Abstract
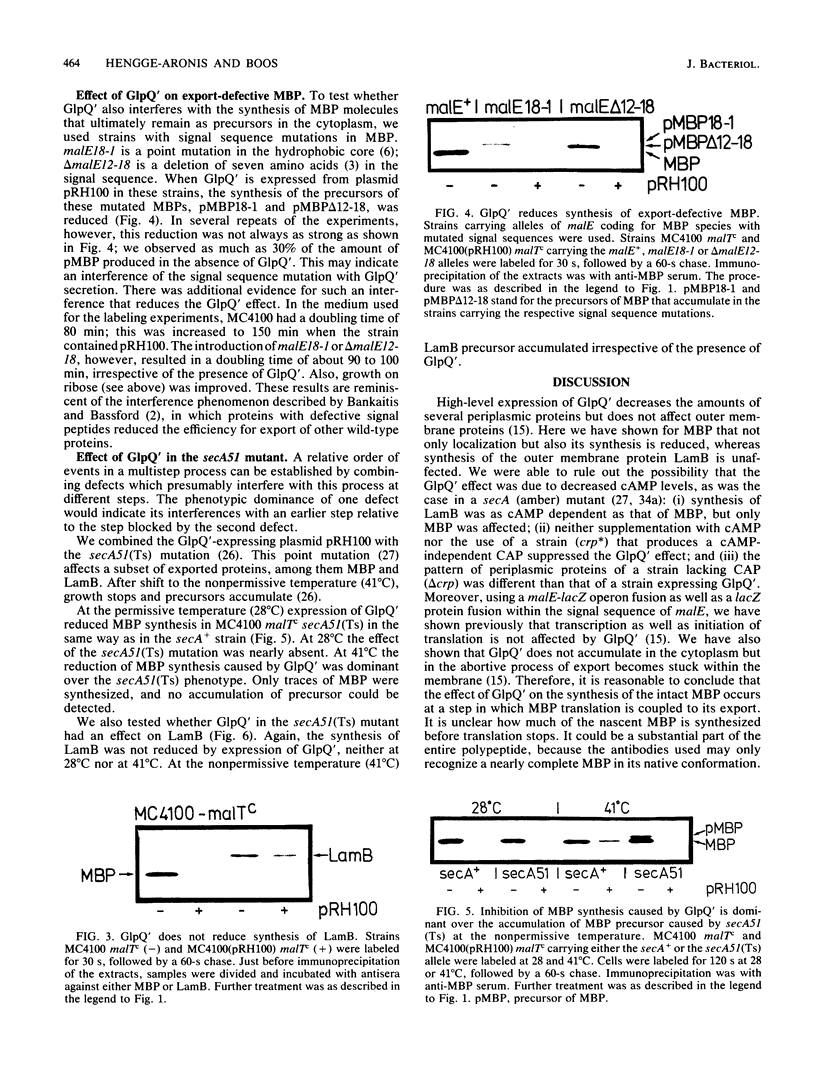
We recently described the suppression of export of a class of periplasmic proteins of Escherichia coli caused by overproduction of a C-terminal truncated periplasmic enzyme (GlpQ'). This truncated protein was not released into the periplasm but remained attached to the inner membrane and was accessible from the periplasm. The presence of GlpQ' in the membrane strongly reduced the appearance in the periplasm of some periplasmic proteins, including the maltose-binding protein (MBP), but did not affect outer membrane proteins, including the lambda receptor (LamB) (R. Hengge and W. Boos, J. Bacteriol., 162:972-978, 1985). To investigate this phenomenon further we examined the fate of MBP in comparison with the outer membrane protein LamB. We found that not only localization but also synthesis of MBP was impaired, indicating a coupling of translation and export. Synthesis and secretion of LamB were not affected. The possibility that this influence was exerted via the level of cyclic AMP could be excluded. Synthesis of MBP with altered signal sequences was also reduced, demonstrating that export-defective MBP which ultimately remains in the cytoplasm abortively enters the export pathway. When GlpQ' was expressed in a secA51(Ts) strain, the inhibition of MBP synthesis caused by GlpQ' was dominant over the precursor accumulation usually caused by secA51(Ts) at 41 degrees C. Therefore, GlpQ' acts before or at the level of recognition by SecA. For LamB the usual secA51(Ts) phenotype was observed. We propose a mechanism by which GlpQ' blocks an yet unknown membrane protein, the function of which is to couple translation and export of a subclass of periplasmic proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Ito K. The SecY membrane component of the bacterial protein export machinery: analysis by new electrophoretic methods for integral membrane proteins. EMBO J. 1985 Dec 1;4(12):3351–3356. doi: 10.1002/j.1460-2075.1985.tb04088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis V. A., Bassford P. J., Jr The synthesis of export-defective proteins can interfere with normal protein export in Escherichia coli. J Biol Chem. 1984 Oct 10;259(19):12193–12200. [PubMed] [Google Scholar]
- Bankaitis V. A., Rasmussen B. A., Bassford P. J., Jr Intragenic suppressor mutations that restore export of maltose binding protein with a truncated signal peptide. Cell. 1984 May;37(1):243–252. doi: 10.1016/0092-8674(84)90320-9. [DOI] [PubMed] [Google Scholar]
- Bassford P. J., Jr, Silhavy T. J., Beckwith J. R. Use of gene fusion to study secretion of maltose-binding protein into Escherichia coli periplasm. J Bacteriol. 1979 Jul;139(1):19–31. doi: 10.1128/jb.139.1.19-31.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedouelle H., Bassford P. J., Jr, Fowler A. V., Zabin I., Beckwith J., Hofnung M. Mutations which alter the function of the signal sequence of the maltose binding protein of Escherichia coli. Nature. 1980 May 8;285(5760):78–81. doi: 10.1038/285078a0. [DOI] [PubMed] [Google Scholar]
- Benson S. A., Bremer E., Silhavy T. J. Intragenic regions required for LamB export. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3830–3834. doi: 10.1073/pnas.81.12.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S. A., Hall M. N., Silhavy T. J. Genetic analysis of protein export in Escherichia coli K12. Annu Rev Biochem. 1985;54:101–134. doi: 10.1146/annurev.bi.54.070185.000533. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Hanley-Way S., Silhavy T. J. Suppressor mutations that restore export of a protein with a defective signal sequence. Cell. 1981 Jan;23(1):79–88. doi: 10.1016/0092-8674(81)90272-5. [DOI] [PubMed] [Google Scholar]
- Ferro-Novick S., Honma M., Beckwith J. The product of gene secC is involved in the synthesis of exported proteins in E. coli. Cell. 1984 Aug;38(1):211–217. doi: 10.1016/0092-8674(84)90542-7. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Gabay J., Schwartz M. Evidence for a coupling of synthesis and export of an outer membrane protein in Escherichia coli. EMBO J. 1983;2(1):15–19. doi: 10.1002/j.1460-2075.1983.tb01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge R., Boos W. Defective secretion of maltose- and ribose-binding proteins caused by a truncated periplasmic protein in Escherichia coli. J Bacteriol. 1985 Jun;162(3):972–978. doi: 10.1128/jb.162.3.972-978.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge R., Larson T. J., Boos W. sn-Glycerol-3-phosphate transport in Salmonella typhimurium. J Bacteriol. 1983 Jul;155(1):186–195. doi: 10.1128/jb.155.1.186-195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Soberon X., Franceschini T., Nakamura K., Itakura K., Inouye M. Role of positive charge on the amino-terminal region of the signal peptide in protein secretion across the membrane. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3438–3441. doi: 10.1073/pnas.79.11.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Ito K., Sato T., Yura T. Synthesis and assembly of the membrane proteins in E. coli. Cell. 1977 Jul;11(3):551–559. doi: 10.1016/0092-8674(77)90073-3. [DOI] [PubMed] [Google Scholar]
- Jackson M. E., Pratt J. M., Stoker N. G., Holland I. B. An inner membrane protein N-terminal signal sequence is able to promote efficient localisation of an outer membrane protein in Escherichia coli. EMBO J. 1985 Sep;4(9):2377–2383. doi: 10.1002/j.1460-2075.1985.tb03942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Nikaido H., Wu H. C. Amino acid sequence homology among the major outer membrane proteins of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1048–1052. doi: 10.1073/pnas.81.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982 Aug;30(1):311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Export of protein in bacteria. Microbiol Rev. 1984 Dec;48(4):290–298. doi: 10.1128/mr.48.4.290-298.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L. Translocation of domains of nascent periplasmic proteins across the cytoplasmic membrane is independent of elongation. Cell. 1983 May;33(1):231–240. doi: 10.1016/0092-8674(83)90352-5. [DOI] [PubMed] [Google Scholar]
- Sabourin D., Beckwith J. Deletion of the Escherichia coli crp gene. J Bacteriol. 1975 Apr;122(1):338–340. doi: 10.1128/jb.122.1.338-340.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz J., Silhavy T. J., Berman M. L., Fiil N., Emr S. D. A previously unidentified gene in the spc operon of Escherichia coli K12 specifies a component of the protein export machinery. Cell. 1982 Nov;31(1):227–235. doi: 10.1016/0092-8674(82)90422-6. [DOI] [PubMed] [Google Scholar]
- Shuman H. A., Silhavy T. J., Beckwith J. R. Labeling of proteins with beta-galactosidase by gene fusion. Identification of a cytoplasmic membrane component of the Escherichia coli maltose transport system. J Biol Chem. 1980 Jan 10;255(1):168–174. [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Kumamoto C. A., Beckwith J. Does secA mediate coupling between secretion and translation in Escherichia coli? J Bacteriol. 1986 May;166(2):505–512. doi: 10.1128/jb.166.2.505-512.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., van Tol H., Lugtenberg B. The ultimate localization of an outer membrane protein of Escherichia coli K-12 is not determined by the signal sequence. EMBO J. 1983;2(8):1275–1279. doi: 10.1002/j.1460-2075.1983.tb01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Gilmore R., Blobel G. Protein translocation across the endoplasmic reticulum. Cell. 1984 Aug;38(1):5–8. doi: 10.1016/0092-8674(84)90520-8. [DOI] [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]