Abstract
The CCAAT/enhancer binding protein (CEBP) family of transcription factors includes five genes. In the ovary, both Cebpa and Cebpb are essential for granulosa cell function. In this study we have explored the role of the Cebpd gene in ovarian physiology by expression and functional studies. Here we report that Cebpd (C/EBPδ) is expressed in the mouse ovary in a highly restricted temporal and spatial pattern. In response to luteinizing hormone (LH/hCG), CEBPD expression is transiently induced in interstitial cells and in theca cells of follicles from the primary to pre-ovulatory stage, and overlaps in part with expression of the alpha-smooth muscle actin protein. Efficient down-regulation of CEBPD was dependent on a functional Cebpb gene. Proliferating human theca cells in culture also express Cebpd. Cells from patients with polycystic ovarian syndrome (PCOS) exhibited higher Cebpd expression levels. However, deletion of Cebpd in mice had no overt effect on ovarian physiology and reproductive function. Very little is known at present about the molecular mechanisms underlying theca/interstitial cell functions. The expression pattern of CEBPD reported here identifies a novel functional unit of mouse theca cells of primary through tertiary follicles responding to LH/hCG together with a subset of interstitial cells. This acute stimulation of CEBPD expression may be exploited to further characterize the hormonal regulation and function of theca and interstitial cells.
Introduction
The ovary is a complex organ undergoing regular phases of re-organization in response to a variety of intrinsic and extrinsic factors. The main functional unit, the follicle is comprised of a central oocyte, mural and cumulus granulosa cell layers, and an outer layer of theca cells. Ovarian follicles reside within the interstitial stroma, which also gives rise to the specialized follicular cell types. The ovulatory surge of luteinizing hormone (LH), produced by the pituitary, triggers a series of dramatic morphological and physiological changes in the pre-ovulatory follicle, culminating in ovulation and subsequent differentiation of the follicular granulosa and theca cells into the luteal cells. While the LH receptor signals primarily through the cAMP/PKA pathway to influence gene expression [1] it also impacts signaling pathways involving Janus and phosphoinositol kinases, chloride currents, and calcium [2]–[5]. Transcription factors known to act downstream of LH receptor activation include STAT1 and STAT5 [2], GATA4 [6], EGR1 [7], and the CCAAT/enhancer binding protein proteins CEBPA and CEBPB [8].
The CEBP family of transcription factors is comprised of five proteins with a highly homologous carboxyterminal leucine-zipper/basic region domain required for dimerization and DNA binding. Each CEBP protein has unique properties regulating cell type-specific growth and differentiation. For example, within the hematopoietic system CEBPA is required for development of granulocytes, while lack of CEBPB affects differentiation of the B-cell lineage and monocytes. During adipocyte differentiation, CEBPB, -D, and A are expressed consecutively [9]. In the mammary gland, CEBPB promotes proliferation and differentiation [10], while CEBPD participates in the initiation of cell death [11]. In the ovary, both CEBPA and CEBPB are expressed in follicular granulosa cells, dynamically regulated by gonadotropins, and essential for follicular development, efficient ovulation and luteal differentiation. In particular, CEBPB is a downstream effector of the LH signaling pathway in granulosa cells [8]. The absence of significant levels of CEBPA and CEBPB in theca cells combined with the importance of these factors in mediating LH-triggered events in granulosa cells suggested the possibility that another member of the family may substitute for their function in theca cells. Therefore, we investigated the expression and function of CEBPD in the ovary.
Cebpd (C/EBPdelta, CELF, CRP3, NFIL-6beta) was first characterized as an acute phase inflammatory response gene. Expression of Cebpd is typically low to undetectable in most cell types and tissues, but is rapidly induced by a variety of extracellular stimuli, (e.g. growth hormone, insulin, IFNgamma, IL-1, IL-6, LPS, TNFalpha, noradrenaline and glutamate) [9], [12]. In vitro and in vivo studies have implicated CEBPD in proliferation of osteoblasts [13], [14], differentiation of lung epithelial cells [15]–[17], and growth arrest of mouse mammary epithelial cells [18]. While Cebpd-deficient mice display no overt phenotype, are fertile and achieve normal life spans, more detailed characterization revealed that the null mutation led to improved performance in the contextual fear conditioning test of long term memory [19], increased mammary ductal branching [20] and delayed mammary gland involution [11]. Furthermore, Cebpd-deficiency exacerbates the differentiation defect of Cebpb-deficient adipocytes [21], and causes genomic instability in fibroblasts [22]. Thus, CEBPD appears to have highly diverse functions depending on cell type and specific physiological stimuli. Furthermore, because its expression is mostly activated by transient signals, the role of CEBPD in vivo may be modulatory and only uncovered when cells are investigated in response to specific stimuli. In this study we have addressed whether CEBPD plays a role in ovarian physiology through expression analyses and a thorough reproductive characterization of Cebpd-deficient mice.
Results
Dynamic regulation of Cebpd mRNA expression in the mouse ovary
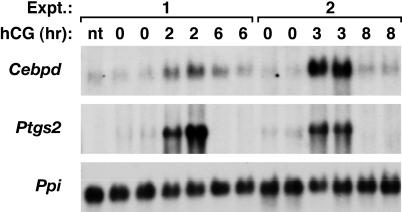
To address the role of CEBPD in the mouse ovary we first assessed its expression pattern in response to LH administration. We used an ovulation protocol in which mice were treated with pregnant mare serum gonadotropin (PMSG) to stimulate the coordinated development of multiple pre-ovulatory follicles, followed two days later by human chorionic gonadotropin (hCG) to mimic the ovulatory surge of LH. Northern blot analysis of whole ovary RNA revealed a highly restricted temporal expression pattern for Cebpd (Fig. 1). Expression of Cebpd mRNA was minimal prior to hCG treatment, but was highly induced 2–3 hours following hCG, before returning to pre-hCG levels by 6 hours where it remained through 8 hours. Similar data were obtained by Western analysis of whole ovary protein extracts (data not shown). Expression of prostaglandin-endoperoxide synthase 2 (Ptgs2, a.k.a. Cox-2, Pghs 2), a gene known to be induced by LH in granulosa cells [23], was evaluated as a positive control (Fig. 1). As expected, ovarian Ptgs2 expression increased following hCG treatment and then declined rapidly. In whole ovary extracts, Cebpd and Ptgs2 mRNA expression were regulated temporally in a similar pattern (Fig. 1). Similar expression data were obtained from 3 week old prepubertal and 5 week old postpubertal mice. Thus, we have used 5–6 week old mice for all other expression analyses in this study.
Figure 1. Cebpd mRNA expression is induced by hCG in mouse ovaries.
Northern blot analysis of whole ovary total RNA isolated from 5 week-old (Exp.1) or 3 week-old (Exp.2) mice treated for 2 days with PMSG followed by the LH analog hCG for the indicated times (n.t. = no treatment). Ptgs2 was used as a positive control for the hCG response. Cyclophilin (Ppi) was used as loading control.
Analysis of Cebpd expression in Cebpb null mice
Since we previously identified an essential role of Cebpb in the LH responsiveness of granulosa cells [24], we assessed Cebpd-expression also in Cebpb-deficient mice (Fig. 2). Cebpd mRNA expression was induced normally by hCG in Cebpb null mice. Expression levels at 0 h and 3 h after hCG treatment were statistically similar in WT and KO ovaries. However, Cebpd mRNA levels remained high in Cebpb knockout mice through five hours of hCG treatment, in contrast to the rapid loss seen in the controls (Fig. 2). By 8 hours following hCG, Cebpb-deficient and control ovaries expressed similar levels of Cebpd mRNA (data not shown). This pattern of normal induction by hCG but delayed down-regulation is similar to the defective down-regulation of Ptgs2 and aromatase mRNA expression in granulosa cells of Cebpb-deficient mice [24].
Figure 2. Down-regulation of Cebpd mRNA requires Cebpb.
Phosphorimage quantitation of Northern blot analyses of whole ovary total RNA from 5 week-old Cebpb null mice (KO) or litter mate controls (WT) treated for 2 days with PMSG followed by hCG for the indicated times. Expression levels of Cebpd were normalized to cyclophilin (Ppi) expression and are shown in arbitrary units (a.u.; mean±SEM; n = 3–4 mice per data point). **P = 0.01 by two-way ANOVA with follow-up comparisons among groups in the form of t-tests at each time point.
Localization of Cebpd expression in theca/interstitial cells
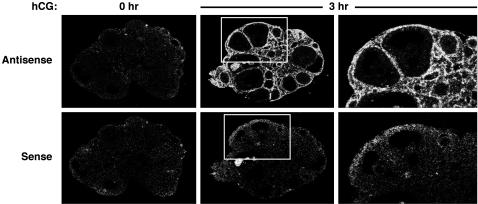
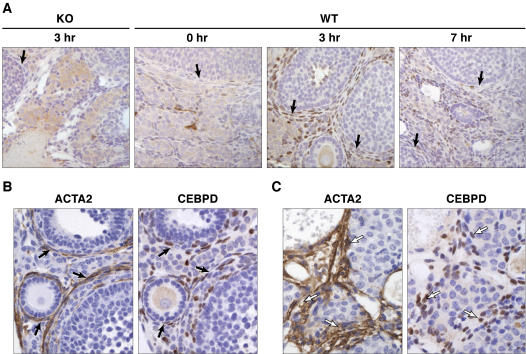
To determine which ovarian cell type(s) Cebpd was expressed in we employed in situ hybridization and immunohistochemistry. Figure 3 confirms the relative lack of Cebpd expression prior to the LH surge, and the dramatic induction of Cebpd mRNA levels following hCG administration. This expression was localized in the theca cell layer of follicles of varying sizes as well as in the stromal compartment. No evidence of granulosa cell expression was observed in any preparation. To better characterize Cebpd expression within the stroma, immunohistochemistry was performed. The anti-CEBPD rabbit polyclonal antibody detected nuclear CEBPD protein expression in theca and interstitial cells specifically in wild-type ovarian tissue but not in Cebpd knockout control tissue (Fig. 4A). As seen with Cebpd mRNA, maximal protein levels were observed 3 h after hCG treatment. However, 7 h following hCG-adminstration to mice CEBPD protein was still detected in interstitial cells, but less so in theca cells (Fig. 4A). Thus, immunohistochemistry revealed expression kinetics that were not evident by analysis of whole ovary mRNA (Fig. 1) or protein extracts (data not shown). Most of the CEBPD-staining interstitial cells exhibit small, elongated nuclei. Theca externa cells express alpha smooth muscle actin (ACTA2) [25], and CEBPD is also expressed in vascular smooth muscle cells [26]. Thus, we sought to determine if CEBPD characterizes ACTA2 expressing interstitial cells. Analysis of parallel sections revealed that ACTA2 and CEBPD stain the same layers in primary to antral follicles (Fig. 4B). Similarly, some stromal areas that stain positive for ACTA2 also contain CEBPD-expressing cells (Fig. 4C).
Figure 3. Cebpd mRNA expression is localized to theca and interstitial cells.
Dark-field photomicrographs of in situ hybridization analyses using Cebpd-specific sense and antisense RNA probes on sections from ovaries of 6 week-old mice treated for 2 days with PMSG followed by hCG for the indicated times. Outlined regions in the center panels are shown at higher magnification on the right.
Figure 4. CEBPD protein expression in mouse ovaries.
(A) Immunohistochemistry for CEBPD in ovaries from wild-type (WT) or Cebpd null mutant (KO) mice at the indicated time points following hCG treatment. (B, C) Immunohistochemistry for CEBPD and alpha smooth muscle actin (ACTA2) in wild-type ovaries harvested 3 h after hCG treatment. Solid arrows indicate theca layers (B), open arrows indicate stromal areas (C) expressing both CEBPD and ACTA2.
Analysis of Cebpd null mutant mice
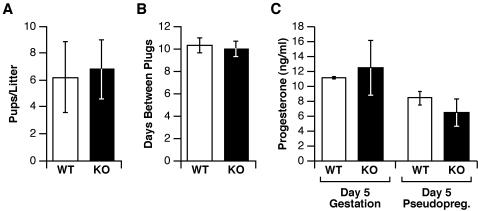
When Cebpd null mutant mice had first been generated [19], females and males were found to be fertile. Here we conducted a more detailed analysis to potentially uncover effects of Cebpd deficiency on ovarian physiology that may not be evident in assays of fertility. Comparison of Cebpd and control mice reproductive parameters are shown in Figure 5. No differences were observed between Cebpd knockout and control mice with respect to the size of their first litters (Fig. 5A). Regulation of the estrous cycle was assessed indirectly by mating the females to sterile males and recording mating activity. Both genotypes mated on average every ten days, indicating normal mating behavior and a normal pseudopregnancy response to mating, which is reliant on luteal progesterone production (Fig. 5B). Levels of progesterone were assessed as a measure of follicular differentiation following ovulation. Cebpd null mice and controls produced similar levels of progesterone on day 5 of gestation and day 5 of pseudopregnancy (Fig. 5C). Lastly, examination of the ovarian histology of mice from age 70 to 400 days did not reveal abnormalities when compared to wild-type littermates (data not shown). Taken together, these data demonstrate that the Cebpd null mutation does not overtly affect reproductive function in female mice.
Figure 5. Cebpd-deficient females exhibit normal reproductive functions (A) Cebpd-deficient females generate normal-sized litters.
Two to three-month old females with wild-type Cebpd (WT, n = 10) or Cebpd null mutation (KO, n = 16) were mated, the male removed subsequent to recording of a vaginal plug, and the number of pups of the following litter were counted within 24 hours after birth. (B) Cebpd-deficient females display regular estrous cycles. Two month-old Cebpd null females (KO, n = 5) and wild-type littermate controls (WT, n = 4) were mated to vasectomized, sterile males and monitored daily for the occurrence of vaginal plugs over a period of 40–50 days. The number of days between 19 plugs per group was averaged. (C) Cebpd-deficient females display normal progesterone levels. Progesterone levels were measured in serum from mice on day 5 of gestation (from experiment in Fig. 5A, n = 5 KO, 3 WT) or on day 5 of pseudopregnancy (from experiment in Fig. 5B, n = 5 KO, 4 WT). Means±S.E.M. are shown.
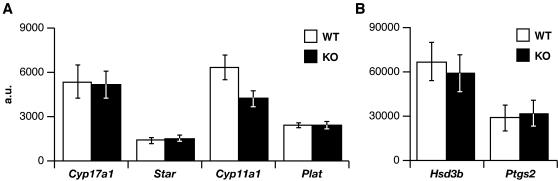
Since a physiological response may proceed normally despite the altered regulation of certain genes, we also analyzed the expression of several marker genes 4 hours after hCG treatment in knockout and wild-type ovaries (Fig. 6). No differences were observed for the mRNA levels of several steroidogenic marker genes, steroidogenic acute regulatory protein (Star), cytochrome P450scc (Cyp11a1), 17-α hydroxylase (Cyp17a1), and 3β-hydroxysteroid dehydrogenase (Hsd3b), as well as the theca-specific tissue-type plasminogen activator (Plat) gene [27]. Furthermore, Ptgs2, a gene primarily expressed in granulosa cells [27], was induced to similar levels in Cebpd knockout and control mice (Fig. 6). These data further confirm normal ovarian function in the absence of CEBPD.
Figure 6. Normal gene expression pattern in Cebpd-deficient ovaries.
Phosphorimage quantification of Northern blot analyses for the indicated genes of whole ovary total RNA from 5 week-old Cebpd null mice (KO) and litter mate controls (WT) treated for 2 days with PMSG followed by hCG for 4 hours. Expression levels were normalized to cyclophilin (Ppi) expression (a.u. = arbitrary units; means±SEM). Panel A: n = 6 WT, 8 KO; Panel B: n = 4 WT, 4 KO.
Expression of CEBPD in cultured human theca cells
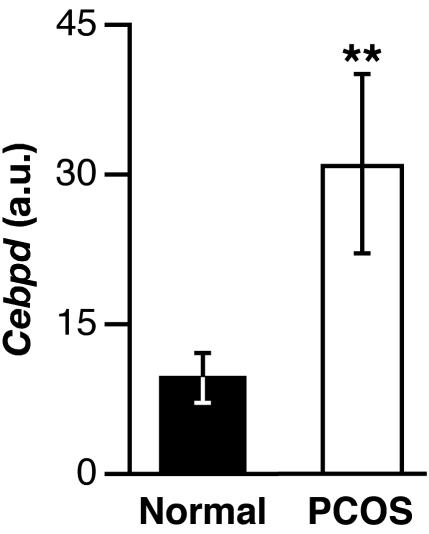
Analysis of cultured proliferating theca cells obtained from patients with polycystic ovarian syndrome (PCOS, n = 4) showed approximately 3-fold higher levels of Cebpd mRNA than theca cells isolated from women not afflicted with this syndrome (Fig. 7). These data confirm that Cebpd expression in theca cells is not a species-specific phenomenon as it occurs in mouse and human. Previous microarray experiments utilizing the same RNA preparations used in our experiments showed increased 17α-hydroxylase (Cyp17a1) expression in these PCOS-theca cells [28], confirming their pathological condition.
Figure 7. Cebpd is overexpressed in theca cells from PCOS patients.
Quantitative real-time RT-PCR results examining Cebpd mRNA expression in theca cells derived from four healthy women and four PCOS patients are shown. Expression levels were normalized by Gapdh values and the relative values are shown (a.u. = arbitrary units). The two-sample independent t-test, for which the values were transformed to their common logs, and the nonparametric Mann-Whitney test, both yielded a statistically significant difference at P<0.03.
Discussion
The CEBP family of transcription factors has many different functions in a variety of physiological processes. In the ovary, CEBPA and CEBPB play key roles in the LH response of the follicle. Cebpa is widely and constitutively expressed in the rat ovary. Cebpa expression levels do not change dramatically upon hormone stimulation, and localize mostly to the granulosa cells and theca cells of preovulatory follicles, with the highest levels in cumulus cells. On the other hand Cebpb expression is rapidly and highly induced by hCG mainly in granulosa cells. Knockdown and targeted mutations have shown that both CEBPA and CEBPB are important for ovulation and Cebpb-deficient mice are sterile presumably due to a granulosa cell defect [8]. We used a combination of expression studies and genetic analysis to assess the function of CEBPD in the mouse ovary. We found that in contrast to CEBPA and CEBPB, the CEBPD protein does not have a critical or non-redundant role in the ovary. However, expression of the Cebpd gene is acutely responsive to the LH surge in a select group of interstitial cells within the ovarian stroma and in theca cells of follicles. The diversity of cell type and hormone-response specific expression of different Cebp genes demonstrates that this gene family has been exploited in evolution to contribute to the development of the ovary by providing unique functions for its variety of cell types. Interestingly, expression of CEBPD in theca cells is not restricted to pre-ovulatory follicles but was seen at all stages of follicular development, beginning with primary follicles. In the stroma, we observed that most alpha smooth muscle actin containing regions harbor CEBPD-expressing cells, and were thus identified as early responders to LH/hCG. To our knowledge, the only other transcription factor gene previously reported as activated by LH/hCG specifically in theca cells, is the zinc finger protein gonadotropin-inducible transcription factor 1 (Giot1). However, activation of Giot1 was detected only in theca interna cells and was not observed in interstitial cells [29]. Remarkably, loss of CEBPD did not have a detrimental effect on thecal cell function, as might be expected, if it was essential for LH-induced steroidogenesis (which is critical for follicular steroidogenesis) or thecal cell development. However, it is possible that loss of CEBPD was compensated by one of the other CEBPs. For example, Cebpe mRNA is detectable in whole ovary RNA, but its hormonal regulation or cellular distribution has not yet been characterized [8].
A role for CEBPB in down regulation of Cebpd mRNA levels was also observed after its initial activation by hCG. Cebpb is mainly expressed in the inner follicular mass of granulosa cells. While we cannot rule out a direct effect of low levels of CEBPB within theca/interstitial cells as the primary mechanism for the observed decline in Cebpd mRNA levels, it is tempting to speculate that thecal Cebpd expression is regulated by juxtacrine/endocrine factors from the granulosa cell compartment. A number of cytokines, including several members of the EGF family, which are induced in the granulosa layer by LH [27], [30], are dramatically downregulated in Cebpb null ovaries (L.K.C., E.S., unpublished data) and, therefore, represent candidate feedback signals.
The observation that the Cebpd gene is not essential for fertility in mice, does not rule out that the protein participates in ovarian gene regulation. Therefore, misexpression or overactivity of CEBPD could impact ovarian function in adverse ways. In humans, hyperactivity of the theca/interstitial compartment is associated with PCOS. In the present study, we evaluated Cebpd levels in PCOS patient derived theca cells. Our results indicate that Cebpd expression was elevated in theca cells that are producing androgen and are in agreement with the chronically elevated androgen levels and Cyp17a1 gene expression seen in PCOS theca cells. However, our data are in contrast to an array-based expression analysis of biopsies, which reports 2-fold lower levels of Cebpd expression in PCOS tissue [31]. There are several potential reasons for this discrepancy. The cultured cells used here were derived from the theca layer of 5–6 mm follicles of both normal and PCOS women, while the biopsies were random and therefore of varying cell type composition that could be quite heterogeneous. In contrast, the cultured theca cells from normal and PCOS women would be more homogenous and comparable cell populations, and cultured cells are known to maintain some of their in vivo characteristics unique to PCOS such as elevated Cyp17a1. However, the potential effect of culture conditions affecting gene expression patterns can not be discounted either. Because of the relatively small number of samples in this analysis we primarily conclude that Cebpd expression in theca cells is not specific to mice but also true for human cells. Our present results lend support but far from confirm that CEBPD is possibly elevated in thecal cells of PCOS women.
In summary, our data show that the Cebpd gene is rapidly induced by LH/hCG in theca cells of primary follicles through pre-ovulatory follicles as well as in interstitial cells. This observation demonstrates some common characteristics in the hormonal regulation between these cell populations. The data also suggest that in contrast to CEBPA and CEBPB, CEBPD function is not directly related to the ovulation process, but may rather have a role in the broad endocrine activity of the theca/interstitial compartment, or possibly the inflammatory response associated with ovulation. The physiologic/developmental role of LH/hCG responsive cells within the ovarian stroma compartment is presently not understood. CEBPD can be a useful marker for the early response of the theca/interstitial compartment to LH/hCG and may help to further characterize these ovarian cell types.
Materials and Methods
Mice
The generation of mice (Mus musculus) with targeted deletion of Cebpd [19] and Cebpb [24] has been described previously. Wild-type and mutant subjects were offspring from heterozygous breeding pairs. Cebpb-deficient mice are not viable on a pure genetic background. Thus, the expression data in Figures 1– 3 were derived from mice of a 129B6-F1 strain background, generated by mating heterozygous mice that had been backcrossed into the 129S1 and C57BL/6 strain background, respectively. All other data were generated with mice on a pure 129S1 strain background, which exhibit similar Cebpd expression as the 129B6-F1 strain. Where indicated, mice were given intraperitoneal injections of pregnant mare serum gonadotropin (PMSG, 5 IU, SIGMA; equivalent to equine chorionic gonadotropines, eCG) or human chorionic gonadotropin (hCG, 5 IU, SIGMA). The mice were housed and bred in a specific pathogen-free facility with a 12 hour light cycle, and with chow and water ad libitum. All procedures were conducted in compliance with the guidelines of the Animal Care and Use Committee of the National Cancer Institute, MD, U.S.A.
In situ analysis
Ovaries were fixed in 4% paraformaldehyde and 5 µm sections were prepared from paraffin embedded tissues. In situ hybridization analysis was performed as described [32], using a cRNA probe representing the coding region for amino acids 1–181 of Cebpd.
Immunohistochemistry
Immunohistochemical staining was performed with the aid of an automated immunostainer (DakoCytomation, Carpinteria, CA). Formalin-fixed paraffin embedded tissue sections were mounted on glass slides and deparaffinized. Prior to staining, heat-induced antigen retrieval was performed by placing the slides into target retrieval solution, high pH (DakoCytomation), and steaming them in a commercial vegetable steamer at full temperature for 30 minutes (CEBPD) or by pressure cooking in TRIS/Citrate (pH 6) for 8 minutes (alpha-smooth muscle actin, ACTA2). Following the antigen retrieval procedure the slides were incubated with a CEBPD rabbit polyclonal antibody (ActiveMotif cat#39006; dilution 1∶500) overnight at 4°C, or a monoclonal mouse antibody against ACTA2 (DAKO, Clone 1A4, dilution 1∶500) for 1 hour. Detection was carried out on the automated system using an HRP/DAB polymer based rabbit detection system (Envision+, DakoCytomation) according to the manufacturer's recommendations.
Progesterone assay
Serum was prepared from orbital eye bleeds and progesterone levels were assessed with the DSL-10-3900 ACTIVE progesterone EIA kit (Diagnostic Systems Laboratories, Inc.), according to the manufacturer's instructions. Each sample was assayed in duplicate. The coefficient of variation across experiments and genotypes was 0.35.
RNA analysis
Total ovarian RNA was prepared by homogenization of tissue in Trizol reagent (Life Technologies, Inc.) according to the manufacturer's protocol. The RNA was analyzed by Northern blotting as described [33] except that hybridizations were carried out in HybPlus solution (SigmaAldrich, Inc.). Radiolabeled DNA probes were prepared from isolated mouse cDNA clones for the indicated genes. The specific signals were recorded and quantified by phosphorimage analysis (Molecular Dynamics, ImageQuant™).
Human theca cell culture and real time PCR analysis
Human theca interna tissue was obtained from follicles of women undergoing hysterectomy, following informed consent under a protocol approved by the Institutional Review Board of the Pennsylvania State University College of Medicine. In the experiments presented in this manuscript, 3rd and 4th passage (31–38 population doublings) theca cells isolated from size-matched follicles obtained from age-matched subjects were used as previously described [34], [35]. All of the PCOS theca preparations studied came from ovaries of women with fewer than six menstrual periods per year and elevated serum total testosterone (T) or bioavailable T levels, as we previously described (26). The control (normal) theca cell preparations came from ovaries of fertile women with normal menstrual histories, menstrual cycles of 21–35 d, and no signs of hyperandrogenemia. The OB/Gyn department at Penn State Medical Center routinely schedules hysterectomies of reproductive aged women during their follicular phase.
Total RNA (5 µg) isolated with Trizol reagent (Life Technologies, Inc.) was treated with DNase I (Promega, Madison, WI) followed by cDNA synthesis using the MMLV reverse transcriptase (Promega) and oligo dT primer (Promega) as previously described [35]. The resulting cDNAs were diluted 1∶100 in sterile water and 1-µl aliquots were used in the quantitative real-time PCRs reactions. The primers used to quantify Cebpd (Forward: 5′-GGTGCCCGCTGCAGTTT-3′; Reverse: 5′-CTCGCAGTTTAGTGGTGGTAAGTC-3′) were designed with Primer Express software package that accompanies the Applied Biosystems Model 7900 sequence detector (PerkinElmer Life Science). The SyBr green reagent PCR Master Mix (Applied Biosystems) was used as previously described [36]. In order to account for differences in starting material, the human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers and probe reagents from Applied Biosystems were used as described by the manufacturer. In order to quantify differences, the samples were compared to standard curves for each target amplicon and the average value for the triplicate was used in all subsequent calculations.
Acknowledgments
We are indebted to Lars Hedin, whose data and support encouraged this study. We thank Barbara Shankle, Mary Beth Hilton, Lori Warg, and Velen L. Nelson-DeGraves for expert technical assistance, Vincenzo Coppola for valuable reagents and advice, Douglas Powel for statistical analysis, and the following colleagues for providing cDNA clones: Anita H. Payne (Cyp17a1), JoAnne S. Richards (Ptgs2, Cyp11a1), and D.M. Stocco (Star). The cDNA clone for Plat [37] was obtained through the American Type Culture Collection.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, and the National Institutes of Child Health and Human Development (HD045519; L.K.C).
References
- 1.Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23:141–174. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho CR, Carvalheira JB, Lima MH, Zimmerman SF, Caperuto LC, et al. Novel signal transduction pathway for luteinizing hormone and its interaction with insulin: activation of Janus kinase/signal transducer and activator of transcription and phosphoinositol 3-kinase/Akt pathways. Endocrinology. 2003;144:638–647. doi: 10.1210/en.2002-220706. [DOI] [PubMed] [Google Scholar]
- 3.Chiang M, Strong JA, Asem EK. Luteinizing hormone activates chloride currents in hen ovarian granulosa cells. Comp Biochem Physiol A Physiol. 1997;116:361–368. doi: 10.1016/s0300-9629(96)00375-1. [DOI] [PubMed] [Google Scholar]
- 4.Sekar N, Veldhuis JD. Concerted transcriptional activation of the low density lipoprotein receptor gene by insulin and luteinizing hormone in cultured porcine granulosa-luteal cells: possible convergence of protein kinase a, phosphatidylinositol 3-kinase, and mitogen-activated protein kinase signaling pathways. Endocrinology. 2001;142:2921–2928. doi: 10.1210/endo.142.7.8287. [DOI] [PubMed] [Google Scholar]
- 5.Gudermann T, Nichols C, Levy FO, Birnbaumer M, Birnbaumer L. Ca2+ mobilization by the LH receptor expressed in Xenopus oocytes independent of 3′,5′-cyclic adenosine monophosphate formation: evidence for parallel activation of two signaling pathways. Mol Endocrinol. 1992;6:272–278. doi: 10.1210/mend.6.2.1314958. [DOI] [PubMed] [Google Scholar]
- 6.Tremblay JJ, Viger RS. Novel roles for GATA transcription factors in the regulation of steroidogenesis. J Steroid Biochem Mol Biol. 2003;85:291–298. doi: 10.1016/s0960-0760(03)00211-5. [DOI] [PubMed] [Google Scholar]
- 7.Russell DL, Doyle KM, Gonzales-Robayna I, Pipaon C, Richards JS. Egr-1 induction in rat granulosa cells by follicle-stimulating hormone and luteinizing hormone: combinatorial regulation by transcription factors cyclic adenosine 3′,5′-monophosphate regulatory element binding protein, serum response factor, sp1, and early growth response factor-1. Mol Endocrinol. 2003;17:520–533. doi: 10.1210/me.2002-0066. [DOI] [PubMed] [Google Scholar]
- 8.Sterneck E, Johnson PF. Expression and Function of CCAAT/Enhancer Binding Proteins (C/EBPs) in the Ovary. In: Adashi e., editor. Ovulation: Evolving Scientific and Clinical Concepts: Springer-Verlag new York Inc; 2000. pp. 277–291. [Google Scholar]
- 9.Takiguchi M. The C/EBP family of transcription factors in the liver and other organs. Int J Exp Pathol. 1998;79:369–391. doi: 10.1046/j.1365-2613.1998.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson GW, Johnson PF, Hennighausen L, Sterneck E. The C/EBPb transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes Dev. 1998;12:1907–1916. doi: 10.1101/gad.12.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thangaraju M, Rudelius M, Bierie B, Raffeld M, Sharan S, et al. C/EBP delta is a critical regulator of pro-apoptotic gene expression during mammary gland involution. Development. 2005;132:4675–4685. doi: 10.1242/dev.02050. [DOI] [PubMed] [Google Scholar]
- 12.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umayahara Y, Ji C, Centrella M, Rotwein P, McCarthy TL. CCAAT/enhancer-binding protein delta activates insulin-like growth factor-I gene transcription in osteoblasts. Identification of a novel cyclic AMP signaling pathway in bone. J Biol Chem. 1997;272:31793–31800. doi: 10.1074/jbc.272.50.31793. [DOI] [PubMed] [Google Scholar]
- 14.Billiard J, Grewal SS, Lukaesko L, Stork PJ, Rotwein P. Hormonal Control of Insulin-like Growth Factor I Gene Transcription in Human Osteoblasts. DUAL ACTIONS OF cAMP-DEPENDENT PROTEIN KINASE ON CCAAT/ENHANCER-BINDING PROTEIN delta. J Biol Chem. 2001;276:31238–31246. doi: 10.1074/jbc.M103634200. [DOI] [PubMed] [Google Scholar]
- 15.Breed DR, Margraf LR, Alcorn JL, Mendelson CR. Transcription factor C/EBPdelta in fetal lung: developmental regulation and effects of cyclic adenosine 3′,5′-monophosphate and glucocorticoids. Endocrinology. 1997;138:5527–5534. doi: 10.1210/endo.138.12.5637. [DOI] [PubMed] [Google Scholar]
- 16.Sugahara K, Sadohara T, Sugita M, Iyama K, Takiguchi M. Differential expression of CCAAT enhancer binding protein family in rat alveolar epithelial cell proliferation and in acute lung injury. Cell Tissue Res. 1999;297:261–270. doi: 10.1007/s004410051354. [DOI] [PubMed] [Google Scholar]
- 17.Cassel TN, Nordlund-Moller L, Andersson O, Gustafsson JA, Nord M. C/EBPalpha and C/EBPdelta activate the clara cell secretory protein gene through interaction with two adjacent C/EBP-binding sites. Am J Respir Cell Mol Biol. 2000;22:469–480. doi: 10.1165/ajrcmb.22.4.3916. [DOI] [PubMed] [Google Scholar]
- 18.O'Rourke JP, Newbound GC, Hutt JA, DeWille J. CCAAT/enhancer-binding protein delta regulates mammary epithelial cell G0 growth arrest and apoptosis. J Biol Chem. 1999;274:16582–16589. doi: 10.1074/jbc.274.23.16582. [DOI] [PubMed] [Google Scholar]
- 19.Sterneck E, Paylor R, Jackson-Lewis V, Libbey M, Przedborski S, et al. Selectively enhanced contextual fear conditioning in mice lacking the transcriptional regulator CCAAT/enhancer binding protein delta. Proc Natl Acad Sci U S A. 1998;95:10908–10913. doi: 10.1073/pnas.95.18.10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gigliotti AP, Johnson PF, Sterneck E, DeWille JW. Nulliparous CCAAT/enhancer binding protein delta (C/EBPdelta) knockout mice exhibit mammary gland ductal hyperplasia. Exp Biol Med (Maywood) 2003;228:278–285. doi: 10.1177/153537020322800306. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 1997;16:7432–7443. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang AM, Montagna C, Sharan S, Yajun N, Ried T, et al. Loss of CCAAT/Enhancer Binding Protein δ Promotes Chromosomal Instability. Oncogene. 2004;23:1549–1557. doi: 10.1038/sj.onc.1207285. [DOI] [PubMed] [Google Scholar]
- 23.Sirois J, Simmons DL, Richards JS. Hormonal regulation of messenger ribonucleic acid encoding a novel isoform of prostaglandin endoperoxide H synthase in rat preovulatory follicles. Induction in vivo and in vitro. J Biol Chem. 1992;267:11586–11592. [PubMed] [Google Scholar]
- 24.Sterneck E, Tessarollo L, Johnson PF. An essential role for C/EBPβ in female reproduction. Genes Dev. 1997;11:2153–2162. doi: 10.1101/gad.11.17.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teubner A, Sobek-Klocke I, Hinssen H, Eichenlaub-Ritter U. Distribution of gelsolin in mouse ovary. Cell Tissue Res. 1994;276:535–544. doi: 10.1007/BF00343950. [DOI] [PubMed] [Google Scholar]
- 26.Fukuoka T, Kitami Y, Okura T, Hiwada K. Transcriptional regulation of the platelet-derived growth factor alpha receptor gene via CCAAT/enhancer-binding protein-delta in vascular smooth muscle cells. J Biol Chem. 1999;274:25576–25582. doi: 10.1074/jbc.274.36.25576. [DOI] [PubMed] [Google Scholar]
- 27.Richards JS. Perspective: the ovarian follicle–a perspective in 2001. Endocrinology. 2001;142:2184–2193. doi: 10.1210/endo.142.6.8223. [DOI] [PubMed] [Google Scholar]
- 28.Wood JR, Nelson VL, Ho C, Jansen E, Wang CY, et al. The molecular phenotype of polycystic ovary syndrome (PCOS) theca cells and new candidate PCOS genes defined by microarray analysis. J Biol Chem. 2003;278:26380–26390. doi: 10.1074/jbc.M300688200. [DOI] [PubMed] [Google Scholar]
- 29.Mizutani T, Yamada K, Yazawa T, Okada T, Minegishi T, et al. Cloning and characterization of gonadotropin-inducible ovarian transcription factors (GIOT1 and -2) that are novel members of the (Cys)(2)-(His)(2)-type zinc finger protein family. Mol Endocrinol. 2001;15:1693–1705. doi: 10.1210/mend.15.10.0718. [DOI] [PubMed] [Google Scholar]
- 30.Park JY, Su YQ, Ariga M, Law E, Jin SL, et al. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 31.Jansen E, Laven JS, Dommerholt HB, Polman J, van Rijt C, et al. Abnormal gene expression profiles in human ovaries from polycystic ovary syndrome patients. Mol Endocrinol. 2004;18:3050–3063. doi: 10.1210/me.2004-0074. [DOI] [PubMed] [Google Scholar]
- 32.Tessarollo L, Parada LF. Oncogene techniques: In situ hybridization. In: Verma IM, Vogt PK, editors. Methods in Enzymology. New York, N.Y.: Academic; 1995. pp. 419–430. [DOI] [PubMed] [Google Scholar]
- 33.Sterneck E, Kaplan D, Johnson PF. Interleukin-6 induces expression of peripherin and cooperates with Trk receptor signaling to promote neuronal differentiation in PC12 cells. J Neurochem. 1996;67:1365–1374. doi: 10.1046/j.1471-4159.1996.67041365.x. [DOI] [PubMed] [Google Scholar]
- 34.Nelson VL, Legro RS, Strauss JF, 3rd, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13:946–957. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- 35.Nelson VL, Qin Kn KN, Rosenfield RL, Wood JR, Penning TM, et al. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86:5925–5933. doi: 10.1210/jcem.86.12.8088. [DOI] [PubMed] [Google Scholar]
- 36.Christenson LK, Stouffer RL, Strauss JF., 3rd Quantitative analysis of the hormone-induced hyperacetylation of histone H3 associated with the steroidogenic acute regulatory protein gene promoter. J Biol Chem. 2001;276:27392–27399. doi: 10.1074/jbc.M101650200. [DOI] [PubMed] [Google Scholar]
- 37.Rickles RJ, Darrow AL, Strickland S. Molecular cloning of complementary DNA to mouse tissue plasminogen activator mRNA and its expression during F9 teratocarcinoma cell differentiation. J Biol Chem. 1988;263:1563–1569. [PubMed] [Google Scholar]