Abstract
8-anilino-1-naphthalenesulfonic acid (ANS) is widely used as a probe for locating binding sites of proteins. To characterize the binding sites of tear lipocalin (TL), we studied ANS binding to apoTL by steady-state and time-resolved fluorescence. Deconvolution of ANS binding revealed that two lifetime components, 16.99 ns and 2.76 ns at pH 7.3, have dissociation constants of 0.58 μM and 5.7 μM, respectively. At pH 3.0, the lifetime components show decreased affinities with dissociation constants of 2.42 μM and ∼21 μM, respectively. Selective displacement of ANS molecules from the ANS-apoTL complex by stearic acid discriminates the internal and external binding sites. Dependence of the binding affinity on ionic strength under various conditions provides strong evidence that an electrostatic interaction is involved. Time-resolved fluorescence is a promising tool to segregate multiple binding sites of proteins.
Keywords: tear lipocalin, time-resolved fluorescence, binding sites, ANS, heterogeneous binding, Lipocalin-1, von Ebner's gland protein, human tear protein
Introduction
The lipocalin family of proteins is characterized by a cup shaped ligand binding fold within a continuously hydrogen-bonded β-barrel that is formed by eight antiparallel β-strands [1]. In fact, studies of lipocalin binding sites and interpretation of function are usually predicated on the assumption that the fold or cavity is the only ligand binding site in the molecule. However, external binding sites with unique characteristics were discovered for two lipocalins, α1-acid glycoprotein and β-lactoglobulin [2-5]. The careful delineation of all binding sites in lipocalins is relevant because a new classification scheme is based on comparative binding affinity analysis using retinol, retinoic acid, as well as the generic molecular probes, 11-(5-(dimethylamino)-1-naphthalene-sulfonylamino)undecanoic acid (DAUDA) and 8-anilino-1-naphtalenesulfonic acid (ANS) [6]. A model invoking a single binding site was used to analyze the steady-state fluorescence titration data for ANS versus 10 human lipocalins. Despite the shared fold in lipocalins, the dissociation constants for ANS binding vary greatly in the range of 1-11 μM, except that retinol binding protein (RBP) showed no ANS binding. The disparity of ANS binding in lipocalins, which share the common fold, may be related to the variation of cavity properties such as accessibility, hydrophobicity and the presence of positively charged side chains at the binding sites. These potential differences have not been thoroughly investigated. Study of external binding sites requires improved resolution of ligand binding that is possible with the detection of multiple fluorescent lifetimes for ANS [3-5].
Tear lipocalin (TL), also known as lipocalin-1 or von Ebner's gland protein, is an example of a human lipocalin with an assortment of endogenous ligands and multiple proposed functions. It is the principal lipid binding protein in tears with ligands that include fatty acids, alkyl alcohols, glycolipids, phospholipids and cholesterol [7]. TL binds a host of other biologically important compounds including sapid molecules [8], retinol [9], potentially harmful lipid oxidation products [10], antioxidants [11], and fungal siderphores [12]. Several putative functions have been assigned to TL such as scavenging lipid from the corneal surface to prevent the formation of lipid induced dry spots [13], solubilization of lipid in tears (2), antimicrobial activity [14], cysteine proteinase inhibition [15], endonuclease activity [16] and scavenging of toxic molecules [10]. The solution structure of TL has been resolved by site directed tryptophan fluorescence and verified by crystallography of TL [17, 18]. A capacious cavity with short interstrand loops C-D and E-F at the entrance confers promiscuity in ligand binding [18] compared to other more selective lipocalins such as such as RBP. Fatty acids are known to bind with the polar head group oriented toward solvent at the calyx mouth and the hydrocarbon tail buried in the cavity of TL [19]. Despite its numerous and diverse endogenous ligands and multiple functions little is known about possible external binding sites of TL.
To characterize the binding sites of TL, we studied ANS binding to TL by steady-state and time-resolved fluorescence. The enhanced resolution of ANS binding via fluorescent lifetime components permitted not only the discrimination of internal and external binding modes but also dissociation constants for each site. The modes of ANS binding are affected differently in low pH and high ionic strength. The elucidation of all binding sites in lipocalins will advance our understanding of binding mechanisms and may be useful for a functional classification within the lipocalin family.
Materials and methods
8-anilino-1-naphthalenesulfonic acid (ANS) and stearic acid were purchased from Sigma.
Expression and purification of recombinant tear lipocalin
The TL cDNA in PCR II (Invitrogen, San Diego, CA), previously synthesized [20], was used as a template to clone the TL gene spanning bases 115-592 of the previously published sequence [9] into pET 20b (Novagen). Flanking restriction sites for NdeI and BamHI were added to produce the native protein sequence as found in tears except for an initiating N-terminal methionine [21]. The TL plasmid was transformed in E. Coli, BL 21 (DE3) and cells were cultured and proteins were expressed according to the manufacturer's protocol (Novagen). Following cell lysis [22], the supernatant was treated with methanol (40% final concentration) at 4° C for 2 1/2 hours. The suspension was centrifuged at 3000g for 30 minutes. The supernatant was dialyzed against 50 mM Tris-HCl pH 8.4. The dialysate was treated with ammonium sulfate 45-75% saturation. The resulting precipitate was dissolved in 50 mM Tris-HCl pH 8.4 and applied to a Sephadex G-100 column (2.5 × 100 cm) equilibrated with 50 mM Tris-HCl, 100 mM NaCl, pH 8.4. The fraction containing TL was dialyzed against 50 mM Tris-HCl, pH 8.4 and applied to a DEAE Sephadex A-25 column. Bound protein was eluted with a 0-0.8 M NaCl gradient. Eluted fractions containing TL were centrifugally concentrated (Amicon, Centricon-10). Purity of mutant proteins was verified by SDS tricine gel electrophoresis [7]. Previously, the proper folding of the recombinant protein was verified by identical near- and far UV CD spectra to the native protein[23, 24]. Laser light scattering studies have confirmed that the recombinant protein is monomeric similar to the native protein [25]. Previously, cysteine labeling studies of the recombinant protein have confirmed that the thiol group on C101 was available for labeling, excluding the possibility of disulfide scrambling [26]. The labeling efficiency of the recombinant protein never exceeded a ratio of 1:1. Taken together these data indicate identical disulfide formation as the native protein. Delipidation was performed by chloroform/methanol extraction, to obtain apoTL, as described previously [7]. The protein concentrations were determined by the biuret method [27].
Steady-state fluorescence
Steady-state fluorescence measurements were made on a Jobin Yvon-SPEX (Edison, NJ) Fluorolog tau-3 spectrofluorometer. Bandwidths for excitation and emission were 2 and 3 nm, respectively. The excitation and emission λ of 335 nm and 460 nm, respectively, were used for ANS fluorescence. 10 mM sodium phosphate and 30 mM sodium citrate buffers (with and without 100 mM NaCl) were used for pH 7.3 and pH 3.0, respectively. Temperature was maintained at 25° C with a thermojacketed cell holder. The fluorescence intensities were corrected for light scattering from buffer.
Fluorescence binding assays
The samples of apoTL (5 μM) in various buffers were titrated with ANS and the fluorescence was measured at emission λmax. The concentration of ethanol stock solution of ANS was confirmed by absorbance at 350 nm, using an extinction coefficient, ε350 = 6300 M-1cm-1. Following each addition of ligand, the solution was mixed and allowed to equilibrate for 3 minutes. At the end of each titration experiment, the ethanol concentration did not exceed 2%. The ANS binding data were analyzed with the following formula for one binding site, which can be derived from the law of mass action:
where F is a fluorescence scaling factor, Kd is the apparent dissociation constant, P is the total protein concentration, Lt is the total ligand concentration, and n is the stoichiometry. The same formula was used to calculate the apparent inhibition constant, Ki, in the competitive binding experiment.
Fluorescence lifetime measurements
The ANS fluorescence decays of samples with various ANS/protein ratios were measured using a PTI Time Master fluorescence lifetime instrument, which consists of a nitrogen laser (GL-3300) linked to a dye laser (GL 302), a frequency doubler (GL 303) and a stroboscopic detector. [2-[2-[4-(dimethylamino)phenyl]ethenyl]-6-methyl-4H-pyran-4-ylidene]-propanedinitrile (DCM) (Exciton, Inc, Dayton, Ohio, USA) dye solution was used to obtain a wavelength of 670 nm. The 335 nm pulses (fwhm ∼ 1.5 ns) from frequency doubled light were used for the excitation of the ANS-protein complexes. The decay curves were analyzed for emission λmax. The emission monochromator slit was 3 nm. The temperature was maintained at 25 °C with a thermojacketed cuvette holder. The instrument response function (IRF) was determined by measuring scattered light from a solution of glycogen. A DPU-15 optical depolarizer (Optics for Research, Caldwell, NJ) was placed before the emission monochromator to eliminate polarization dependence of the detection train. Each data point on a lifetime decay curve represents the average of nine laser flashes, and each decay represents 300 of these data points evenly spaced over the collection time interval.
The fluorescence intensity decays were analyzed using the software supplied with the PTI instrument. A multiexponential decay law was used in the analysis:
where I is fluorescence intensity, αi and τi are the normalized pre-exponential factors, and decay time, respectively. The lifetimes were considered a global parameter in fluorescence decay analysis. The goodness of fit was assessed by the chi2 criterion. The fractional fluorescence intensity of each component was defined as fi = αiτi / Σαjτj and was used for deconvolution of binding curves. The relative contribution of each lifetime component to the steady-state fluorescence intensity (ISS) was calculated as Ii = fiISS.
Results and Discussion
ANS binding to TL by steady-state fluorescence, effect of pH and ionic strength
The steady-state fluorescence characteristics of ANS-apoTL binding are evident in Figure 1. ANS fluoresces weakly in aqueous solutions. Fluorescence is enhanced when ANS binds to apoTL. The enhancement of fluorescence intensity upon ANS binding to apoTL is greater at pH 3.0 compared to that at pH 7.3 [23]. Yet the binding affinity of ANS to apoTL is about 10 fold higher at pH 7.3 (Kd= 0.41 μM) than that obtained at pH 3.0 (Kd= 4.0 μM). ApoTL undergoes a molten globule transition at pH 3.0 that has been documented by changes in tertiary structure of the protein and ANS fluorescence enhancement [23]. However, little is known about ANS binding properties of TL at pH 3.0. To understand the mechanism of the binding at both pH values, ANS-apoTL titration experiments were performed with and without 100 mM NaCl (Fig. 1). The ionic strength of the solution differentially influences the binding affinities of ANS at pH 3.0 and pH 7.3. At pH 3.0, 100 mM NaCl decreases the binding affinity of ANS to apoTL about four fold, although no significant change is detected for the binding affinity at pH 7.3 (Fig. 1).
Figure 1.
ANS fluorescence titration in the presence of apoTL (5 μM) at various buffer conditions. Solid curves are generated by fitting the experimental data to a one binding site model.
Resolution of ANS binding to TL by time-resolved fluorescence
The resolution of ANS binding to proteins by steady-state measurements is limited particularly when the fluorescence intensity of ANS for one binding site is a fraction of the fluorescence intensity of a second binding site. In this case, a small enhancement of ANS fluorescence intensity upon binding does not necessarily reflect a low binding affinity to proteins [3-5].
Steady-state fluorescence intensity is the sum of the contributions of the individual lifetime components. Therefore, when both steady state and time resolved fluorescence are applied together, the steady-state fluorescence binding curve can be deconvolved into the binding components, each of which is associated with a particular fluorescence lifetime. The ANS fluorescence lifetime components can be linked to various binding sites. For TL, it has been shown that ANS binding to TL is heterogeneous and yields two fluorescence lifetime components [28]. The two lifetime components may represent different binding modes. Therefore, to segregate modes of ANS binding, fluorescence lifetimes were measured by titration of ANS with apoTL. At pH 7.3 (with and without 100 mM NaCl), fluorescence decay curves at various ANS concentrations could be fit satisfactorily by two global decay times, which correspond to two different binding sites (Figure 2 and Table 1). Figures 3 and 4 represent deconvolutions of ANS binding curves at pH 7.3 and pH 3.0, respectively, which are constructed from the global decay time analysis. It is evident that ANS has two binding modes at pH 7.3 (Fig. 3A). The strongly (Kd= 0.58 μM) and weakly (Kd= 5.7 μM) bound ANS show fluorescence lifetimes of 16.99 ns and 2.76 ns, respectively. Free ANS molecules (τ= 0.25 ns [29]) are not detected under these experimental conditions. The fractional contribution of the weakly bound ANS molecules to the steady-state intensity is very low, about 9 % at an ANS concentration of 15 μM (Table 1). Several lines of data suggest that the long lifetime (16.99 ns) represents ANS molecules that are bound inside the cavity of TL. Previous data indicated that the short lifetime (2.76 ns) species are consistent with ANS molecules bound to the external binding sites of TL [28, 29]. The ionic strength of a solution differently influences the binding properties of the species. The ionic strength of 0.1 M (NaCl) minimally alters the lifetime and binding constant of ANS, which is bound to the putative internal binding site (Fig. 3B). However, the Kd for the external binding site of ANS has increased about two fold, despite the slightly increased contribution of the short lifetime species to the steady-state fluorescence. For the external binding site, the short lifetime and sensitivity of binding to a change in ionic strength are consistent with an electrostatic mechanism of binding in which the sulfonate group of ANS interacts with positively charged side chains of TL. Mechanisms that lead to increased ANS fluorescence intensity and lifetime, as well as a blue shift of fluorescence have been discussed previously [29]. Even at a fully exposed site, the interaction between the sulfonate group of ANS and the positively charged side chains (Arg, Lys) alter intra- and intermolecular charge-transfer (CT) processes that lead to a blue shift and increased fluorescence of ANS. Decreased solvent dynamics at the protein surface will also diminish the intermolecular CT rate of ANS that further increases the fluorescence intensity [30].
Figure 2.
ANS fluorescence intensity decays (as an example) at various ANS concentrations with apoTL (5 μM). Solid curves are global double-decay time fit. Buffer: 10mM sodium phosphate, pH 7.3. In the figure, the number of the experimental data points has been cut in half to view clearly the differences in the ANS fluorescence intensity decays at various ANS concentrations.
Table 1.
The ANS fluorescence decay parameters at various ANS concentrations with apoTL (5 μM) recovered by global double- decay time analysis.
τ1= 2.76 ns and τ2= 16.99 ns are global parameters
Global Chi2= 1.4
| ANS (μM) | α1 | f1 | α2 | f2 |
|---|---|---|---|---|
| 0.5 | 0.24 | 0.05 | 0.76 | 0.95 |
| 1 | 0.24 | 0.05 | 0.76 | 0.95 |
| 1.5 | 0.25 | 0.05 | 0.75 | 0.95 |
| 2 | 0.23 | 0.05 | 0.77 | 0.95 |
| 3 | 0.27 | 0.06 | 0.73 | 0.94 |
| 4 | 0.27 | 0.06 | 0.73 | 0.94 |
| 5 | 0.30 | 0.07 | 0.7 | 0.93 |
| 7 | 0.32 | 0.07 | 0.68 | 0.93 |
| 11 | 0.34 | 0.08 | 0.66 | 0.92 |
| 15 | 0.38 | 0.09 | 0.62 | 0.91 |
Figure 3.
Deconvolution of ANS fluorescence titration curves into lifetime components at pH 7.3 without (A) and with (B) 100 mM NaCl. The concentration of apoTL is 5 μM. Solid curves are generated by fitting of the experimental data to one binding site model.
Figure 4.
Deconvolution of ANS fluorescence titration curves into lifetime components at pH 3.0 without (A) and with (B) 100 mM NaCl. The concentration of apoTL is 5 μM. Solid curves are generated by fitting of the experimental data to one binding site model.
The resolution of ANS binding at low pH
ANS binding to apoTL exhibits quite different properties at pH 3.0 than at neutral pH (Fig. 4). For global analysis, it was necessary to add one more lifetime component, 0.25 ns, to account for free ANS molecules. In the transition from pH 7.3 to pH 3.0, the ANS fluorescence lifetime of the 16.99 ns component is slightly decreased to 16.04 ns. The lifetime of the 2.76 ns component, however, is increased about 2.3 fold (Fig. 3A and 4A). The ANS fluorescence intensities of the long- and short lifetime components are significantly higher (about 5 and 11 fold, respectively) at pH 3.0 compared to that of pH 7.3 (Fig. 3A and 4A). However, despite the high fluorescence intensity, both species of ANS molecules that have long- and short lifetimes show lower affinities to apoTL at pH 3.0 than at pH 7.3. Dissociation constants are increased about 4 fold for both species. At pH 3.0, in contrast to pH 7.3, 0.1 M ionic strength decreases affinity of the long lifetime components of ANS molecules. The dissociation constant is increased about 5 fold (Fig. 4B). Saturation was not observed in the titration curve for the short lifetime species at pH 3.0 and 0.1 M ionic strength. The lifetime of the 6.30 ns component at 0.1 M ionic strength is decreased to 5.27 ns (Fig. 4). At pH 3.0, decreased affinity of ANS (16.04 ns species) to apoTL can result from changes in both binding enthalpy and entropy. The native to molten globule transition of TL disrupts tertiary interactions between side chains in a site specific manner [23, 31] that may decrease binding enthalpy. On the other hand, in a molten globule state hydrophobic sites are more exposed, which may decrease the entropic contribution. Therefore, the decreased ANS binding affinity at pH 3.0 is consistent with a molten globule state of TL. In agreement with the increased exposure, ANS binding affinity to the internal binding site becomes susceptible to the ionic strength at pH 3.0.
Assignment of intracavitary binding of ANS by a competitive displacement assay
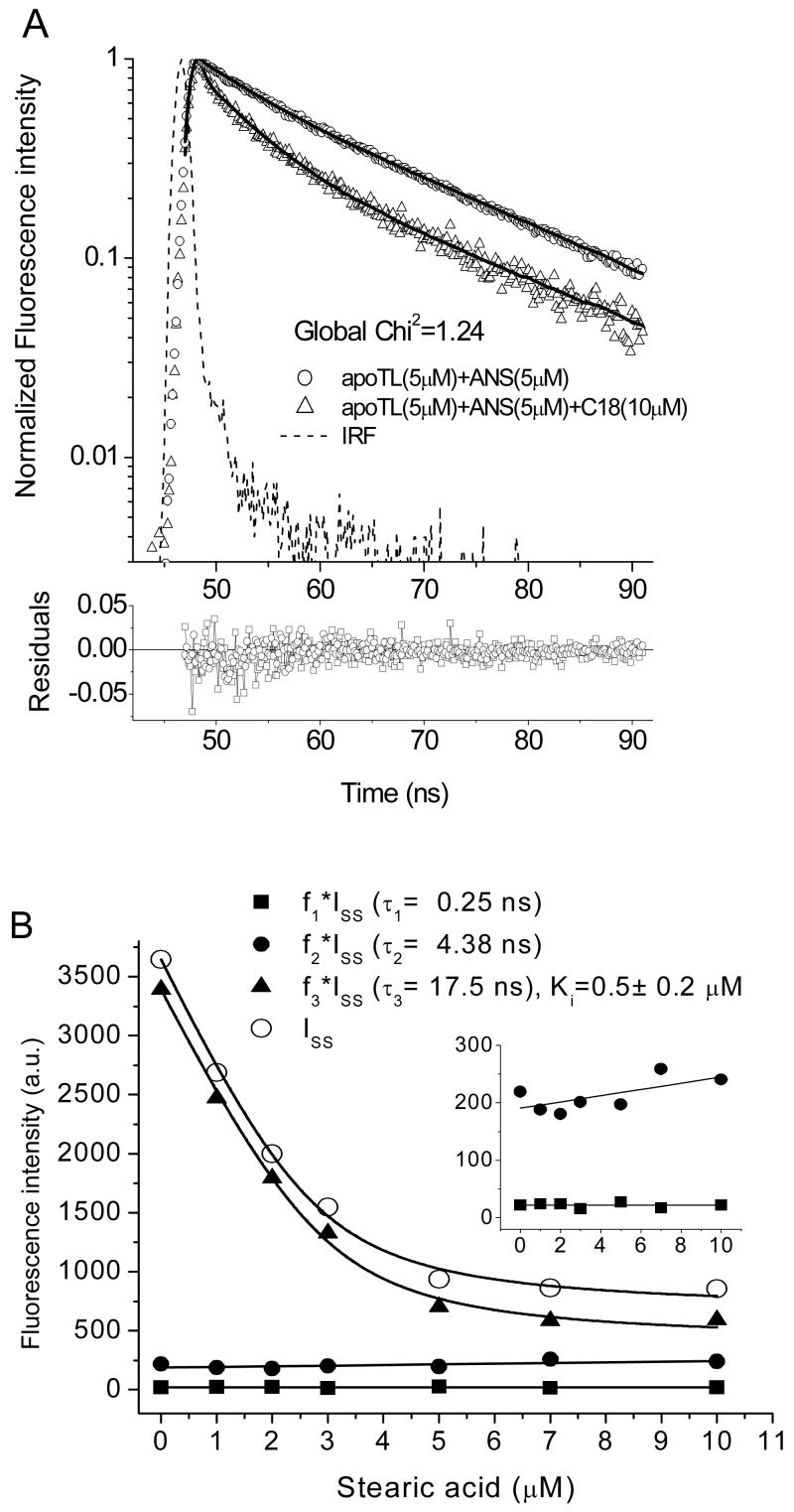
Because fatty acids bind to the internal cavity of TL [19], it is possible to segregate binding modes of ANS using stearic acid to competitively displace ANS from the ANS-apoTL complex while monitoring both steady-state and lifetime fluorescence (Fig. 5). Decay curves of ANS fluorescence intensity at various concentrations of stearic acid could be fit satisfactorily by triple global decay times (Fig. 5A). The displacement curve and its deconvolution into lifetime components are shown in Fig. 5B. Stearic acid displaces only the ANS species (Ki= 0.5 μM) that has the long lifetime. Therefore, the long- and short lifetime species can be attributed to ANS bound to the internal and external binding sites of TL, respectively. The inset in Fig. 5B is shown to highlight the slight increase in the short lifetime component with increasing stearic acid concentration. The most likely explanation is that ANS molecules, displaced from the internal binding site, are available to bind to the external binding site. Therefore, the displacement of ANS from the internal binding site increases the population of the short lifetime component.
Figure 5.
Displacement of ANS form ANS (5 μM)-apoTL (5 μM) complex by stearic acid (A) ANS fluorescence intensity decays (as an example) of apoTL-ANS complex without and with stearic acid (18 μM). Solid curves are global triple-decay time fit. (B) The deconvolution of the ANS displacement curve into lifetime components. (B, inset) The free (0.24 ns) and short (4.38 ns)- lifetime components of the displacement curve. The units are same as in the main figure. Buffer: 10mM sodium phosphate, 100 mM NaCl, pH 7.3
The experiments of ionic strength versus binding of ANS to apoTL also lend support to the intracavitary assignment for the long lifetime species. At pH 7.3, the long lifetime component and the binding affinity are unaltered by 100 mM NaCl. This finding does not necessarily imply a purely hydrophobic interaction. Electrostatic interactions occurring through the protein medium are simply less sensitive to salt because counterions cannot penetrate into the protein. Therefore, ion pair formation between a sulfonate group of ANS and an intracavitary positively charged side chain, can not be excluded, particularly in light of the fact that a positively charged residue Lys 114 is located inside the cavity [17, 18]. However, several inferences can be drawn from these experiments. The mode of binding for the external site of TL has an electrostatic contribution because affinity decreases in 0.1 M NaCl. The low fluorescence intensity and the short lifetime for ANS molecules bound externally indicate that the ion pair is exposed to solvent. Even for a solvent exposed site, the effectiveness of the salt screening will be reduced if the two charged groups are very close to each other or not fully exposed to solvent [32].
Mechanistic implications for ANS binding and correlation with fluorescence parameters
In proteins the major determinant for ANS binding is an electrostatic interaction between the sulfonate group of ANS and the positively charged side chain [33]. However, the application of time-resolved fluorescence to ANS binding with arginine and lysine derivatives indicates that electrostatic interactions between ANS derivatives and positively charged side chains do not account for high binding affinity. In addition to ion pairing, complementary interactions such as van der Waals, should be considered for binding affinity with Kd < 1mM in external binding sites of proteins [29]. Therefore, for the external binding site, it is reasonable to conclude that the sulfonate group of ANS and a positively charged side chain of TL are fully exposed to solvent. It is interesting to note that bound ANS molecules, which have a long lifetime, become sensitive to ionic strength at pH 3.0. It has been shown that TL undergoes a molten globule transition at pH 3.0, suggesting loss of tertiary interactions and exposure of hydrophobic sites. Taken together these findings suggest that the intracavitary binding of ANS also has an electrostatic contribution that becomes more solvent exposed at pH 3.0. However, the long lifetime is minimally decreased, suggesting that solvent reorientation dynamics must be very low at this site. It has been shown that in aqueous solvent, the ANS excited state depopulates efficiently via intermolecular electron transfer, which results in a decreased fluorescence yield of ANS [34, 35]. A (H2O)4±1 cluster, rather than a single molecule of water, is the effective charge acceptor [30, 36]. Therefore, at pH 3.0, a rapid cluster formation (during the fluorescence lifetime of ANS) of water molecules adjacent to bound ANS with the long lifetime can be excluded. Although the internal cavity of TL becomes more exposed in a molten globule state, the solvent dynamics at this site are greatly reduced compared with that of the protein surface. Alternatively, ANS binding to the internal cavity of TL at pH 3.0 may induce extensive molecular compaction as suggested previously for various proteins [37] and for the central sheet of TL [31].
Implications for lipocalin family
These findings have implications for other lipocalins. ANS binding properties of TL can be compared to β-lactoglobulin. In contrast to TL, at low pH a molten globule transition was not observed for β-lactoglobulin. Significant tertiary structure still can be observed at pH 2.3 [5]. Furthermore, a low pH transition increases affinity of ANS to β-lactoglobulin about 40 fold. At pH 2.3, ionic strength, 0.1 M, results in decreased affinity of ANS to β-lactoglobulin for both binding sites but not at pH 6.3 or pH 8.2,. Despite the significant differences in ANS fluorescence lifetimes, the two binding sites of β-lactoglobulin yield similar binding constants at all tested pH values [5]. In addition to the differences mentioned above, the ANS binding affinity for apoTL is about 3 orders of magnitude greater than that of β-lactoglobulin. It is possible that the presence of the unconserved positively charged residue, Lys 114, inside the cavity of TL confers higher ANS affinity to apoTL than the homologous residue, Gln 120, in β-lactoglobulin. The role of Lys 114 of TL in the ANS binding will be pursued in future studies. This comparison shows that despite the similar motif of the lipocalin fold, the family members exhibit diverse binding properties to the ligand. Presumably, the ligand binding reflects functional differences. At least by steady-state fluorescence another closely related lipocalin, human plasma RBP does not show ANS binding in the native state. ANS bound to an external binding site would have low steady-state fluorescence intensity that is not necessarily a sign of low affinity, e.g. β-lactoglobulin [5]. Our data suggest that application of time-resolved fluorescence to the ligand binding studies with RBP and other ligand-carrying members of the lipocalin family may reveal additional external binding sites. Retinol binding to RBP was studied by steady-state fluorescence that relies on significant fluorescence enhancement when retinol is transferred from the solvent to the hydrophobic cavity. However, this method would fail to detect the retinol bound to presumed external binding sites. Time-resolved fluorescence is a promising tool to detect multiple binding sites of proteins, particularly when the proteins exhibit multiple functions.
Acknowledgments
Supported by U.S. Public Health Service Grants NIH EY 11224 and EY 00331 as well as the Edith and Lew Wasserman Endowed Professorship in Ophthalmology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flower DR. Biochem J. 1996;318(Pt 1):1–14. doi: 10.1042/bj3180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Essassi D, Zini R, Tillement JP. J Pharm Sci. 1990;79:9–13. doi: 10.1002/jps.2600790104. [DOI] [PubMed] [Google Scholar]
- 3.Collini M, D'Alfonso L, Baldini G. Protein Sci. 2000;9:1968–1974. doi: 10.1110/ps.9.10.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collini M, D'Alfonso L, Molinari H, Ragona L, Catalano M, Baldini G. Protein Sci. 2003;12:1596–1603. doi: 10.1110/ps.0304403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Alfonso L, Collini M, Baldini G. Biochim Biophys Acta. 1999;1432:194–202. doi: 10.1016/s0167-4838(99)00105-3. [DOI] [PubMed] [Google Scholar]
- 6.Breustedt DA, Schonfeld DL, Skerra A. Biochim Biophys Acta. 2006;1764:161–173. doi: 10.1016/j.bbapap.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Glasgow BJ, Abduragimov AR, Farahbakhsh ZT, Faull KF, Hubbell WL. Curr Eye Res. 1995;14:363–372. doi: 10.3109/02713689508999934. [DOI] [PubMed] [Google Scholar]
- 8.Blaker M, Kock K, Ahlers C, Buck F, Schmale H. Biochim Biophys Acta. 1993;1172:131–137. doi: 10.1016/0167-4781(93)90279-m. [DOI] [PubMed] [Google Scholar]
- 9.Redl B, Holzfeind P, Lottspeich F. J Biol Chem. 1992;267:20282–20287. [PubMed] [Google Scholar]
- 10.Lechner M, Wojnar P, Redl B. Biochem J. 2001;356:129–135. doi: 10.1042/0264-6021:3560129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glasgow BJ, Abduragimov AR, Gassymov OK, Yusifov TN, Ruth EC, Faull KF. Adv Exp Med Biol. 2002;506:567–572. doi: 10.1007/978-1-4615-0717-8_79. [DOI] [PubMed] [Google Scholar]
- 12.Fluckinger M, Haas H, Merschak P, Glasgow BJ, Redl B. Antimicrob Agents Chemother. 2004;48:3367–3372. doi: 10.1128/AAC.48.9.3367-3372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glasgow BJ, Marshall G, Gasymov OK, Abduragimov AR, Yusifov TN, Knobler CM. Invest Ophthalmol Vis Sci. 1999;40:3100–3107. [PubMed] [Google Scholar]
- 14.Selsted ME, Martinez RJ. Exp Eye Res. 1982;34:305–318. doi: 10.1016/0014-4835(82)90079-3. [DOI] [PubMed] [Google Scholar]
- 15.van't Hof W, Blankenvoorde MF, Veerman EC, Amerongen AV. J Biol Chem. 1997;272:1837–1841. doi: 10.1074/jbc.272.3.1837. [DOI] [PubMed] [Google Scholar]
- 16.Yusifov TN, Abduragimov AR, Gasymov OK, Glasgow BJ. Biochem J. 2000;347(Pt 3):815–819. [PMC free article] [PubMed] [Google Scholar]
- 17.Breustedt DA, Korndorfer IP, Redl B, Skerra A. J Biol Chem. 2005;280:484–493. doi: 10.1074/jbc.M410466200. [DOI] [PubMed] [Google Scholar]
- 18.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Biochemistry. 2001;40:14754–14762. doi: 10.1021/bi0110342. [DOI] [PubMed] [Google Scholar]
- 19.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Protein Sci. 2000;9:325–331. doi: 10.1110/ps.9.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glasgow BJ, Heinzmann C, Kojis T, Sparkes RS, Mohandas T, Bateman JB. Curr Eye Res. 1993;12:1019–1023. doi: 10.3109/02713689309029229. [DOI] [PubMed] [Google Scholar]
- 21.Glasgow BJ. Graefes Arch Clin Exp Ophthalmol. 1995;233:513–522. doi: 10.1007/BF00183433. [DOI] [PubMed] [Google Scholar]
- 22.Marston FAO. DNA Cloning. IRL Press; Oxford, England: 1987. A Practical Approach. [Google Scholar]
- 23.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Biochim Biophys Acta. 1998;1386:145–156. doi: 10.1016/s0167-4838(98)00092-2. [DOI] [PubMed] [Google Scholar]
- 24.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Biochem Biophys Res Commun. 1999;265:322–325. doi: 10.1006/bbrc.1999.1668. [DOI] [PubMed] [Google Scholar]
- 25.Gasymov OK, Abduragimov A, Merschak P, Redl B, Glasgow BJ. BBA - Proteins & Proteomics. 2007 doi: 10.1016/j.bbapap.2007.1007.1014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glasgow BJ, Gasymov OK, Abduragimov AR, Yusifov TN, Altenbach C, Hubbell WL. Biochemistry. 1999;38:13707–13716. doi: 10.1021/bi9913449. [DOI] [PubMed] [Google Scholar]
- 27.Bozimowski D, Artiss JD, Zak B. J Clin Chem Clin Biochem. 1985;23:683–689. doi: 10.1515/cclm.1985.23.10.683. [DOI] [PubMed] [Google Scholar]
- 28.Gasymov OK, Abduragimov A, Glasgow B. Photochem Photobiol. 2007 doi: 10.1111/j.1751-1097.2007.00180.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gasymov OK, Glasgow BJ. BBA- Proteins and Proteomics. 2007;1774:403–411. doi: 10.1016/j.bbapap.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore RA, Lee J, Robinson GW. J Phys Chem. 1985;89:3648–3654. [Google Scholar]
- 31.Gasymov OK, Abduragimov AR, Glasgow BJ. Biochem Biophys Res Commun. 2007;357:499–504. doi: 10.1016/j.bbrc.2007.03.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luisi DL, Snow CD, Lin JJ, Hendsch ZS, Tidor B, Raleigh DP. Biochemistry. 2003;42:7050–7060. doi: 10.1021/bi027202n. [DOI] [PubMed] [Google Scholar]
- 33.Matulis D, Lovrien R. Biophys J. 1998;74:422–429. doi: 10.1016/S0006-3495(98)77799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kosower EM, Kanety H. J Am Chem Soc. 1983;105:6236–6243. [Google Scholar]
- 35.Kosower EM. Ann Rev Phys Chem. 1986;37:127–156. [Google Scholar]
- 36.Lee J, Robinson GW. J Am Chem Soc. 1985;107:6153–6156. [Google Scholar]
- 37.Matulis D, Baumann CG, Bloomfield VA, Lovrien RE. Biopolymers. 1999;49:451–458. doi: 10.1002/(SICI)1097-0282(199905)49:6<451::AID-BIP3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]