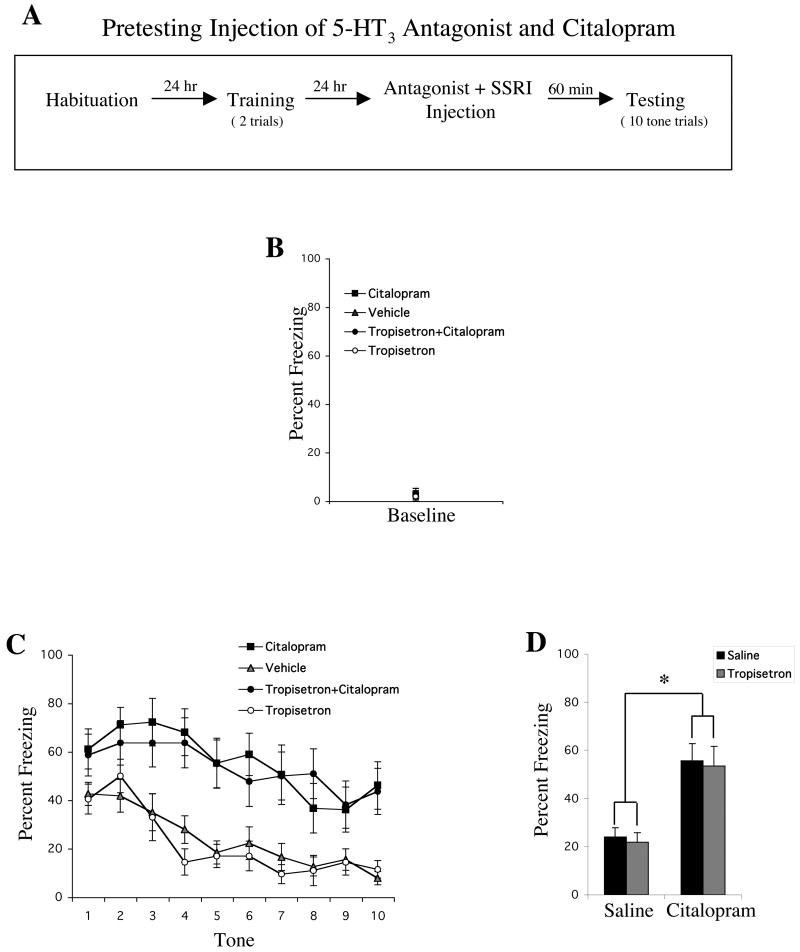
An Injection of a 5-HT3 Antagonist Does Not Block the Effect of Citalopram on Conditioned Fear Expression
Injecting the 5-HT3 antagonist, tropisetron (0.1 mg/kg, i.p.), with citalopram (10 mg/kg, i.p.) did not alter the enhancing effects of citalopram (10 mg/kg, i.p.) on conditioned fear expression (Figure 3). We performed a three-way ANOVA with factors: antagonist (tropisetron vs. saline), SSRI (citalopram vs. saline), and tone trial (1–10; repeated measures). There was a significant main effect of SSRI [F1,45=29.12, p<.01] and tone trial [F9,405=16.85, p<.01] but no significant main effect of antagonist [F1,45=.14, p=.71]. There was a significant SSRI × tone trial interaction [F9,405=2.39, p<.05], but the other two-way interactions were not significant and the three-way interaction was also not significant. An analysis on baseline freezing revealed no differences between any of the groups.
Figure 3.
The 5-HT3 receptor antagonist, tropisetron (0.1 mg/kg, i.p.), did not block the enhancing effects of citalopram (10 mg/kg, i.p.) on conditioned fear expression. (A) General behavioral procedures: 24 hours after habituation, rats were fear conditioned with two tone-shock pairings. The next day, animals were given a single injection containing citalopram, tropisetron, citalopram+tropisetron, or saline 60 minutes before they were tested to 10 presentations of the tone alone. (B) Mean ± SEM percent freezing of rats treated with citalopram (n=9), vehicle (n=19), tropisetron+citalopram (n=11), or tropisetron (n=10) during the baseline period prior to the first tone trial. (C) Mean ± SEM percent freezing of each group during each tone trial. (D) Mean ± SEM percent freezing of each group averaged across all 10 tones. * indicates main effect of citalopram, p<.01.
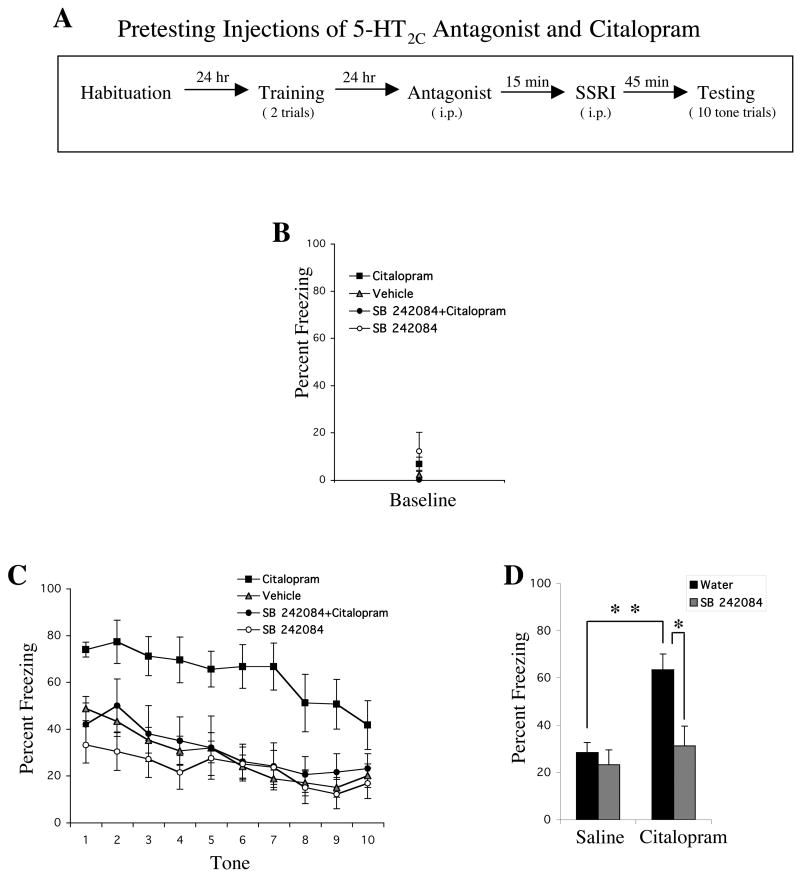
Pretreatment with a 5-HT2C Antagonist Blocks the Effect of Citalopram on Conditioned Fear Expression
Pre-treatment with the 5-HT2C antagonist, SB-242084 (0.2 mg/kg, i.p.), significantly blocked the enhancing effects of citalopram (10 mg/kg, i.p.) on conditioned fear expression (Figure 4). We performed a three-way ANOVA with factors: antagonist (SB 242084 vs. water), SSRI (citalopram vs. saline), and tone trial (1–10; repeated measures). There were significant main effects of antagonist [F1,52=8.61, p<.01], SSRI [F1,52=11.35, p<.01] and tone trial [F9,468=15.05, p<.01] and a significant antagonist × SSRI interaction [F1,52=4.48, p<.05]. The other two-way interactions were not significant and the three-way interaction was also not significant. Post hoc comparison using Tukey’s HSD confirmed the previously described significant effect of citalopram (p<.01). It also indicated that SB 242084 had no significant effect on its own (p=.90), but did reverse the effects of citalopram (p<.05). An analysis on baseline freezing revealed no differences between any of the groups.
Figure 4.
Pre-treatment with the 5-HT2C receptor antagonist, SB 242084 (0.2 mg/kg, i.p.), blocked the enhancing effects of citalopram (10 mg/kg, i.p.) on conditioned fear expression. (A) General behavioral procedures: 24 hours after habituation, rats were fear conditioned with two tone-shock pairings. The next day, animals were given 2 injections. The first injection of SB 242084 or water was given 15 minutes before the second injection of citalopram or saline. Forty-five minutes later, rats were tested to 10 presentations of the tone alone. When one of the two injections contained vehicle, only the drug condition is indicated in Figures B and C. (B) Mean ± SEM percent freezing of rats treated with citalopram (n=9), vehicle (n=23), SB 242084+citalopram (n=10), or SB 242084 (n=14) during the baseline period prior to the first tone trial. (C) Mean ± SEM percent freezing of each group during each tone trial. (D) Mean ± SEM percent freezing of each group averaged across all 10 tones. * indicates that p<.05 and ** indicates p<.01 using Tukey’s HSD post hoc test.
Discussion
Effects of SSRIs on Conditioned Fear Expression
Previously, we reported that a single pre-training injection of the SSRI citalopram enhanced the acquisition of auditory fear conditioning (Burghardt et al 2004). Here we show that a single pre-testing injection of citalopram or the SSRI fluoxetine enhanced conditioned fear expression. Given that animals in the present set of experiments were given drug treatment after fear acquisition, and patients are typically treated with SSRIs for their anxiety symptoms after the disorder has already developed, the current findings examine the effects of SSRIs in a manner that resembles the clinical setting. Although fear and anxiety are distinct, our results showing that acute SSRI treatment increases fear are consistent with reports that symptoms of anxiety are often exacerbated during early stages of SSRI treatment (Mir 1997; Spigset 1999).
The acute effects of SSRIs have also been explored in a number of different animal models of anxiety. While our findings are consistent with the anxiogenic-like effects of acute SSRI treatment reported using the social interaction test (Bagdy et al 2001; Dekeyne et al 2000), the elevated plus maze (Silva and Brandao 2000), and the open field test (Matto and Allikmets 1999), these effects are not found in all animal models of anxiety (Matto and Allikmets 1999; Poltronieri et al 2003; Schreiber et al 1998).
Interestingly, pre-testing citalopram treatment decreases freezing to a fear conditioned context, using the same dose as that used in the present study (Hashimoto et al 1996). Therefore, acute SSRI treatment may have the opposite effect on contextual and auditory fear conditioning. A similar discrepancy was described for the effects of acute SSRI treatment on the acquisition of conditioned fear. While we found that a single pre-training injection of citalopram enhanced the acquisition of auditory fear conditioning (Burghardt et al 2004), others report that the same drug treatment impaired the acquisition of contextual fear conditioning (Inoue et al 1996a). Although there are a number of factors that may account for these differential effects (see below), the enhanced conditioned fear expression we report using auditory fear conditioning more closely resembles the initial anxiogenic effects reported clinically. As a result, auditory fear conditioning, but not contextual fear conditioning, can be used as a model for understanding the anxiogenic effects of SSRI treatment.
These differential effects of acute SSRI treatment on auditory and contextual fear conditioning may be attributable to differences in the neural circuits that are recruited by these tasks and their respective serotonergic inputs. Lesion studies indicate that both the hippocampus and amygdala are involved in the processing of complex, polymodal stimuli required for contextual fear conditioning (Kim and Fanselow 1992; Phillips and LeDoux 1992), while the amygdala, but not the hippocampus, is involved in the processing of simple, modality-specific information required for auditory fear conditioning (Phillips and LeDoux 1992). The hippocampus receives its serotonergic input primarily from the median raphe (Vertes et al 1999), while the serotonergic inputs to the amygdala arise primarily from the dorsal raphe (Vertes 1991; Vertes et al 1999). Consequently, differences in the effects of acute SSRI administration on contextual and auditory fear conditioning may reflect differences in the primary sources of serotonin to the brain regions that underlie these tasks.
An analysis of freezing behavior prior to tone onset indicates that there were no differences in baseline freezing between animals treated with citalopram or fluoxetine and their respective control groups. Therefore drug treatment did not increase freezing in a non-specific manner. A number of other studies assessing the effects of citalopram or fluoxetine on movement found no evidence of a motor impairment with the doses used in this study (Belzung et al 2001; Hashimoto et al 1996). The low levels of freezing across groups during the pre-CS time period also indicate that there was no generalization of fear from the training to the testing context. Rather, the increase in freezing resulting from SSRI treatment appears to tone-specific. Furthermore, our earlier study (Burghardt et al 2004) shows that citalopram does not increase freezing responses to a tone before the tone is associated with a shock, suggesting that the increase in freezing reported here reflects enhanced expression of conditioned fear and not a non-specific change in reactivity to the tone.
Effects of Tianeptine on Conditioned Fear Expression
Tianeptine is purported to be a serotonin reuptake enhancer (Datla and Curzon 1993; Fattaccini et al 1990), although recent studies suggest that it is involved in modulating glutamatergic transmission (Kole et al 2002; Reagan et al 2004). Clinically, tianeptine has been found to be just as effective as SSRIs in treating depression and symptoms of anxiety in depressed patients (Lepine et al 2001). Unlike fluoxetine, tianeptine has been associated with a decrease in the requirement for concomitant anxiolytic prescription (Alby 1993). Although such findings indicate that it may have anxiolytic properties, tianeptine treatment has not been explored in patients with anxiety disorders. Animal studies have found that daily treatment with tianeptine (10 mg/kg) prevents stress-induced dendritic atrophy in the hippocampus (Conrad et al 1999), and stress-induced spatial memory impairments in the Y-maze (Conrad et al 1996).
Using the same dose shown to be sufficient in the remodeling of dendrites, we found no effect of a single pre-testing injection of tianeptine (10 mg/kg) on conditioned fear expression. Similarly, we found no effect of a pre-training tianeptine on the acquisition of auditory fear conditioning in our previous study (Burghardt et al 2004), and others have found no acute tianeptine effects using the social interaction test (File and Mabbutt 1991), immobility time test, or tests of spatial memory (Morris et al 2001; Nowakowska et al 2000). Although we found no acute effect of tianeptine, the anxiolytic potential of chronic administration at this dose is suggested by our previous findings that daily tianeptine treatment decreases the acquisition of auditory fear conditioning (Burghardt et al 2004). If tianeptine is found to effectively treat anxiety disorders, then the lack of an acute effect on fear expression suggests that tianeptine may be preferable to SSRIs.
Effects of Tomoxetine on Conditioned Fear Expression
Serotonin/norepinephrine reuptake inhibitors (SNRIs) have been shown to be at least as effect as SSRIs in the treatment of anxiety disorders (Silverstone 2004). Interestingly, SNRIs have not been found to exacerbate symptoms of anxiety in patients with anxiety disorders or depression, despite the expectation that such side effects would result from activation of the norepinephrine system (Silverstone 2004). To further evaluate the role of norepinephrine in modulating the fear system, we tested the effects of tomoxetine, a selective norepinephrine reuptake enhancer, on the expression of conditioned fear. We found that a pre-testing injection of tomoxetine, at a dose that significantly increases norepinephrine levels without affecting serotonin (Bymaster et al 2002), had no significant effect on freezing responses to the tone. Similarly, it was reported that a pre-testing injection of other norepinephrine reuptake inhibitors did not affect contextual fear conditioning (Hashimoto et al 1996). These findings indicate that selectively increasing extracellular norepinephrine does not have an anxiogenic effect. Rather, this effect seems to be specifically mediated by increases in serotonin resulting from SSRI treatment.
Effects of a 5-HT3 Receptor Antagonist on Conditioned Fear Expression
Preclinical studies indicating that selective 5-HT3 antagonists are anxiolytic (Costall and Naylor 1992; Kilfoil et al 1989), although the results of additional studies have been less consistent (Cutler et al 1997; Rodgers et al 1997). Using a dose that others found to significantly reduce contextual fear conditioning (Yoshioka et al 1995), we found no effect of pre-testing tropisetron, a 5-HT3 antagonist, on auditory fear conditioning. Perhaps these differential effects can be accounted for by the aforementioned differences in the way serotonin modulates the dissociable neural circuits that mediate these tasks.
To examine the functional relationship between SSRIs and the 5-HT3 receptor we administered citalopram and tropisetron together and tested their effects on conditioned fear expression. We found that blocking the 5-HT3 receptor did not reverse the citalopram-induced enhancement of fear expression, indicating that the 5-HT3 receptor may not be involved in mediating this effect.
The Effects of 5-HT2C Receptor Antagonists on Conditioned Fear Expression
Clinical and preclinical studies suggest a role for 5-HT2 receptors in anxiety. For example, the non-selective 5-HT2 agonist mCPP increases symptoms of anxiety in patients with panic disorder and agoraphobia (Charney et al 1987). Conversely, the mixed 5-HT2A/2C antagonist ritanserin is anxiolytic in patients with generalized anxiety disorder (Ceulemans et al 1985) and agoraphobia (Humble 1986), and decreases conditioned fear responses in healthy humans (Hensman et al 1991). Animal studies indicate a similar anxiogenic effect of mCPP administration (Bagdy et al 2001; Guitton and Dudai 2004), and an anxiolytic effect of the selective 5-HT2C receptor antagonist, SB 242084 (Martin et al 2002), implicating the involvement of this specific receptor subtype in anxiety.
We tested the effects of SB 242084 on the expression of auditory fear conditioning using the minimal dose shown to be effective in increasing social interaction (Bagdy et al 2001), and found that it did not significantly reduce freezing. Similarly, others report that 5-HT2 antagonists had no effect on contextual fear conditioning (Inoue et al 1996b), or tail suspension responses (Cremers et al 2004).
The Involvement of 5-HT2C Receptors in the Acute Effects of Citalopram
It has been shown that selective blockade of the 5-HT2C receptor with SB 242084 blocks the anxiogenic effects of SSRIs in the social interaction test (Bagdy et al 2001; Dekeyne et al 2000), although 5-HT2 receptor antagonists do not reverse the anxiogenic effects of SSRIs in the free-exploration test. To address whether the relationship between 5-HT2C receptors and SSRIs described using the social interaction test can be generalized to other fear/anxiety related behaviors, we assessed the effects of 5-HT2C receptor blockade on acute SSRI-induced increases in conditioned fear expression. We found that pretreatment with the selective 5-HT2C antagonist, SB 242084, reversed the citalopram-induced enhancement of fear expression. It is unlikely that these results are attributable to non-specific effects of the antagonist, since there were no differences between any treatment groups in baseline freezing, no effects of SB 242084 alone on conditioned fear expression, and other groups report that the dose of SB 242084 used in this study does not impair movement (Kennett et al 1997; Martin et al 2002). The lack of an effect of SB 242084 alone indicates that 5-HT2C blockade may only have effects on auditory fear conditioning under conditions of elevated serotonin. Our results suggest that 5-HT2C receptors may play a significant role in mediating the anxiogenic effects of SSRI treatment found clinically, and that blockade of these receptors could be therapeutically advantageous.
Our past and present results showing that systemic SSRI treatment modulates auditory fear conditioning implicate the amygdala as a possible site of action for these drugs. Given that direct infusions of a 5-HT2 receptor agonist into the amygdala has anxiogenic effects (Campbell and Merchant 2003), it is possible that the enhancement we find in conditioned fear expression following SSRI treatment is attributable to activation of these particular amygdala serotonin receptors. Future studies involving intra-amygdala infusions of 5-HT2C receptor antagonists, in animals treated systemically with an SSRI, are required before this conclusion can be drawn.
Summary and Conclusions
In summary, we extended our previous findings that acute SSRI treatment enhances the acquisition of auditory fear conditioning by showing that acute SSRIs also enhance the expression of conditioned fear. This effect was found with two different SSRIs, citalopram and fluoxetine, but not with tianeptine, a purported serotonin reuptake enhancer, or tomoxetine, a norepinephrine reuptake inhibitor. Therefore the effect seems to be specific to SSRIs. We also show that the SSRI-induced enhancement in fear expression is blocked by systemic administration of a 5-HT2C, but not a 5-HT3, receptor antagonist. These findings indicate that the 5-HT2C receptors may be a mechanism through which the anxiogenic effects of SSRIs are mediated in humans. Clinically, our findings suggest that co-administration of 5-HT2C receptor antagonists with SSRIs may help prevent the increase in symptoms of anxiety often reported during early stages of treatment.
Acknowledgments
This work was supported by the NIH grant P50 MH58911.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alby JM, Ferreri M, Cabanne J, De Bodinat C, Dagens V. Efficacy of tianeptine (Stablon) for the treatment of major depression and dysthymea with somatic complaint. A comparative study versus fluoxetine (Prozac) Ann Psychiatry. 1993;8:136–144. [Google Scholar]
- Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. Int J Neuropsychopharmacol. 2001;4:399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- Belzung C, Le Guisquet AM, Barreau S, Calatayud F. An investigation of the mechanisms responsible for acute fluoxetine-induced anxiogenic-like effects in mice. Behav Pharmacol. 2001;12:151–62. doi: 10.1097/00008877-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Bingefors K, Isacson DG. Concomitant prescribing of tranquilizers and hypnotics among patients receiving antidepressant prescriptions. Ann Pharmacother. 1998;32:531–5. doi: 10.1345/aph.17211. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Quirion R, Meaney MJ. A comparison of the effects of diazepam versus several typical and atypical anti-depressant drugs in an animal model of anxiety. Psychopharmacology. 1989;97:277–9. doi: 10.1007/BF00442264. [DOI] [PubMed] [Google Scholar]
- Bondareff W, Alpert M, Friedhoff AJ, Richter EM, Clary CM, Batzar E. Comparison of sertraline and nortriptyline in the treatment of major depressive disorder in late life. Am J Psychiatry. 2000;157:729–36. doi: 10.1176/appi.ajp.157.5.729. [DOI] [PubMed] [Google Scholar]
- Bosker FJ, Cremers TI, Jongsma ME, Westerink BH, Wikstrom HV, den Boer JA. Acute and chronic effects of citalopram on postsynaptic 5-hydroxytryptamine(1A) receptor-mediated feedback: a microdialysis study in the amygdala. J Neurochem. 2001;76:1645–53. doi: 10.1046/j.1471-4159.2001.00194.x. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57:832–40. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficity/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–8. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Campbell BM, Merchant KM. Serotonin 2C receptors within the basolateral amygdala induce acute fear-like responses in an open-field environment. Brain Res. 2003;993:1–9. doi: 10.1016/s0006-8993(03)03384-5. [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proc Natl Acad Sci U S A. 2005;102:12224–9. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannistraro PA, Wright CI, Wedig MM, et al. Amygdala responses to human faces in obsessive-compulsive disorder. Biol Psychiatry. 2004;56:916–20. doi: 10.1016/j.biopsych.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Ceulemans DL, Hoppenbrouwers ML, Gelders YG, Reyntjens AJ. The influence of ritanserin, a serotonin antagonist, in anxiety disorders: a double-blind placebo-controlled study versus lorazepam. Pharmacopsychiatry. 1985;18:303–5. doi: 10.1055/s-2007-1017385. [DOI] [PubMed] [Google Scholar]
- Charney DS, Woods SW, Goodman WK, Heninger GR. Serotonin function in anxiety. II. Effects of the serotonin agonist MCPP in panic disorder patients and healthy subjects. Psychopharmacology (Berl) 1987;92:14–24. doi: 10.1007/BF00215473. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–34. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–13. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Costall B, Naylor RJ. Anxiolytic potential of 5-HT3 receptor antagonists. Pharmacol Toxicol. 1992;70:157–62. doi: 10.1111/j.1600-0773.1992.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Costall B, Naylor RJ. Anxiolytic effects of 5-HT3 antagonists in animals. In: Rodgers RJ, Cooper SJ, editors. 5-HT1A Agonists, 5-HT3 Antagonists and Benzodiazepines: Their Comparative Behavioral Pharmacology. Chichester, UK: Wiley; 1991. pp. 133–157. [Google Scholar]
- Cremers TI, Giorgetti M, Bosker FJ, et al. Inactivation of 5-HT(2C) receptors potentiates consequences of serotonin reuptake blockade. Neuropsychopharmacology. 2004;29:1782–9. doi: 10.1038/sj.npp.1300474. [DOI] [PubMed] [Google Scholar]
- Cutler MG, Rodgers RJ, Jackson JE. Behavioural effects in mice of subchronic buspirone, ondansetron and tianeptine. I. Social interactions. Pharmacol Biochem Behav. 1997;56:287–93. doi: 10.1016/s0091-3057(96)00241-9. [DOI] [PubMed] [Google Scholar]
- Datla KP, Curzon G. Behavioural and neurochemical evidence for the decrease of brain extracellular 5-HT by the antidepressant drug tianeptine. Neuropharmacology. 1993;32:839–45. doi: 10.1016/0028-3908(93)90138-s. [DOI] [PubMed] [Google Scholar]
- Dekeyne A, Denorme B, Monneyron S, Millan MJ. Citalopram reduces social interaction in rats by activation of serotonin (5-HT)(2C) receptors. Neuropharmacology. 2000;39:1114–7. doi: 10.1016/s0028-3908(99)00268-3. [DOI] [PubMed] [Google Scholar]
- Del-Ben CM, Deakin JF, McKie S, et al. The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: an FMRI study. Neuropsychopharmacology. 2005;30:1724–34. doi: 10.1038/sj.npp.1300728. [DOI] [PubMed] [Google Scholar]
- Fattaccini CM, Bolanos-Jimenez F, Gozlan H, Hamon M. Tianeptine stimulates uptake of 5-hydroxytryptamine in vivo in the rat brain. Neuropharmacology. 1990;29:1–8. doi: 10.1016/0028-3908(90)90076-4. [DOI] [PubMed] [Google Scholar]
- Feighner JP, Boyer WF. Selective serotonin re-uptake inhibitors : the clinical use of Citalopram, Fluoxetine, Fluvoxamine, Paroxetine, and Sertraline. Chichester; New York: Wiley; 1991. [Google Scholar]
- File S, Mabbutt P. Effects of tianeptine in animal models of anxiety and on learning and memory. Drug Development Research. 1991;23:47–56. [Google Scholar]
- Gorman JM, Liebowitz MR, Fyer AJ, et al. An open trial of fluoxetine in the treatment of panic attacks. J Clin Psychopharmacol. 1987;7:329–32. [PubMed] [Google Scholar]
- Gregor KJ, Riley JA, Downing DK. Concomitant use of anxiolytics and hypnotics with selective serotonin reuptake inhibitors. Clin Ther. 1996;18:521–7. doi: 10.1016/s0149-2918(96)80034-8. discussion 520. [DOI] [PubMed] [Google Scholar]
- Griebel G, Moreau JL, Jenck F, Misslin R, Martin JR. Acute and chronic treatment with 5-HT reuptake inhibitors differentially modulate emotional responses in anxiety models in rodents. Psychopharmacology. 1994;113:463–70. doi: 10.1007/BF02245224. [DOI] [PubMed] [Google Scholar]
- Guitton MJ, Dudai Y. Anxiety-like state associates with taste to produce conditioned taste aversion. Biol Psychiatry. 2004;56:901–4. doi: 10.1016/j.biopsych.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala.[comment] Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Inoue T, Koyama T. Serotonin reuptake inhibitors reduce conditioned fear stress-induced freezing behavior in rats. Psychopharmacology (Berl) 1996;123:182–6. doi: 10.1007/BF02246175. [DOI] [PubMed] [Google Scholar]
- Hensman R, Guimaraes FS, Wang M, Deakin JF. Effects of ritanserin on aversive classical conditioning in humans. Psychopharmacology. 1991;104:220–4. doi: 10.1007/BF02244182. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Joharchi N, Sellers EM. Behavioral effects of the 5-hydroxytryptamine3 receptor agonists 1-phenylbiguanide and m-chlorophenylbiguanide in rats. J Pharmacol Exp Ther. 1993;264:1440–9. [PubMed] [Google Scholar]
- Humble M, Asberg-Wistedt A, Wistedt B, Bertilsson L. A pilot study of ritanserin (a selective serotonin-2 receptor antagonist) in panic disorder and agoraphobia. Clinical and biochemical effects. CINP Congress; Puerto Rico. December 14–17; 1986. p. 165. [Google Scholar]
- Inoue T, Hashimoto S, Tsuchiya K, Izumi T, Ohmori T, Koyama T. Effect of citalopram, a selective serotonin reuptake inhibitor, on the acquisition of conditioned freezing. European Journal of Pharmacology. 1996a;311:1–6. doi: 10.1016/0014-2999(96)00391-3. [DOI] [PubMed] [Google Scholar]
- Inoue T, Tsuchiya K, Koyama T. Serotonergic activation reduces defensive freezing in the conditioned fear paradigm. Pharmacol Biochem Behav. 1996b;53:825–31. doi: 10.1016/0091-3057(95)02084-5. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, et al. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:609–20. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- Kent JM, Coplan JD, Gorman JM. Clinical utility of the selective serotonin reuptake inhibitors in the spectrum of anxiety. Biol Psychiatry. 1998;44:812–24. doi: 10.1016/s0006-3223(98)00210-8. [DOI] [PubMed] [Google Scholar]
- Kilfoil T, Michel A, Montgomery D, Whiting RL. Effects of anxiolytic and anxiogenic drugs on exploratory activity in a simple model of anxiety in mice. Neuropharmacology. 1989;28:901–5. doi: 10.1016/0028-3908(89)90188-3. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kole MH, Swan L, Fuchs E. The antidepressant tianeptine persistently modulates glutamate receptor currents of the hippocampal CA3 commissural associational synapse in chronically stressed rats. Eur J Neurosci. 2002;16:807–16. doi: 10.1046/j.1460-9568.2002.02136.x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lepine JP, Altamura C, Ansseau M, et al. Tianeptine and paroxetine in major depressive disorder, with a special focus on the anxious component in depression: an international, 6-week double-blind study dagger. Hum Psychopharmacol. 2001;16:219–227. doi: 10.1002/hup.289. [DOI] [PubMed] [Google Scholar]
- Maren S. Is there savings for pavlovian fear conditioning after neurotoxic basolateral amygdala lesions in rats? Neurobiology of Learning & Memory. 2001;76:268–83. doi: 10.1006/nlme.2001.4042. [DOI] [PubMed] [Google Scholar]
- Martin JR, Ballard TM, Higgins GA. Influence of the 5-HT2C receptor antagonist, SB-242084, in tests of anxiety. Pharmacol Biochem Behav. 2002;71:615–25. doi: 10.1016/s0091-3057(01)00713-4. [DOI] [PubMed] [Google Scholar]
- Martin P, Puech AJ. Antagonism by benzodiazepines of the effects of serotonin-, but not norepinephrine-, uptake blockers in the learned helplessness paradigm in rats. Biol Psychiatry. 1996;39:882–90. doi: 10.1016/0006-3223(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Matto V, Allikmets L. Acute and chronic citalopram treatment differently modulates rat exploratory behavior in the exploration box test: no evidence for increased anxiety or changes in the [3H]raclopride binding. Pharmacology. 1999;58:59–69. doi: 10.1159/000028269. [DOI] [PubMed] [Google Scholar]
- Matto V, Harro J, Allikmets L. The effects of cholecystokinin A and B receptor antagonists, devazepide and L 365260, on citalopram-induced decrease of exploratory behaviour in rat. J Physiol Pharmacol. 1996;47:661–9. [PubMed] [Google Scholar]
- McKie S, Del-Ben C, Elliott R, et al. Neuronal effects of acute citalopram detected by pharmacoMRI. Psychopharmacology (Berl) 2005;180:680–6. doi: 10.1007/s00213-005-2270-y. [DOI] [PubMed] [Google Scholar]
- Milham MP, Nugent AC, Drevets WC, et al. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biol Psychiatry. 2005;57:961–6. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Mir S, Taylor D. The adverse effects of antidepressants. Current Opinion in Psychiatry. 1997;10:88–94. [Google Scholar]
- Morelli M, Pinna A, Ruiu S, Del Zompo M. Induction of Fos-like-immunoreactivity in the central extended amygdala by antidepressant drugs. Synapse. 1999;31:1–4. doi: 10.1002/(SICI)1098-2396(199901)31:1<1::AID-SYN1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Morris RG, Kelly S, Burney D, Anthony T, Boyer PA, Spedding M. Tianeptine and its enantiomers: effects on spatial memory in rats with medial septum lesions. Neuropharmacology. 2001;41:272–81. doi: 10.1016/s0028-3908(01)00058-2. [DOI] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–91. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Nader K, LeDoux J. The dopaminergic modulation of fear: quinpirole impairs the recall of emotional memories in rats. Behavioral Neuroscience. 1999;113:152–65. doi: 10.1037//0735-7044.113.1.152. [DOI] [PubMed] [Google Scholar]
- Nowakowska E, Kus K, Chodera A, Rybakowski J. Behavioural effects of fluoxetine and tianeptine, two antidepressants with opposite action mechanisms, in rats. Arzneimittelforschung. 2000;50:5–10. doi: 10.1055/s-0031-1300156. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. Benzodiazepine use, abuse, and dependence. J Clin Psychiatry. 2005;66(Suppl 2):28–33. [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pineyro G, Blier P. Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol Rev. 1999;51:533–91. [PubMed] [Google Scholar]
- Pollack MH, Doyle AC. Treatment of panic disorder: focus on paroxetine. Psychopharmacol Bull. 2003;37(Suppl 1):53–63. [PubMed] [Google Scholar]
- Poltronieri SC, Zangrossi H, Jr, de Barros Viana M. Antipanic-like effect of serotonin reuptake inhibitors in the elevated T-maze. Behav Brain Res. 2003;147:185–92. doi: 10.1016/s0166-4328(03)00151-7. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cutler MG, Jackson JE. Behavioural effects in mice of subchronic buspirone, ondansetron and tianeptine. II. The elevated plus-maze. Pharmacol Biochem Behav. 1997;56:295–303. doi: 10.1016/s0091-3057(96)00242-0. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike? Psychopharmacology. 1997;129:197–205. doi: 10.1007/s002130050181. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learning & Memory. 1999;6:97–110. [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Melon C, De Vry J. The role of 5-HT receptor subtypes in the anxiolytic effects of selective serotonin reuptake inhibitors in the rat ultrasonic vocalization test. Psychopharmacology. 1998;135:383–91. doi: 10.1007/s002130050526. [DOI] [PubMed] [Google Scholar]
- Silva RC, Brandao ML. Acute and chronic effects of gepirone and fluoxetine in rats tested in the elevated plus-maze: an ethological analysis. Pharmacology, Biochemistry & Behavior. 2000;65:209–16. doi: 10.1016/s0091-3057(99)00193-8. [DOI] [PubMed] [Google Scholar]
- Silverstone PH. Qualitative review of SNRIs in anxiety. J Clin Psychiatry. 2004;65(Suppl 17):19–28. [PubMed] [Google Scholar]
- Spigset O. Adverse reactions of selective serotonin reuptake inhibitors: reports from a spontaneous reporting system. Drug Saf. 1999;20:277–87. doi: 10.2165/00002018-199920030-00007. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Placebo-controlled comparison of the selective serotonin reuptake inhibitors citalopram and sertraline. Biol Psychiatry. 2000;48:894–901. doi: 10.1016/s0006-3223(00)00957-4. [DOI] [PubMed] [Google Scholar]
- Stein C, Davidowa H, Albrecht D. 5-HT(1A) receptor-mediated inhibition and 5-HT(2) as well as 5-HT(3) receptor-mediated excitation in different subdivisions of the rat amygdala. Synapse. 2000;38:328–37. doi: 10.1002/1098-2396(20001201)38:3<328::AID-SYN12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Stahl S. Serotonin and anxiety: current models. Int Clin Psychopharmacol. 2000;15(Suppl 2):S1–6. doi: 10.1097/00004850-200008002-00002. [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, McEwen BS, LeDoux JE. Serotonin modulation of sensory inputs to the lateral amygdala: dependency on corticosterone. Journal of Neuroscience. 1998;18:9529–38. doi: 10.1523/JNEUROSCI.18-22-09529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JG, Coolen LM, Spooren WJ, et al. Patterns of c-fos expression induced by fluvoxamine are different after acute vs. chronic oral administration. Eur Neuropsychopharmacol. 1998;8:213–26. doi: 10.1016/s0924-977x(97)00072-2. [DOI] [PubMed] [Google Scholar]
- Verster JC, Volkerts ER. Clinical pharmacology, clinical efficacy, and behavioral toxicity of alprazolam: a review of the literature. CNS Drug Rev. 2004;10:45–76. doi: 10.1111/j.1527-3458.2004.tb00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. Journal of Comparative Neurology. 1991;313:643–68. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. Journal of Comparative Neurology. 1999;407:555–82. [PubMed] [Google Scholar]
- Yoshioka M, Matsumoto M, Togashi H, Saito H. Effects of conditioned fear stress on 5-HT release in the rat prefrontal cortex. Pharmacol Biochem Behav. 1995;51:515–9. doi: 10.1016/0091-3057(95)00045-x. [DOI] [PubMed] [Google Scholar]