Abstract
Studies on patients and large animal models suggest the importance of atrial fibrosis in the development of atrial fibrillation (AF). To investigate whether increased fibrosis is sufficient to produce a substrate for AF, we have studied cardiac electrophysiology (EP) and inducibility of atrial arrhythmias in MHC-TGFcys33ser transgenic mice (Tx), which have increased fibrosis in the atrium but not in the ventricles. In anesthetized mice, wild-type (Wt) and Tx did not show significant differences in surface ECG parameters. With transesophageal atrial pacing, no significant differences were observed in EP parameters, except for a significant decrease in corrected sinus node recovery time in Tx mice. Burst pacing induced AF in 14 of 29 Tx mice, whereas AF was not induced in Wt littermates (P<0.01). In Langendorff perfused hearts, atrial conduction was studied using a 16-electrode array. Epicardial conduction velocity was significantly decreased in the Tx RA compared with the Wt RA. In the Tx LA, conduction velocity was not significantly different from Wt, but conduction was more heterogeneous. Action potential characteristics recorded with intracellular microelectrodes did not reveal differences between Wt and Tx mice in either atrium. Thus, in this transgenic mouse model, selective atrial fibrosis is sufficient to increase AF inducibility.
Keywords: atrial fibrillation, fibrosis, growth factors
Atrial fibrillation (AF) is a commonly occurring arrhythmia, present in ≈5% of people older than age 65 years. Clinically, increased vulnerability to AF is also associated with underlying heart disease, such as congestive heart failure (CHF) and mitral valve disease.1 Increased inducibility of AF has been observed in animal models of aging,2,3 CHF,4 atrial tachycardia-induced cardiomyopathy,5,6 and chronic atrial dilatation caused by mitral regurgitation.7
Theoretical models have implicated atrial interstitial fibrosis as a substrate for AF.8,9 Atrial interstitial fibrosis increases with age in humans and has been observed in patients with AF10,11 and in animal models of aging,2,3 mitral regurgitation,7 and CHF.4 With the unknown cause of atrial fibrosis in humans and the presence of compounding factors in animal models, the contribution of atrial fibrosis to AF substrate formation remains unclear. Studies to date have been limited by lack of animal models of selective atrial fibrosis to study the effects of fibrosis without the presence of heart failure or other underlying heart disease.
The purpose of this study was to determine the effect of atrial fibrosis on the AF vulnerability. We have studied a transgenic mouse model with cardiac overexpression of a constitutively active form of transforming growth factor (TGF)-β1, MHC-TGFcys33ser.12 This model has been previously demonstrated to have elevated TGF-β1 activity in the atria and ventricles. Cardiac development and morphology appear normal, except for increased interstitial fibrosis in the atrial myocardium. Ventricular size and histology is normal.12 We have used this model to study the impact of selective atrial fibrosis on cardiac electrophysiology and the substrate of atrial arrhythmias.
Methods
Animals
MHC-TGFcys33ser mice were generated and bred as described.12 Experiments were performed on wild-type (Wt) and transgenic (Tx) littermates. Genotypes were determined by polymerase chain reaction. In total, 30 Wt and 30 Tx mice were used, ranging in age from 90 to 365 days, with an average age of 193±90 and 184±92 days, respectively. Studies were in accordance with National Institutes of Health (NIH) guidelines.
Studies on Intact Mice
Electrophysiology studies were performed as previously described.13 Urethane (2 g/kg) was injected intraperitoneally for anesthesia. After reflexes had disappeared, mice were fixed to a temperature-regulated operating table. Platinum electrodes were inserted subcutaneously in the limbs and connected to a custom-built electrocardiogram (ECG) amplifier for a standard 6-lead ECG. The trachea was incised and a cannula was inserted, connected to a rodent ventilator (Palmer Ltd.), set to 130 cycles per minute with a tidal volume of ≈0.5 mL, limited to 5 mL water pressure. For atrial stimulation, a 4-French quadripolar catheter was advanced through the esophagus and placed at the site with the lowest threshold for atrial capture.
To measure the atrial effective refractory period (AERP) in a subgroup of 13 Wt and 10 Tx mice, the chest was opened in a V-shape from the xiphoid processus to the front legs. Atrial recording electrodes were made of 0.03 mm MP35N wire (Fort Wayne Metals, Fort Wayne Ind), bent at the tip to form a small hook. After opening the pericardium, a closely spaced electrode pair was attached to the myocardium at the RA.
Programmed Electrical Stimulation
Transesophageal stimulation electrodes were connected to a True-Type-Logic triggered stimulus isolation unit with variable current output. A stimulus amplitude of 1.5× diastolic capture threshold was used, with a stimulus duration of 1 ms.
Sinus node recovery time (SNRT) was measured after a 2-second pacing train with a basic cycle length (BCL) of 100 ms. The SNRT was defined as the interval between the last stimulus in the pacing train and the onset of first sinus return beat. The Wenckebach period (WCL) was determined by applying 2-second pacing trains with variable BCL. The atrial and atrioventricular nodal effective refractory periods (AERP and AVERP] were determined with a 2-second pacing train with a BCL of 100 ms, followed after a variable delay by an extrastimulus.
Inducibility of atrial arrhythmias was tested by applying 2-second bursts, using the automated stimulator that was part of the data acquisition software. The first 2-second burst had a cycle length (CL) of 40 ms, decreasing in each successive burst with a 2-ms decrement down to a CL of 20 ms. This series of bursts was repeated once. AF was defined as a period of rapid irregular atrial rhythm lasting at least 2 seconds. If 1 or more bursts in the 2 series of bursts evoked an AF episode, AF was considered to be inducible in that animal; otherwise, AF was considered to be noninducible.
Studies on Langendorff Perfused Hearts
After intraperitoneal injection of heparin (0.5 U/g) and urethane (2 mg/g), hearts from 7 Wt and 7 Tx mice were rapidly excised and placed in a tissue chamber at 36°C±1°C. The aorta was cannulated for retrograde perfusion at a pressure of 80 cm H20 with modified Tyrode solution (in mmol/L: NaCl 130, NaHCO3 24, NaH2PO4 1.2, MgCl2 1, glucose 5.6, KCl 4.0, CaCl2 1.8, gassed with 95% O2/5% CO2). Two chlorinated silver wires were placed in the bath as indifferent and common ground electrodes. Atrial unipolar electrograms were recorded using a 1.2×1.2-mm array of 4×4 unipolar recording electrodes with a pair of stimulation electrodes at the side of the array. The electrode array was pressed against the RA or LA surface, covering a large portion of the anterior aspect of the atrial appendage and free wall. During normal sinus rhythm, the maximum duration of electrograms was measured. Atrial conduction was assessed during continuous pacing with 1 of the bipolar stimulus pairs at BCLs 150, 120, 90, and 60 ms at a stimulus amplitude of 1.5× diastolic threshold and a stimulus duration of 1 ms. At the same BCLs, AERP was determined with a 2-second drive train, followed by a variable delay by an extrastimulus.
Histology
Whole hearts from 7 Tx and 7 Wt were mounted in tissue freezing medium (Triangle Biomedical Science, Durham, NC). Cryosections (5 μm) were fixed with formalin and stained with either Sirius red/fast green or Masson trichrome. Images were digitized using a Spot camera (Diagnostics Instruments). To quantify collagen deposition, red pixel content of digitized photos was measured relative the total tissue area (red and green pixels) using Adobe Photoshop 7 software.
Microelectrode Studies
In 5 mice in each group, microelectrode recordings were made in the left atrium (LA) and right atrium (RA). After excision of the heart, the ventricle was removed beneath the atrioventricular ring. The atrium was pinned down with the endocardial side up in a tissue bath. Modified Tyrode solution was superfused at 36°C±1°C. Glass microelectrodes with a resistance of 15 to 40 MΩ were filled with 3 mol/L KCl. Impalements were made on the endocardium of the appendage and free wall of both atria during pacing with a bipolar stimulation electrode with a pulse duration of 1 ms at a BCL of 150 ms. Microelectrode signals were recorded using a Duo 773 amplifier (WPI, Sarasota, Fla). The maximum upstroke velocity of action potential (Vmax), resting membrane potential, and the action potential durations (APDs) at 30%, 60%, and 90% repolarization (APD30, APD60, APD90) were measured off-line.
Data Sampling and Analysis
For open-chest experiments, signals from the ECG recorder and differential amplifiers were filtered at 500 Hz and sampled at 1 kHz using a 1401-Plus AD/DA converter (Cambridge Electronic Design Ltd). Data sampling, programmed electrical stimulation, and off-line analysis were controlled by custom-made software in Spike2 language (Cambridge Electronic Design Ltd). In measurements of atrial conduction, unipolar signals were bandpass-filtered at 0.1 Hz to 5 kHz using 2 Iso-DAM8 amplifiers (World Precision Instruments, Sarasota, Fla) and sampled at 8 kHz using a Gould ACQ-16 data acquisition interface.
To assess atrial conduction properties in Langendorff perfused hearts, activation time points were determined at each of the 16 electrodes as the point of maximal negative dV/dt. Activation, vector, and phase maps were constructed from these activation time points using custom-made software. Conduction vectors were used to calculate the average conduction velocity in the recording location. For phase maps, the difference in activation time point between an electrode and its neighboring electrodes was divided by the interelectrode distance to yield the phase, in ms/mm, for each electrode.4,14 The “heterogeneity range” was defined as the difference between the maximal and minimal phase within a phase map. The “heterogeneity index” was defined as the heterogeneity range divided by the median phase of the phase map.
Data were analyzed with a multivariate ANOVA and post hoc Newman-Keuls test, and values of P<0.05 were considered statistically significant. Data are represented as mean±SD unless mentioned otherwise.
Results
Surface ECG
Conventional 6-lead ECGs were recorded in anesthetized intact mice. Figure 1 depicts representative examples of an ECG in a Wt mouse (Figure 1A) and Tx mouse (Figure 1B). The most striking difference in surfaces ECG was the reduced P-wave amplitude in Tx mice. In lead aVR, a lead with a negative consistently monophasic P-wave, the average P-wave amplitude was 1.14±0.03 mV for Wt mice and 0.083±0.02 mV for Tx mice (P<0.01). Other surface ECG parameters are summarized in Figure 1C. The P duration, PQ interval, RR interval, QRS and QT durations, and QRS morphology in Wt and Tx were not significantly different between Wt and Tx mice.
Figure 1.
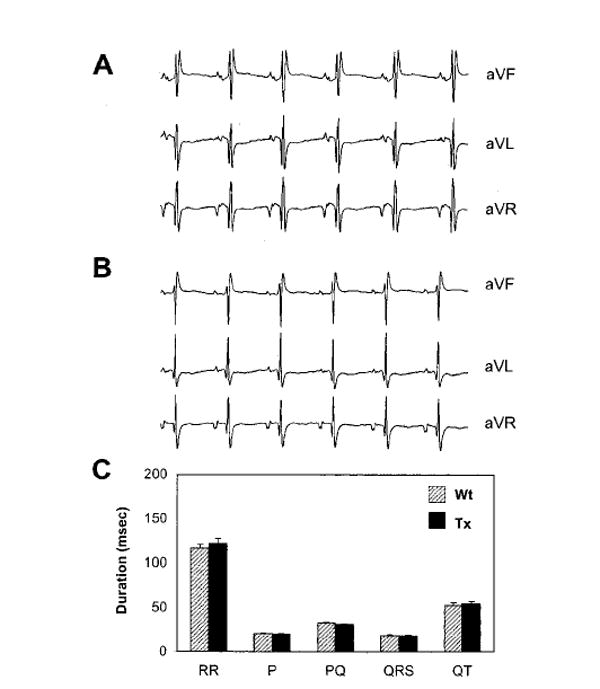
Surface ECGs in Wt and Tx mice. A, Example of an ECG of a Wt mouse. Einthoven leads I, II, and III. B, Example of an ECG in Tx mouse. C, Overview of ECG intervals and durations in Wt and Tx mice (n=30 in both groups). Data are mean±SEM.
Open Chest Experiments
Transesophageal stimulation was used to determine the SNRT, AVERP, WCL, and inducibility of atrial arrhythmias. In a subgroup of the study population, epicardial-recording electrodes were attached to the RA to determine AERP.
Examples of transesophageal electrical stimulation protocols are shown in Figure 2A (SNRT) and Figure 2B (WCL). As shown in Figure 2C, the AERP, AVERP, and WCL were not significantly different between Wt and Tx mice. However, the corrected SNRT (CSNRT) was significantly shorter in Tx mice compared with Wt (CSNRT=SNRT−sinus cycle length [SCL]).
Figure 2.
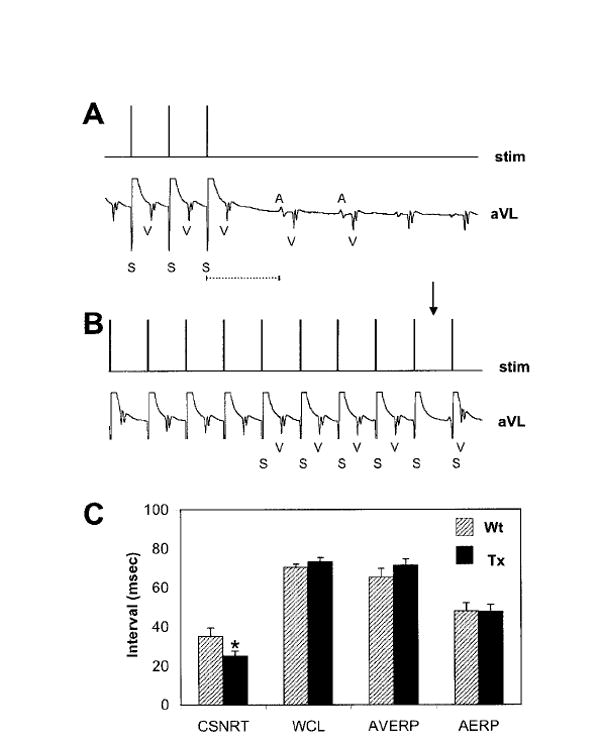
Programmed electrical stimulation. A, Determination of SNRT in a Wt mouse. During transesophageal stimulation, large stimulation artifacts are recorded on the surface ECG. The SNRT was defined as the interval between the last stimulus of the train and the onset of the subsequent P-wave. B, WCL determination in a Wt mouse. Arrow indicates the absence of a QRS complex after progressive A-V prolongation during pacing at the WCL. C, Overview of atrial pacing parameters. For all parameters, n=28 for Wt and n=29 for Tx, except for the AERP, in which n=13 for Wt and n=10 for Tx. *Significant reduction of the CSNRT in Tx mice, P=0.03. Data are mean±SEM.
Spontaneous episodes of AF were not observed in any of the Wt or Tx mice during experiments on intact mice. However, with transesophageal burst pacing of the LA, episodes of AF could be induced in some Tx mice. Figure 3A shows an example of a Tx mouse in which 2-second transesophageal burst evoked an episode of AF, which spontaneously converted to sinus rhythm after 5 seconds. AF was defined as an episode of rapid irregular atrial rhythm lasting >2 seconds. In 14 of 29 Tx mice (48%), AF could be induced but it could not be induced in any of the 28 Wt mice tested (χ2, P<0.01). Mean AF episode duration in Tx mice with inducible AF was 12±27 seconds. In a number of mice, a bipolar atrial electrogram was recorded during AF. Figure 3B shows examples of atrial electrograms in 2 Tx mice. During the arrhythmic episode, atrial activity was rapid and irregular, characteristic of AF.
Figure 3.

AF inducibility in Wt and Tx mice. A, Example of an induced AF episode in a Tx mouse. Upper traces show stimulation and lead II. Inset, Before the burst (a), the mouse was in sinus rhythm. Inset: After termination of the burst (b), the mouse displayed an irregular atrial rhythm with irregular ventricular response. Inset, After 5 seconds (c), the AF episode terminated spontaneously and sinus rhythm resumed. B, Bipolar atrial electrograms and surface ECG in 2 Tx mice. In these examples, mean AF cycle lengths were 43±6 ms (a) and 23±3 ms (b).
Histology
Masson trichrome staining of whole-heart sections confirms that morphologically, Tx hearts (Figure 4A and 4C, respectively) were normal and did not show atrial dilatation. Overexpression of TGF-β1 in this Tx leads to increased fibrosis in the atrium, but not in the ventricle, as reported earlier.12 At high magnification (Figure 4B and 4D), it was apparent that the increase in fibrosis was more pronounced in the Tx RA than in Tx LA. To quantify the extent of atrial fibrosis, the relative area of fibrosis was calculated from atria of 7 Wt and 7 Tx mice stained with Sirius Red. The area occupied by fibrosis was 12.9%±4.7% in the Wt RA, 11.1%±2.4% in the Wt LA, 50.4%±14.2% in the Tx RA and 30.6%±5.9% in the Tx LA. These numbers were significantly different for Wt RA versus Tx RA (P<0.005), for Wt LA versus Tx LA (P<0.005), and for Tx RA versus Tx LA (P<0.01). Between adjacent myocytes in Tx atria, the gap junction proteins Cx40 and Cx43 were still abundantly expressed at levels comparable to Wt atria (see online data supplement at http://circres.ahajournals.org).
Figure 4.

Histology of Wt and Tx hearts. A, Wt heart stained with Masson trichrome. B, Sirius red staining of a Wt RA (upper panel) and Wt LA (lower panel). C, Tx heart stained with Masson trichrome. D, Sirius red staining of a Tx RA (upper panel) and Tx LA (lower panel). The level of fibrosis in the Tx is similar to Wt in the ventricle but highly increased in the atria (red signal in B and D). Scale bars represent 1 mm.
Atrial Conduction in Perfused Hearts
Atrial conduction in Wt and Tx mice was further investigated using a 4×4 array of unipolar extracellular electrodes (Figure 5A) in Langendorff perfused hearts. Compared with the Wt, electrograms in the Tx RA and LA had lower amplitudes (Figure 5B). Whereas the duration of unipolar electrograms was similar for the Wt and Tx RA, electrograms in the Tx LA were significantly prolonged compared with the Wt LA (Figure 5C). In addition, fragmented electrograms were frequently observed in the Tx LA (Figure 5B, arrow).
Figure 5.

Electrogram morphology in Wt and Tx atria during normal sinus rhythm. A, The 4×4 electrode array used for recording of unipolar electrograms. B, Electrograms from the Wt RA, Tx RA, Wt LA, and Tx LA, respectively. Electrograms in the Tx had lower amplitude than in the Wt. The Tx LA frequently showed fragmented electrograms (arrow). C, Electrogram duration in the Wt and Tx, measured as the duration of the high-frequency component of individual unipolar signals. Electrograms in the Tx LA were significantly longer than in the Wt RA, Wt LA, and Tx RA. Data are mean SEM.
AERP was determined at a number of BCLs using bipolar stimulation at the side of the array. The AERP did not show dependence on the BCL in either Wt or Tx over the range tested. In both the RA and LA, AERPs were not significantly different between Wt and Tx mice at any BCL (Figure 6A).
Figure 6.

ERP, CV, and WL in perfused mouse hearts. A, ERP as a function of the BCL in the Wt and Tx RA (left panel) in the Wt and Tx LA (right panel). No significant differences in ERP were observed between the groups. B, CV as a function of the BCL in the Wt and Tx RA (left panel) and in the Wt LA and Tx LA (right panel). CV was significantly slower in the Tx RA than in the Wt RA at all BCLs tested, and CV in the Wt LA and Tx LA were not significantly different at any BCL. C, WL as a function of BCL in the Wt and Tx RA (left panel) and in the Wt and Tx LA (right panel). WL was calculated as the product of ERP and WL for individual recording locations. There were no significant differences in WL between the groups. Data are mean±SEM.
From the activation time points at the recording electrodes, conduction vector maps were constructed to calculate the average conduction velocity in the recording area. At all BCLs investigated, CV in the Tx RA was significantly lower than in the Wt RA (Figure 6B, left panel). In the Tx LA, CV was not significantly different from CV in the Wt LA (Figure 6B, right panel). The wavelength (WL), calculated as the product of AERP and CV in each mouse, was not significantly different in Tx RA and Tx LA compared with Wt (Figure 6C).
Activation maps of the Wt RA and LA displayed homogeneous conduction (Figure 7A). In contrast, the Tx atria showed areas of local isochrone crowding, particularly in the LA (Figure 7A, last panel). Conduction heterogeneity was further assessed using the distribution of phase differences in the recorded areas. The heterogeneity range, the range between maximal and minimal phase differences within recording locations, a measure for absolute heterogeneity, was not significantly different between Wt RA and Tx RA (Figure 7B, left panel). However, the heterogeneity range was significantly higher than in the Wt LA compared with the Tx LA at all BCLs investigated (Figure 7B, right panel). The “heterogeneity index” (the “heterogeneity range” divided by the median phase), a measure for relative heterogeneity within recording locations, was not significantly different between Wt RA and Tx RA (Figure 7C, left panel) but was significantly higher in the Tx LA compared with the Wt LA (Figure 7C, right panel).
Figure 7.

Activation patterns and conduction heterogeneity in perfused mouse hearts. A, Activations maps for Wt RA, Tx RA, Wt LA, and Tx LA. Wt RA and LA displayed homogeneous conduction. Tx RA and especially Tx LA showed areas of isochrone crowding. B, Heterogeneity range (maximum phase − minimum phase within recording areas) for Wt and Tx RA (left panel) and Wt and Tx LA (right panel). Heterogeneity ranges in the Tx LA were significantly higher than in the Wt LA at all BCLs. C, Heterogeneity index (ratio of phase range and median phase) for Wt and Tx RA as a function of BCL (left panel) and Wt and Tx LA as a function of BCL (right panel). Heterogeneity indices were significantly higher in the Tx LA compared with the Wt LA. Data are mean±SEM.
Microelectrode Recordings
Representative microelectrode recordings from Wt and Tx mice are shown in Figure 8 for the RA and LA. There were no systematic differences in action potential shapes between Wt and Tx. The resting membrane potential, action potential amplitude, maximum upstroke velocity (Vmax), and action potential duration at 30%, 60%, and 90% repolarization (APD30, APD60, and APD90) of the Tx did not significantly differ from those of the Wt in both atria (Figure 8C).
Figure 8.

Action potentials recorded from RA and LA of Wt and Tx. Atrial preparations were paced at a BCL of 150 ms. A, Action potentials recordings from the Wt and Tx RA. B, Action potentials recordings from LA. C, Action potential characteristics: resting membrane potential (RMP), action potential amplitude (APA), maximum upstroke velocity (Vmax), and action potential durations at 30%, 60%, and 90% repolarization (APD30, 60, 90). Number of cells/number of mice: Wt RA: 10/ 5, Tx RA: 9/ 5, Wt LA: 10/ 5, and Tx LA 10/ 5. Data are mean± SEM.
Discussion
In recent years, several mouse models with increased inducibility of atrial tachyarrhythmias have been described. Several studies have reported an increased vulnerability for AF in response to administration of cholinergic agonists in normal mice,15,16 and in 1 study, AF could be induced in normal mice without pharmacological intervention.17 In mice lacking the gap junction protein connexin40, atrial conduction velocity was decreased and episodes of AF could be evoked by atrial burst pacing.13,18 The present study demonstrates that increased atrial fibrosis in mice leads to vulnerability to AF. These studies illustrate that, despite its small size, the mouse atrium can be used successfully to study the basic mechanisms of AF substrate formation.
Previously, overexpression of constitutively active TGF-β1 has been shown to cause selective atrial fibrosis in a transgenic mouse model.12 In this study, we investigate the physiologic consequences of this fibrosis and show that inducibility of AF is strongly increased. The absence of spontaneous AF episodes suggests that although some substrate for AF is present in Tx mice, initiating triggers do not occur frequently.
Apart from increased atrial fibrosis, Tx hearts appear anatomically and histologically normal. Surface ECGs also showed little indication of structural heart disease in the ventricle or conduction system; no significant differences between Wt and Tx were found in SCL, PQ interval, QRS duration and morphology, and QT time. The P-wave duration was not significantly different between Wt and Tx. However, the P-wave amplitude was significantly reduced in the Tx. The reduction in P-wave amplitude on surface ECGs and in electrogram amplitude in epicardial recordings may reflect a reduction in the amount of excitable atrial tissue as a result of replacement fibrosis: fewer remaining myocytes would mean less atrial excitable mass and therefore a decrease in voltage generated during the P wave.
The lack of difference in PQ interval, AVERP, and WCL suggests that the AV node was not implicated in the fibrotic process and that atrial fibrosis did not affect AV nodal inputs. However, in Tx mice, the CSNRT was significantly shorter than in the Wt. It is conceivable that fibrosis in the RA working myocardium caused some degree of entrance block into the sinus node during atrial pacing, thereby decreasing the extent of overdrive suppression of the sinoatrial (SA) node.19 The SA nodal pacemaking process itself is not necessarily affected in Tx mice, as reflected by the lack of difference in SCL between Wt and Tx.
A decrease in effective refractory period (ERP) is generally thought to be proarrhythmic for AF.20 However, both in open chest studies and in Langendorff perfused mouse hearts, the ERP did not differ significantly between Wt and Tx atria and did not exhibit cycle length dependence in the range of BCLs tested. As a crude measure, this lack of difference suggests that the action potential duration of atrial myocytes is not altered by overexpression of TBF-β1. This is further substantiated by microelectrode recordings, which did not show any differences between the Tx and Wt mice.
In the Tx RA, CV was significantly decreased compared with Wt. By contrast, CV in the Tx LA was not significantly different from Wt. However, in the Tx LA, conduction is more heterogeneous than in the Wt LA, as apparent from activation maps and as quantified from phase maps. Analysis of phase maps did not show a significant increase in the Tx RA compared with the Wt RA. In addition, fragmented electrograms were frequently observed in the Tx LA, but not in the Tx RA. The level of fibrosis is highly increased in the Tx LA (30% of the tissue area versus 11% for the Wt LA) and even more in the Tx RA (50% versus 13% for the Wt RA). It is conceivable that the differential effect of increased fibrosis between the LA and RA reflects differences in tissue organization in the LA and RA recording areas, where the increased fibrosis in the Tx may have unmasked underlying heterogeneity in fiber orientation.
The multiple wavelet theory20 has been widely accepted as an explanation for the mechanism of AF sustenance. According to this theory, fibrillation requires several reentrant wavelets to coexist where the wavelength (WL) of 1 reentrant wavelet is the product of ERP and CV. However, in this view, fibrillation in hearts smaller than a “critical mass” would not be possible.21,22 It was recently reported that the mouse ventricle is able to sustain fibrillation, although its WL (15 to 30 mm) should not allow multiple wavelets to coexist.23 In the present study, WL in the LA and RA of both Wt and Tx was ≈15 mm. The length of normal mouse atria from the tip of the appendage to back of the atrium measures ≈3 mm and Tx mice did not show atrial dilatation. If the atria in the Tx mouse were a homogeneous substrate, it could probably not accommodate >1 reentrant wavelet. However, the increased interstitial fibrosis makes the Tx atria a structurally heterogeneous substrate. Both for canine atrial preparation and computer models, Spach et al have demonstrated that structural changes in the atrial myocardium during aging, which include increased interstitial (micro)fibrosis and its concomitant decrease in side-to-side electrical coupling, can cause a shift from uniform anisotropy to nonuniform anisotropy in atrial conduction.9,24 In nonuniformly anisotropic tissue, slow and heterogeneous conduction may be observed during transverse propagation in the absence of variations in intrinsic membrane properties, making it possible for reentry to occur in relatively small circuits.8 Therefore, interstitial fibrosis observed in the Tx mouse could make AF possible in a tissue area much smaller than would be expected based on wavelength or “critical mass” considerations.
The rapid atrial pacing model of AF, generally considered to be a animal model for “lone AF” or AF without underlying heart disease in humans, does not display increased fibrosis.4,25 However, increased fibrosis was observed in atrial biopsies from patients with “lone AF” diagnosed.10,26 Other animal models, more representative for AF with underlying heart disease, have increased atrial fibrosis. For rat and dog models of aging, increased fibrosis in both atria was observed.2,3 In a dog model of CHF4 and of atrial dilatation caused by mitral regurgitation,7 atrial interstitial fibrosis was increased in the LA. For all these models, an increased vulnerability for AF has been reported. In the canine CHF model, Li et al have correlated the presence of atrial fibrosis to increased conduction heterogeneity4 and have shown that enalapril reduces CHF-induced fibrosis,27 conduction heterogeneity, and AF stability. Moreover, recent studies have demonstrated that after reversal of CHF in dogs, CHF-induced alterations in cellular electrophysiology disappear,28 but atrial fibrosis, increased conduction heterogeneity, and the substrate for AF remain.29
Together, the reports indicate that atrial fibrosis is common in animal models of AF and in patients with AF. However, many of these models have compounding factors that can contribute to AF substrate formation. Canine models of aging and CHF display changes in atrial cellular electrophysiology.3,30 In human patients with “lone AF,” other histological abnormalities including cellular hypertrophy, myocarditis, and necrosis have been reported.10,26 The Tx mouse in this study does not show inflammation, necrosis, cellular hypertrophy, or other histological changes, and, based on the lack of difference in microelectrode recordings and in ERP, it does not appear to have altered action potential duration.
The increased AF inducibility in this model of atrial fibrosis suggests that atrial fibrosis in itself can be sufficient to form a substrate for AF. This indicates that atrial fibrosis can be an important contributor to the AF substrate in other animal models and would be a significant predictor for AF vulnerability in patients with pronounced atrial interstitial fibrosis, even in the absence of other proarrhythmic factors.
Even with the small size of the mouse atria, this transgenic mouse will allow further investigation of the genesis and maintenance of AF. As was recently demonstrated in this model, intracellular calcium transients can be measured in individual atrial myocytes within perfused hearts.31 With this method, the AF substrate in this model could be studied at a very high level of detail.
Limitations of This Study
It is conceivable that overexpression of TGF-β1 causes changes in cellular electrophysiology of atrial myocytes. However, the lack of alteration in ERP and the lack of differences in action potentials recorded by microelectrodes suggest that there are no major primary cellular electrophysiological effects of TGF-β1. However, we cannot exclude the presence of more subtle alterations in cellular electrophysiology, which could conceivably contribute to AF vulnerability.
Conclusions
In a transgenic mouse of selective atrial fibrosis, vulnerability to AF is increased in the absence of a change in atrial ERP. In this model, alterations in atrial myocardial structure lead to increased conduction heterogeneity in the LA and a decrease in CV in the RA. This study indicates that the alterations in atrial conduction produced by atrial interstitial fibrosis alone are sufficient to produce a substrate for AF, even in the relatively small mouse atrium.
Acknowledgments
Supported by R01-HL66362 (J.O.).
Footnotes
The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circres.ahajournals.org/cgi/content/full/94/11/1458
References
- 1.Levy S. Factors predisposing to the development of atrial fibrillation. Pacing Clin Electrophysiol. 1997;20:2670–2674. doi: 10.1111/j.1540-8159.1997.tb06115.x. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi H, Wang C, Miyauchi Y, Omichi C, Pak HN, Zhou S, Ohara T, Mandel WJ, Lin SF, Fishbein MC, Chen PS, Karagueuzian HS. Aging-related increase to inducible atrial fibrillation in the rat model. J Cardiovasc Electrophysiol. 2002;13:801–808. doi: 10.1046/j.1540-8167.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- 3.Anyukhovsky EP, Sosunov EA, Plotnikov A, Gainullin RZ, Jhang JS, Marboe CC, Rosen MR. Cellular electrophysiologic properties of old canine atria provide a substrate for arrhythmogenesis. Cardiovasc Res. 2002;54:462–469. doi: 10.1016/s0008-6363(02)00271-7. [DOI] [PubMed] [Google Scholar]
- 4.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 5.Morillo CA, Klein GJ, Jones DL, Guiraudon CM. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation. 1995;91:1588–1595. doi: 10.1161/01.cir.91.5.1588. [DOI] [PubMed] [Google Scholar]
- 6.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 7.Verheule S, Wilson E, Everett TT, Shanbhag S, Golden C, Olgin J. Alterations in atrial electrophysiology and tissue structure in a canine model of chronic atrial dilatation due to mitral regurgitation. Circulation. 2003;107:2615–2622. doi: 10.1161/01.CIR.0000066915.15187.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spach MS, Josephson ME. Initiating reentry: the role of nonuniform anisotropy in small circuits. J Cardiovasc Electrophysiol. 1994;5:182–209. doi: 10.1111/j.1540-8167.1994.tb01157.x. [DOI] [PubMed] [Google Scholar]
- 9.Spach MS, Boineau JP. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol. 1997;20:397–413. doi: 10.1111/j.1540-8159.1997.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 10.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 11.Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. 2002;54:361–379. doi: 10.1016/s0008-6363(02)00273-0. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima H, Nakajima HO, Salcher O, Dittie AS, Dembowsky K, Jing S, Field LJ. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res. 2000;86:571–579. doi: 10.1161/01.res.86.5.571. [DOI] [PubMed] [Google Scholar]
- 13.Verheule S, van Batenburg CA, Coenjaerts FE, Kirchhoff S, Willecke K, Jongsma HJ. Cardiac conduction abnormalities in mice lacking the gap junction protein connexin40. J Cardiovasc Electrophysiol. 1999;10:1380–1389. doi: 10.1111/j.1540-8167.1999.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 14.Lammers WJ, Schalij MJ, Kirchhof CJ, Allessie MA. Quantification of spatial inhomogeneity in conduction and initiation of reentrant atrial arrhythmias. Am J Physiol. 1990;259:H1254–63. doi: 10.1152/ajpheart.1990.259.4.H1254. [DOI] [PubMed] [Google Scholar]
- 15.Wakimoto H, Maguire CT, Kovoor P, Hammer PE, Gehrmann J, Triedman JK, Berul CI. Induction of atrial tachycardia and fibrillation in the mouse heart. Cardiovasc Res. 2001;50:463–473. doi: 10.1016/s0008-6363(01)00264-4. [DOI] [PubMed] [Google Scholar]
- 16.Kovoor P, Wickman K, Maguire CT, Pu W, Gehrmann J, Berul CI, Clapham DE. Evaluation of the role of I(KACh) in atrial fibrillation using a mouse knockout model. J Am Coll Cardiol. 2001;37:2136–2143. doi: 10.1016/s0735-1097(01)01304-3. [DOI] [PubMed] [Google Scholar]
- 17.Schrickel JW, Bielik H, Yang A, Schimpf R, Shlevkov N, Burkhardt D, Meyer R, Grohe C, Fink K, Tiemann K, Luderitz B, Lewalter T. Induction of atrial fibrillation in mice by rapid transesophageal atrial pacing. Basic Res Cardiol. 2002;97:452–460. doi: 10.1007/s003950200052. [DOI] [PubMed] [Google Scholar]
- 18.Hagendorff A, Schumacher B, Kirchhoff S, Luderitz B, Willecke K. Conduction disturbances and increased atrial vulnerability in Connexin40-deficient mice analyzed by transesophageal stimulation. Circulation. 1999;99:1508–1515. doi: 10.1161/01.cir.99.11.1508. [DOI] [PubMed] [Google Scholar]
- 19.Prinsze FJ, Bouman LN. The cellular basis of intrinsic sinus node recovery time. Cardiovasc Res. 1991;25:546–557. doi: 10.1093/cvr/25.7.546. [DOI] [PubMed] [Google Scholar]
- 20.Allessie MA. Atrial electrophysiologic remodeling: another vicious circle? J Cardiovasc Electrophysiol. 1998;9:1378–1393. doi: 10.1111/j.1540-8167.1998.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 21.Allessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The “leading circle” concept: a new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ Res. 1977;41:9–18. doi: 10.1161/01.res.41.1.9. [DOI] [PubMed] [Google Scholar]
- 22.Winfree AT. Electrical turbulence in three-dimensional heart muscle. Science. 1994;266:1003–1006. doi: 10.1126/science.7973648. [DOI] [PubMed] [Google Scholar]
- 23.Vaidya D, Morley GE, Samie FH, Jalife J. Reentry and fibrillation in the mouse heart. A challenge to the critical mass hypothesis. Circ Res. 1999;85:174–181. doi: 10.1161/01.res.85.2.174. [DOI] [PubMed] [Google Scholar]
- 24.Spach MS, Miller WT, 3rd, Dolber PC, Kootsey JM, Sommer JR, Mosher CE., Jr The functional role of structural complexities in the propagation of depolarization in the atrium of the dog. Cardiac conduction disturbances due to discontinuities of effective axial resistivity. Circ Res. 1982;50:175–191. doi: 10.1161/01.res.50.2.175. [DOI] [PubMed] [Google Scholar]
- 25.Ausma J, Wijffels M, Thone F, Wouters L, Allessie M, Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. 1997;96:3157–3163. doi: 10.1161/01.cir.96.9.3157. [DOI] [PubMed] [Google Scholar]
- 26.Frustaci A, Caldarulo M, Buffon A, Bellocci F, Fenici R, Melina D. Cardiac biopsy in patients with “primary” atrial fibrillation. Histologic evidence of occult myocardial diseases. Chest. 1991;100:303–306. doi: 10.1378/chest.100.2.303. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Shinagawa K, Pang L, Leung TK, Cardin S, Wang Z, Nattel S. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001;104:2608–2614. doi: 10.1161/hc4601.099402. [DOI] [PubMed] [Google Scholar]
- 28.Cha TJ, Ehrlich JR, Zhang L, Shi YF, Tardif JC, Leung TK, Nattel S. Dissociation between ionic remodeling and ability to sustain atrial fibrillation during recovery from experimental congestive heart failure. Circulation. 2004;109:412–418. doi: 10.1161/01.CIR.0000109501.47603.0C. [DOI] [PubMed] [Google Scholar]
- 29.Shinagawa K, Shi YF, Tardif JC, Leung TK, Nattel S. Dynamic nature of atrial fibrillation substrate during development and reversal of heart failure in dogs. Circulation. 2002;105:2672–2678. doi: 10.1161/01.cir.0000016826.62813.f5. [DOI] [PubMed] [Google Scholar]
- 30.Li D, Melnyk P, Feng J, Wang Z, Petrecca K, Shrier A, Nattel S. Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation. 2000;101:2631–2638. doi: 10.1161/01.cir.101.22.2631. [DOI] [PubMed] [Google Scholar]
- 31.Rubart M, Wang E, Dunn KW, Field LJ. Two-photon molecular excitation imaging of Ca2+ transients in Langendorff-perfused mouse hearts. Am J Physiol Cell Physiol. 2003;284:C1654–68. doi: 10.1152/ajpcell.00469.2002. [DOI] [PubMed] [Google Scholar]


