Abstract
The RET receptor tyrosine kinase is activated by GDNF and controls outgrowth and invasion of the ureteric bud epithelia in the developing kidney. In renal epithelial cells and in enteric neuronal precursor cells, activation of RET results in chemotaxis as Ret expressing cells invade the surrounding GDNF expressing tissue. One potential downstream signaling pathway governing RET mediated chemotaxis may require phosphatidylinositol 3-kinase (PI3K), which generates PI(3,4,5) triphosphate. The PTEN tumor suppressor gene encodes a protein and lipid phosphatase that regulates cell growth, apoptosis and many other cellular processes. PTEN helps regulate cellular chemotaxis by antagonizing the PI3K signaling pathway through dephosphorylation of phosphotidylinositol triphosphates. In this report, we show that PTEN suppresses RET mediated cell migration and chemotaxis in cell culture assays, that RET activation results in asymmetric localization of inositol triphosphates and that loss of PTEN affects the pattern of branching morphogenesis in developing mouse kidneys. These data suggest a critical role for the PI3K/PTEN axis in shaping the pattern of epithelial branches in response to RET activation.
Keywords: PTEN, RET, GDNF, Chemotaxis, Branching morphogenesis
Introduction
The process of epithelial branching morphogenesis occurs in a variety of developing mammalian tissues, including mammary gland, the prostate, the lung and the kidney, and consists of common mechanistic elements (Costantini, 2006; Lu et al., 2006). Initial outgrowth of an invasive bud is followed by proliferation and cell migration to generate an ampullae that bi- or trifurcates to form new buds that will repeat the process and generate a complex arborized epithelial network. The pattern of arborization depends on positive regulation by growth factors and receptors to promote proliferation and migration and negative regulation to inhibit excessive budding and bifurcation. How potential positive and negative signals are integrated to control cell movement, while retaining the essential character of an invasive epithelia, remains unclear.
In the developing kidney, the ureteric bud diverticulum grows out of the nephric duct and invades a group of adjacent cells, the metanephric mesenchyme (Dressler, 2006). This outgrowth is stimulated by glial derived neurotrophic factor (GDNF), which is expressed in the mesenchyme and activates the RET receptor tyrosine kinase on the ureteric bud epithelia through a co-receptor GFRα1 (Costantini and Shakya, 2006). Loss of RET, GDNF or the co-receptor GFRα1 results in renal agenesis in mice, due to inhibition of ureteric bud growth and branching (Cacalano et al., 1998; Moore et al., 1996; Pichel et al., 1996; Sanchez et al., 1996; Schuchardt et al., 1994). In cell culture, GDNF is a chemoattractant for RET expressing epithelial cells (Tang et al., 1998) and is sufficient to promote ureteric bud outgrowth (Brophy et al., 2001; Sainio et al., 1997). Similarly, the RET/GDNF pathway is required for migration of enteric neuron precursor cells into the gut, also through a chemotactic mechanism (Natarajan et al., 2002).
Chemotaxis in response to extracellular gradients has been well described in Dictyostelium, in which asymmetry in the distribution of phosphatidylinositol (3,4,5)-triphosphate, (PI (3,4,5)P3), can promote directional migration (Funamoto et al., 2002; Huang et al., 2003; Iijima and Devreotes, 2002). Coincident with the activation of phosphatidylinositol 3-kinase (PI3K) at the leading edges of migrating cells is the localization of the lipid phosphatase, PTEN, at the trailing ends. Pten is a tumor suppressor gene whose loss is detected in a variety of human cancers (Cairns et al., 1998; Li et al., 1997; Steck et al., 1997). PTEN has a dual specificity protein phosphatase and lipid phosphatase activity that can dephosphorylate PI(3,4,5)P3 (Myers et al., 1997, 1998). Thus, PTEN antagonizes PI3K activity to suppress protein kinase B (Akt/PKB) dependent pathways, which can regulate cell migration, proliferation and apoptosis (Stambolic et al., 1998). Akt/PKB contains a pleckstrin homology (PH) domain that mediates binding to PI(3,4,5)P3 to stimulate intracellular signaling pathways (Lemmon and Ferguson, 2000). Furthermore, the protein phosphatase activity of PTEN includes dephosphorylation and inactivation of focal adhesion kinase (FAK), which can modify the interactions between the extracellular matrix and the cytoskeleton (Tamura et al., 1998). PTEN can down-regulate integrin-mediated cell spreading and focal adhesion formation by a phosphatase-dependent manner, suggesting that PTEN functions in controlling cell surface interactions in which integrins, focal adhesion kinase and cell migration are involved (Tamura et al., 1998). Similarly, the lipid phosphatase Ship1, which can also dephosphorylate PI(3,4,5)P3, is essential for neutrophil migration in response to chemotactic gradients (Nishio et al., 2007).
While the RET/GDNF pathway is essential for early kidney development, the intracellular mechanism regulating epithelial cell migration and branching morphogenesis are not entirely clear. Ligand dependent activation of RET results in the autophosphorylation of multiple tyrosine residues of which Y1062 is the most critical for renal development (Wong et al., 2005). Subsequent recruitment of multiple intracellular signaling partners, including Shc, Grb2, p85(PI3K), enigma and Dok-6 (Besset et al., 2000; Crowder et al., 2004; Degl’Innocenti et al., 2004; Durick et al., 1998), has been reported. Previously, we have utilized renal epithelial cells that stably express RET to demonstrate GDNF mediated chemotaxis (Tang et al., 1998). In this system, RET activation increased PI3K activity. Suppression of PI3K by pharmacological inhibitors inhibited GDNF mediated chemotaxis in epithelial cells and ureteric bud outgrowth in embryonic organ cultures (Tang et al., 2002). If the pattern of branching morphogenesis is shaped by PI3K activation in response to RET/GDNF, then the lipid phosphatase PTEN may also be required to down-regulate the signaling responses and fine tune the pattern during development. To address this question, we show that PTEN suppresses GDNF/RET mediated cell migration and chemotaxis in epithelial cell culture. RET activation results in asymmetric distribution of PI(3,4,5)P3 as cells approach a gradient of GDNF. Furthermore, loss of PTEN in the ureteric bud and developing collecting duct system results in aberrant branching patterns, mislocalization of glomeruli and lethality. These data indicate an essential role for PTEN in shaping the pattern of branching morphogenesis in the developing kidney and suggest that PI(3,4,5)P3 is an important signaling mediator for the GDNF/RET pathway.
Materials and methods
Plasmids
PTEN and PTEN-C124S expression plasmids were kindly provided by J. Dixon (UCSD, San Diego, CA). PTEN(C124S) is a mutant form in which serine at 124 is substituted for cysteine and its DNA sequence was confirmed. The fragments of PTEN-IRES-EGFP or PTEN(C124S)-IRES-EGFP from the previous constructs were also subcloned into CMV vector (CB6+) to produce CMV-PTEN-IRES-EGFP and CMV-PTEN(C124S)-IRES-EGFP, respectively, and used for chemotaxis study. Akt/PKB PH domain-GFP fusion (PH-GFP) was kindly provided by T. Meyer (Stanford Univ., Palo Alto, CA).
Stable transfection of MDCK cell line
RET-overexpressing MDCK (Ret9) cells were cotransfected with PGK-Hygro and CMV-PTEN-IRES-EGFP, CMV-PTEN(C124S)-IRES-EGFP or PH-GFP, respectively, and selected for 10 days with 0.3 mg/ml hygromycin in Dulbecco’s modified Eagles medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin under humidified 5% CO2/95% air at 37 °C. At least 3 clones from each construct were obtained and frozen down at −140 °C.
Western blotting
Cells were lysed in PK-lysis buffer (Cai et al., 2002) and protein levels were quantified by the Bio-Rad colorimetric assay (Bio-Rad, Hercules, CA). SDS/PAGE sample buffer was added and samples were boiled for 5 min. Samples were run on a 8% polyacrylamide gel, transferred to PVDF membrane (Perkin-Elmer, Boston, MA) and blocked with 5% non-fat milk in Tris-buffered saline. Membranes were immunoblotted with anti-HA antibody (Covance, Richmond, CA), anti-PTEN antibody (Cell signaling, Beverly, MA) and anti-p-Akt antibody (Cell signaling, Beverly, MA) in 10 mM Tris, pH 7.5, 100 mM NaCl, 0.1% Tween 20, 1% non-fat milk. Secondary antibodies conjugated to horseradish peroxidase were used at a 1:10,000 dilution, and the signal was visualized by chemiluminescence (Amersham, Buckinghamshire, England). For quantitation, fluorescent conjugated secondary antibodies and a Li-Cor Odyssey infrared imager were used to scan the membranes.
Chemoattraction and cell migration assays
For chemoattraction assay, preparation of type I collagen gel and GDNF bead was described by Tang et al. (1998). RET-PTEN, RET-PTEN(C124S) or PH-GFP cells were seeded on the surface of the gel at 40,000 cells/well with DMEM containing 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin, 0.3 mg/ml G418, 0.2 mg/ml hygromycin and supplemented with 250 ng/ml GFRα1. Cells around the beads were photographed over time at 24 h intervals. For cell migration assay, 1×104 cells were plated onto the transwell filters containing 8 μm pores (Corning, Inc., Corning, NY) with the same media previously described. GDNF and GFRα1 were added to the bottom chamber or both the bottom and the top chambers at 100 ng/ml and 250 ng/ml, respectively. After a 24-h incubation, cells on the filters were fixed with 4% paraformaldehyde and stained with DAPI. All experiments were done in triplicate and averages presented with one standard deviation from the mean.
PTEN tissue specific mutants
The pten floxed allele was obtained from T. Mak and genotyped as described (Suzuki et al., 2001). The HoxB7-Cre transgene was obtained from C. Bates and utilized as described previously (Zhao et al., 2004). For organ cultures, E11.5 kidneys were dissected free and placed on transwell filters in serum free media. After 48 h of culture, organ rudiments were fixed in methanol and stained whole with mouse anti-pan-cytokeratin (Sigma) and rabbit anti-laminin (Sigma).
Results
Given the potential for PI3K to promote RET mediated chemotaxis, we asked whether overexpression of the phosphatase PTEN could inhibit GDNF/RET mediated cell migration and chemotaxis in cell culture. If activation of PI3K by RET is essential for directional mobility, then overexpression of the phosphatase PTEN should inhibit directional chemotaxis and/or cell migration in general. PTEN can dephosphorylate PI(3,4,5)P3 and thus disrupt Akt localization at the cell membrane. Wild-type PTEN or a phosphatase deficient form PTEN-C124S were introduced into MDCK cells stably expressing RET (Ret9 cells) and multiple clones identified by Western blotting (data not shown). Cells were assayed for their ability to respond to GDNF/GFRα1 by examining RET and Akt phosphorylation (Fig. 1A). Immunoprecipitation of HA-tagged RET after GDNF treatment revealed that in Ret9 cells and all PTEN derivatives, RET was tyrosine phosphorylated at near equal amounts. Parental MDCK cells do not express RET and show no P-Tyr proteins after immunoprecipitation with anti-HA. To assess the activation of the PI3K pathway, we utilized P-Akt levels as an indirect readout. Antibodies against P-Akt were used to probe total lysates before and after GDNF treatment. Ret9 cells show a strong response as indicated by a doublet recognized by the anti-P-Akt antibodies. Cells expressing PTEN show an attenuated response as the higher migrating P-Akt species is not seen. Ret9 derivatives that express mutant PTEN-C124S also show high P-Akt levels in response to GDNF, with the slower migrating form present. These data show that PTEN expression does not effect RET phosphorylation, but can suppress the level of P-Akt present, whereas the C124S mutant PTEN has little affect on P-Akt levels.
Fig. 1.

Ret activation in PTEN expressing cells. (A) Ret9 cells and derivatives expressing wild-type PTEN or mutant PTEN-C124S all show tyrosine phosphorylation of 170 kDa Ret protein after GDNF/GFRα1 addition. Equal amounts of RET were immunoprecipitated with anti-HA. Total lysates were probed with anti-P-Akt. Note that PTEN expressing cells had attenuated levels of P-Akt as indicated by the absence of the slower migrating form. (B) MDCK, Ret9, RET-PTEN and RET-PTEN (C124S) were grown on plastic in media alone or with GDNF and soluble GFRα1 (GDNF/GFRα1). Dissociation of cell–cell junctions and outgrowth of cellular processes were observed in Ret9 and RET-PTEN(C124S) cells but not in MDCK and RET-PTEN expressing cells.
Extension of cellular processes and dissociation of cells from their neighbors are a prerequisite for the chemotactic response mediated by RET activation. To determine if PTEN suppression can suppress cell scattering and lamellipodia formation, we utilized the Ret9 cells and the PTEN expressing derivatives in several assays. In the absence of RET activation, none of the cells deviated markedly from the parental MDCK phenotype. Cells on plastic grew as tightly associated islands. Addition of GDNF and GFRα1 promoted cell scattering and extension of lamellipodia in Ret9 cells and in PTEN-C124S mutant cell lines, but less so in cells expressing PTEN (Fig. 1B). We also plated these cells on thin collagen gels containing GDNF soaked beads and monitored cell migration towards the beads over time. As expected, PTEN expressing cells were unable to migrate directionally towards a source of GDNF in the bead assay (Fig. 2). The Ret9 and PTEN-C124S mutant cells had no problem migrating towards and eventually completely surrounding the GDNF beads. To quantify chemotaxis more precisely, we utilized a transwell migration assay. Cells were seeded at high density onto membranes with 8-micron pores. GDNF was added to the bottom chamber or to the bottom and the top chamber and the number of cells migrating through the pores was counted (Fig. 3). Ret9 cells expressing PTEN had little chemotactic response to GDNF and were as immobile as parental MDCK cells that do not express RET. As published previously, Ret9 cells migrated through the pores when stimulated with GDNF. More cells were found migrating through the filter when GDNF was added to the bottom chamber only, compared to addition of GDNF to the top and bottom. This is an important distinction that separates random migration from directional movement. Interestingly, Ret9 cells expressing the mutant PTEN-C124S showed a small but significant increase in migration that was evident when GDNF was added only to the bottom chamber, suggesting that this mutant may have some dominant negative effects.
Fig. 2.

PTEN inhibits chemotaxis of Ret9 cells on collagen gels. GDNF beads were placed within thin collagen gels upon which 40,000 cells were seeded and grown for 4 days. Both the Ret9 and PTEN-C124S derivatives were able to scatter and migrate towards the GDNF beads, eventually surrounding the bead itself. Parental MDCK cells and PTEN expressing Ret9 cells were unable to dissociate and migrate.
Fig. 3.

Quantitative transfilter migration assay. Cells were seeded onto the transwell inserts with 8-micron polycarbonate filters. GDNF and GFRα1 were added to the bottom chamber only (B) or to both top and bottom chambers (TB). Controls (C) were cultured in the absence of GDNF/GFRα1. After 24 h, the cells migrating through the filter were counted. The average numbers of cells per field are shown with the standard error bars. The Student’s t test was performed between the indicated samples to determine statistical significance.
In order to directly visualize the polarized activation of PI3K in Ret9 cells, we utilized a GFP reporter to examine the cellular localization of activated PI3K in GDNF dependent cell migration. The pleckstrin homology (PH) domain of AKT binds to PI(3,4)-diphosphate and PI(3,4,5)-triphosphate and when fused to GFP can be used to visualize activated PI3-kinase (Arrieumerlou and Meyer, 2005; Haugh et al., 2000). MDCK renal epithelial cells expressing RET (Ret9) were transfected with GFP-AKT(PH) and examined after addition of GDNF/GFRa1. Activation of RET resulted in GFP localization at the leading edges of lamellipodia and membrane ruffles (Fig. 4A) which was not observed in untreated cells (Fig. 4B). The Ret9-Akt-GFP cells were also plated onto thin collagen gels in which GDNF containing heparin acrylamide beads were embedded. Previous experiments had indicated that Ret9 cells dissociated and migrated towards the source of GDNF (Tang et al., 1998). Using standard fluorescent microscopy, we examined cells as they approached the GDNF bead to gauge whether and where PI3K was active (Figs. 4C–F). As cells extended lamellipodia towards the beads, GFP fluorescence could be detected at the leading edges. In fact, GFP was consistently observed on that side of the cell and on the lamellipodia extending towards the bead, but not in those extending away from the GDNF bead. Occasionally, the end of the lamellipodia was bifurcated in two directions and GFP was seen only on the leading edge facing the bead and not on the edge moving away from the bead (Figs. 4E, F). These data suggest that RET signaling and PI3K activation are responsive to extracellular directional cues and result in polarized PI3-kinase activation only at the leading edges of the cell.
Fig. 4.

Localization of active PI3K by AKT-GFP fusion proteins. Ret9 cells were stably transfected with GFP-AKT(PH). (A) Addition of GDNF/GFRα1 stimulated numerous lamellipodia that show GFP localization at the leading edges (arrows). (B) Untreated Ret9/GFP-AKT cells grow as island with little GFP localization at the edges. (C–F) Ret9/GFP-AKT cells were plated on collagen gels near GDNF coated beads and examined for GFP localization over time. The position of the GDNF bead is marked by an asterisk. Note GFP localization along leading edges (C, arrows) but not on the opposite side of the cell (arrowheads). Also, cells show multiple GFP positive spikes on the side of the cell facing the GDNF bead (D, arrows). GFP localization within a cellular process is asymmetrically localized at the tip (E, E′) strong at the edge facing the bead (arrow) and weak on the opposite edge (arrowhead). A bifurcated extension (F, F′) again shows strong GFP on the end growing towards the bead (arrow) but not on the end moving away (arrowhead).
RET expression is found along the nephric duct and in the ureteric bud as it invades the metanephric mesenchyme. As the ureteric bud branches, RET is restricted to the tips of the buds. The levels of RET at the tips can be enhanced by GDNF, suggesting that RET protein expression is autoregulated (Pepicelli et al., 1997). To test the ability of PTEN to impact early ureteric bud branching morphogenesis, we generated a tissue specific PTEN loss-of function kidney by deleting floxed PTEN alleles (Suzuki et al., 2001) with a HoxB7Cre transgenic driver (Srinivas et al., 1999; Zhao et al., 2004). The HoxB7Cre transgene is active in the ureteric bud epithelial and all its derivatives, enabling us to test the function of PTEN only in the branching epithelial component of the developing kidney. Mice homozygous for the floxed allele (ptenL/L) were crossed to ptenL/+ heterozygous mice that also carried the HoxB7-Cre transgene. Offspring were analyzed at various stages, from E11.5 to weaning.
At E11.5, the ureteric bud has invaded the metanephric mesenchyme and branched into a T-shaped structure. No significant differences were observed in PTEN− kidneys at that time. To examine the pattern of branching morphogenesis, we cultured kidneys in serum free media for 48 h on transwell filters. The branching pattern was examined by staining with anti-laminin and anti-cytokeratins (Fig. 5). PTEN− kidneys showed subtle alterations in branching patterns, particularly at the tips. While PTEN+ ureteric bud tips were generally rounded, symmetrical and covered by a smooth laminin containing basement membrane, PTEN− ureteric bud tips were often dilated, asymmetric and exhibited discontinuous and gnarly basement membranes. In PTEN− kidneys, the ureteric bud stalks showed increased kinks and small ectopic bud outgrowths. Condensation of the mesenchyme was not grossly affected, neither was glomerulogenesis.
Fig. 5.
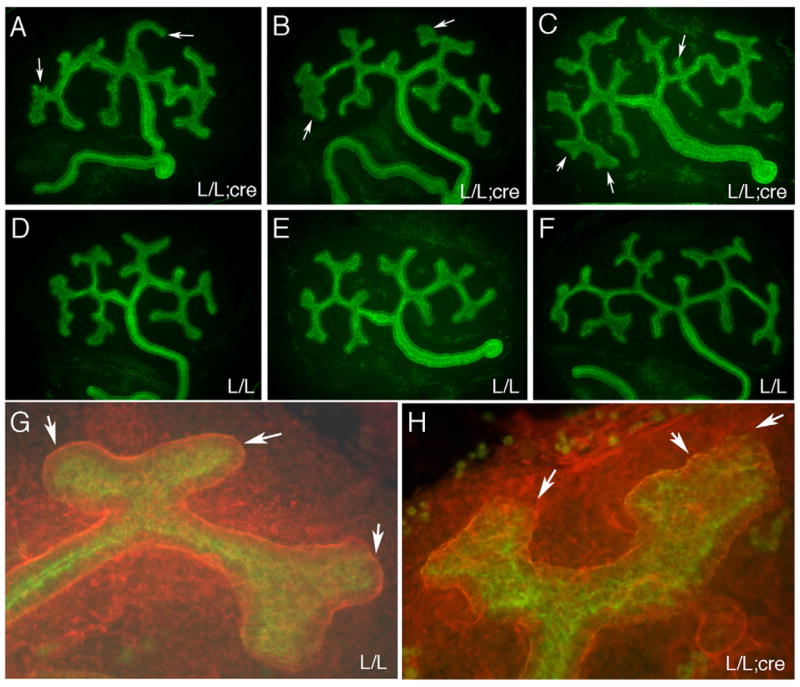
In vitro branching morphogenesis of PTEN:HoxB7Cre kidney rudiments. E11.5 kidneys were cultured for 48 h in serum free media and stained for cytokeratin (green) and laminin (red). (A–C) Three different PTEN− kidney rudiments showing subtle branching defects. Note the asymmetric end buds (A, arrows), the abnormally large misshapen ampullae (B, arrows) and ectopic minor branches (C, arrow). The ureteric bud stalks are excessively kinked. (D–E) Control kidney rudiments lacking the Cre transgene. The PTEN genotype is indicated. (G) A typical wild-type terminal end bud is smooth and covered by a continuous basement membrane, with rounded termini (arrows). (H) A typical PTEN− ureteric bud lacks the smooth appearance of the wild-types. The termini show an enlarged ampullae with rough edges and jagged basement membrane that can be discontinuous (arrows).
At the newborn stage, we examined the pattern of branching morphogenesis kidneys by immunostaining with anti-cytokeratins which are specific for the ureteric bud derived epithelia (Figs. 6A–D). The PTEN− kidneys had terminal end buds that were often dilated and connected to misshaped stalks. PTEN+ end buds did not exhibit these dilations and the stalks were generally straight along the radial axis of the kidney. We also examined older mice at 14 and 20 days post-partum. At 14 days, the density of glomeruli within the outermost cortical layer was significantly increased, from 6.4±1.9 to 10.7±2.5 per unit area (Fig. 7A). Some glomeruli in the PTEN− mice sat directly under the capsule (Fig. 6F), something that is never seen in a wild-type kidney. By 14 days, significant glomerular pathology was evident. Most glomeruli in PTEN− mice were small, with little evidence of capillary blood flow and still unevenly positioned within the cortex (Figs. 6E–H).
Fig. 6.

Patterning defects in PTEN:HoxB7Cre kidneys. (A–D) Newborn kidney sections were stained for ureteric bud derivatives with anti-cytokeratin; genotypes are as indicated. Note the enlarged lumen in PTEN− ureteric bud tips (arrows) and the kinked collecting ducts (arrowhead). (E–H) 14-day-old kidneys were embedded in paraffin, sectioned and stained with Periodic Acid Shiff. PTEN− kidneys show unusual subcapsular glomeruli (arrow, F). Also, note high density of cortical glomeruli in PTEN− kidneys. Boxes in G and H represent areas counted for quantitation as shown in Fig. 7.
Fig. 7.

Quantitative analysis of PTEN:HoxB7Cre kidneys. (A) Peripheral glomeruli were counted in sections from 14-day-old kidneys within a fixed area (0.25 mm2) at a range from 0 to 0.4 mm from the capsule. More than 100 data points were collected from 5 different kidneys for each genotype. Results are presented as the mean with one standard deviation (p<0.01). (B) Survival curve of PTEN+ and PTEN− (n=14) animals. (C) Levels of PTEN protein were measured by Western blot analysis and normalized to either Akt or E-cadherin levels using fluorescent conjugated secondary antibodies and a Li-Cor Odyssey infrared imager. Mean protein ratios from 3 separate kidneys are shown with error bars being one standard deviation from the mean (p<0.02).
None of the ptenL/L;HoxB7-Cre mice (PTEN−) survived more than 26 days postnatally (Fig. 7B). These PTEN− mice were easily phenotyped by 2 weeks due to smaller body size and sickly appearance. To confirm a reduction in PTEN protein expression, quantitative Western blotting from total kidney lysates was done. In the newborns, the levels of PTEN protein in the HoxB7Cre mice were reduced by 30% when compared to total Akt, or 50% when compared to E-cadherin (Fig. 7C). The remaining PTEN protein levels in the PTEN− mice are likely due to the cell types not expressing HoxB7Cre, such as endothelial cells, glomeruli and more proximal tubule cells. To better visualize the loss of PTEN, immunohistochemistry was performed on paraffin sections from 14-day-old littermates (Fig. 8). Diffuse cytoplasmic and nuclear staining is seen in most cells from a PTEN+ kidney, whereas PTEN− sections show epithelial structures with no PTEN staining (Figs. 8A, C). Particularly the epithelial cells surrounding small, subcapsular cyst shows complete lack of PTEN protein. These cysts are DBA lectin positive epithelia, consistent with their derivation from the ureteric buds (Figs. 8B, D).
Fig. 8.

Localization of PTEN in 14-day postnatal kidneys. (A) Cortex of PTEN+ kidney stained with rabbit anti-PTEN and immunoperoxidase conjugated secondary antibodies shows diffuse cytoplasmic and nuclear staining throughout cortex. (B) Collecting tubules (red) of PTEN+ section. Nuclei are stained with DAPI (blue). (C) PTEN− cortex shows fewer PTEN positive cells, particularly within the microcystic epithelia of the collecting tubules (arrows). (D) DBA (red) staining of collecting tubules within the cortex or PTEN− kidneys again shows higher density and cystic morphology.
These data demonstrate that subtle branching defects, which begin early in development, can lead to significant patterning defects within the branching collecting duct system and the nephrons that are induced by these branching tips.
Discussion
In this report, we provide evidence that the PI3K and the PTEN phosphatase drive RET mediated chemotaxis in renal epithelial cells. Several previous reports suggest PI3K as a downstream effector of RET signaling in both renal and neural cell chemotaxis (Natarajan et al., 2002; Srinivasan et al., 2005; Tang et al., 2002). However, these studies relied solely on pharmacological inhibitors whose specificity may be concentration dependent and not complete. That PI3K responds to GDNF in Ret9 cells is illustrated by the asymmetrical localization of Akt-GFP fusion proteins to the leading edges of migrating cells, closest to the source of GDNF. In fact, lamellipodia could be discerned that showed multiple extensions at the tips, whereby only the tips growing towards GDNF showed strong PI3K activation. The data suggest that RET activation responds to positional cues and can interpret a GDNF gradient such that cells move towards the source. Ret mediated chemotaxis can be suppressed by overexpressing the phosphatase PTEN, further supporting an essential role for PI(3,4,5)P3.
Outgrowth of the ureteric bud in the developing kidney can be modeled as a chemotactic driven process at the whole tissue level. In isolated cells and model organisms such as Dictyostelium, the two principle events leading to cell migration are polymerization of filamentous F-actin followed by protrusion of cell surface at the leading edge and assembly of myosin II resulting in contraction at the posterior (Levi et al., 2002). Binding of chemoattractant ligands to receptors at the leading edge activates PI3K and produces local spikes of high PI(3,4,5)P3 concentrations (Arrieumerlou and Meyer, 2005). These local spikes, within the context of an already polarized cell, can lead to small changes in directional turning of lamellipodia and directed migration towards high concentrations of ligand (Arrieumerlou and Meyer, 2005). Binding of the PH domain protein Akt/PKB to PI(3,4,5)P3 results in asymmetric signaling responses that drive the chemotactic process (Meili et al., 1999). In our cell system, Akt-GFP fusion protein localization to lamellipodia closest to the source of GDNF also indicates asymmetric activation of RET and subsequent activation of PI3K. Cells transfected with Akt-GFP showed varying amounts of fluorescence. Many cells had high nuclear and perinuclear fluorescence, yet GFP at the cell surface was generally low. Diffusion of GDNF from beads embedded in thin collagen gels presumably generates a gradient within the gel that can be sensed by cells migrating on top of the gel. These cells showed GFP expression on the cell surface, with enhanced levels on the leading edges of lamellipodia and membrane ruffles closest to the GDNF source. Even within a bifurcated tip of a single lamellipodia, GFP localized to the side closest to the source of GDNF. These data support a role for RET in sensing local concentrations of GDNF as a prerequisite for directional migration.
Concurrent with activation of PI3K at the leading edges close to the chemoattractant source, the opposing sides of a migrating cell can show high PTEN activity, which reduces the levels of PI (3,4,5)P3, further polarizing the cell (Funamoto et al., 2002; Iijima and Devreotes, 2002; Janetopoulos et al., 2004). Reduction of PTEN gene dosage in B cells sensitizes cells to cytokine mediated chemotaxis and Akt activation (Fox et al., 2002), whereas complete PTEN deletion in mouse embryo fibroblasts stimulates cell motility by activation of Rac and cdc42 (Liliental et al., 2000). The deletion of the lipid phosphatase Ship1 also results in chemotactic defects, primarily in the homing of neutrophils to sites of inflammation (Nishio et al., 2007). In our cell migration assays, expression of wild-type PTEN inhibits migration to such an extent that chemotaxis can no longer be examined specifically. However, there is some evidence that the PTEN-C124S mutant can act as a dominant negative (Maehama and Dixon, 1998), which is consistent with our observation that Ret9 cells expressing this mutant form actually show a slight but statistically significant enhancement of chemotaxis.
In the developing kidney, RET is expressed at the tips of the ureteric bud and is essential for branching morphogenesis (Schuchardt et al., 1996). The activity of RET is controlled at multiple levels, by transcription factors that regulate receptor and ligand expression (Brophy et al., 2001; Clarke et al., 2006; Kume et al., 2000), by extracellular signaling factors such as Robo/Slit (Grieshammer et al., 2004) and Bmp4 (Brophy et al., 2001; Miyazaki et al., 2000) and by intracellular inhibitors of tyrosine kinase activity such as Sprouty (Basson et al., 2005). If PI3K activation and localized PI(3,4,5)P3 are important for RET mediated branching morphogenesis, then deletion of PTEN would increase PI(3,4,5)P3 throughout the cell, but would not eliminate the ligand dependent activation of RET nor the restricted expression pattern at the bud tips. Thus, the PTEN− kidneys generated using the HoxB7Cre transgenic driver are fundamentally different than several other mutants or transgenic strains that deregulate RET activity. However, the distorted ureteric bud tips are similar in morphology to those observed with high doses of ectopic GDNF (Pepicelli et al., 1997; Sainio et al., 1997) or expression of a constitutively active form of RET in the ureteric bud epithelium (de Graaff et al., 2001).
One revealing series of experiments by Shakya et al. (2005) expressed GDNF using a HoxB7 transgene, essentially putting the ligand and receptor within the same cell and crossing this transgene into a GDNF null background. Thus, by eliminating mesenchymal specific GDNF and replacing it with ureteric bud expressed GDNF, many of the kidneys were still able to develop with only minor defects. The authors conclude that mesenchymal GDNF is not required as a chemotactic signal. However in those particular transgenic kidneys, the pattern of branching morphogenesis could still be shaped by negative regulators of RET activity that act both outside and within the ureteric bud epithelia. That ectopic ureteric buds in HoxB7-GDNF transgenic embryos generally grew in the same direction may also indicate that the surrounding mesenchymal environment is conducive to invasion only along the dorso-lateral side or that there are negative regulators of RET expressed medio-ventrally. Similarly, in the PTEN− kidneys described here, many additional negative regulators of RET, including Sprouty, Bmp4 and Robo/Slit, are still acting on the epithelial cells. The end result is in fact quite subtle and is probably best observed within the early organ cultures at the bud tips. The smooth, rounded ampullae are replaced by a dilated and rough looking bud tip that in places shows discontinuity of the laminin basement membrane. This translated to a patterning defect in adult kidneys whereby glomeruli are not evenly distributed within the cortex.
In summary, the data presented in this report indicate a role for PI3K in RET mediated chemotaxis of renal epithelial cells. Expression of the tumor suppressor PTEN can block the effects of RET activation by counteracting the activation of PI3K to inhibit cell migration. The mechanism of PTEN mediated suppression of RET has implications for the development of the collecting duct system as tissue specific PTEN knockouts show subtle branching defects that result in mislocalization of glomeruli and lethality. Furthermore, given the role of oncogenic RET in tumor growth and invasion (Melillo et al., 2005), the ability of PTEN to suppress this process also merits investigation.
Acknowledgments
We thank T. Mak for the PTEN floxed mice, C. Bates for the HoxB7Cre transgenic mice, J. Dixon for the PTEN expression plasmids and T. Meyer for the Akt-GFP expression construct. This work was supported in part by NIH grant DK062914 to G. R.D. and a Fellowship from the American Foundation for Urological Disease to D.K.
References
- Arrieumerlou C, Meyer T. A local coupling model and compass parameter for eukaryotic chemotaxis. Dev Cell. 2005;8:215–227. doi: 10.1016/j.devcel.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Basson MA, et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell. 2005;8:229–239. doi: 10.1016/j.devcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Besset V, et al. Signaling complexes and protein–protein interactions involved in the activation of the Ras and phosphatidylinositol 3-kinase pathways by the c-Ret receptor tyrosine kinase. J Biol Chem. 2000;275:39159–39166. doi: 10.1074/jbc.M006908200. [DOI] [PubMed] [Google Scholar]
- Brophy PD, et al. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development. 2001;128:4747–4756. doi: 10.1242/dev.128.23.4747. [DOI] [PubMed] [Google Scholar]
- Cacalano G, et al. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, et al. Phosphorylation of Pax2 by the c-Jun N-terminal kinase and enhanced Pax2-dependent transcription activation. J Biol Chem. 2002;277:1217–1222. doi: 10.1074/jbc.M109663200. [DOI] [PubMed] [Google Scholar]
- Cairns P, et al. Point mutation and homozygous deletion of PTEN/MMAC1 in primary bladder cancers. Oncogene. 1998;16:3215–3218. doi: 10.1038/sj.onc.1201855. [DOI] [PubMed] [Google Scholar]
- Clarke JC, et al. Regulation of c-Ret in the developing kidney is responsive to Pax2 gene dosage. Hum Mol Genet. 2006;15:3420–3428. doi: 10.1093/hmg/ddl418. [DOI] [PubMed] [Google Scholar]
- Costantini F. Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation. 2006;74:402–421. doi: 10.1111/j.1432-0436.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- Costantini F, Shakya R. GDNF/Ret signaling and the development of the kidney. Bioessays. 2006;28:117–127. doi: 10.1002/bies.20357. [DOI] [PubMed] [Google Scholar]
- Crowder RJ, et al. Dok-6, a Novel p62 Dok family member, promotes Ret-mediated neurite outgrowth. J Biol Chem. 2004;279:42072–42081. doi: 10.1074/jbc.M403726200. [DOI] [PubMed] [Google Scholar]
- Degl’Innocenti D, et al. Differential requirement of Tyr1062 multi-docking site by RET isoforms to promote neural cell scattering and epithelial cell branching. Oncogene. 2004;23:7297–7309. doi: 10.1038/sj.onc.1207862. [DOI] [PubMed] [Google Scholar]
- de Graaff E, et al. Differential activities of the RET tyrosine kinase receptor isoforms during mammalian embryogenesis. Genes Dev. 2001;15:2433–2444. doi: 10.1101/gad.205001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- Durick K, et al. Shc and Enigma are both required for mitogenic signaling by Ret/ptc2. Mol Cell Biol. 1998;18:2298–2308. doi: 10.1128/mcb.18.4.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JA, et al. Disruption of a single Pten allele augments the chemotactic response of B lymphocytes to stromal cell-derived factor-1. J Immunol. 2002;169:49–54. doi: 10.4049/jimmunol.169.1.49. [DOI] [PubMed] [Google Scholar]
- Funamoto S, et al. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Grieshammer U, et al. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell. 2004;6:709–717. doi: 10.1016/s1534-5807(04)00108-x. [DOI] [PubMed] [Google Scholar]
- Haugh JM, et al. Spatial sensing in fibroblasts mediated by 3′ phosphoinositides. J Cell Biol. 2000;151:1269–1280. doi: 10.1083/jcb.151.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YE, et al. Receptor-mediated regulation of PI3Ks confines PI (3,4,5)P3 to the leading edge of chemotaxing cells. Mol Biol Cell. 2003;14:1913–1922. doi: 10.1091/mbc.E02-10-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. doi: 10.1016/s0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- Janetopoulos C, et al. Chemoattractant-induced phosphatidylinositol 3,4,5-trisphosphate accumulation is spatially amplified and adapts, independent of the actin cytoskeleton. Proc Natl Acad Sci U S A. 2004;101:8951–8956. doi: 10.1073/pnas.0402152101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume T, et al. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development. 2000;127:1387–1395. doi: 10.1242/dev.127.7.1387. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Ferguson KM. Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem J. 2000;350(Pt 1):1–18. [PMC free article] [PubMed] [Google Scholar]
- Levi S, et al. Myosin II dynamics in Dictyostelium: determinants for filament assembly and translocation to the cell cortex during chemoattractant responses. Cell Motil Cytoskelet. 2002;53:177–188. doi: 10.1002/cm.10068. [DOI] [PubMed] [Google Scholar]
- Li J, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Liliental J, et al. Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr Biol. 2000;10:401–404. doi: 10.1016/s0960-9822(00)00417-6. [DOI] [PubMed] [Google Scholar]
- Lu P, et al. Comparative mechanisms of branching morphogenesis in diverse systems. J Mammary Gland Biol Neoplasia. 2006;11:213–228. doi: 10.1007/sl0911-006-9027-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Meili R, et al. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 1999;18:2092–2105. doi: 10.1093/emboj/18.8.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melillo RM, et al. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest. 2005;115:1068–1081. doi: 10.1172/JCI22758. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Miyazaki Y, et al. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest. 2000;105:863–873. doi: 10.1172/JCI8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MW, et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Myers MP, et al. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci U S A. 1997;94:9052–9057. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MP, et al. The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc Natl Acad Sci U S A. 1998;95:13513–13518. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan D, et al. Requirement of signalling by receptor tyrosine kinase RET for the directed migration of enteric nervous system progenitor cells during mammalian embryogenesis. Development. 2002;129:5151–5160. doi: 10.1242/dev.129.22.5151. [DOI] [PubMed] [Google Scholar]
- Nishio M, et al. Control of cell polarity and motility by the PtdIns(3,4,5) P3 phosphatase SHIP1. Nat Cell Biol. 2007;9:36–44. doi: 10.1038/ncb1515. [DOI] [PubMed] [Google Scholar]
- Pepicelli CV, et al. GDNF induces branching and increased cell proliferation in the ureter of the mouse. Dev Biol. 1997;192:193–198. doi: 10.1006/dbio.1997.8745. [DOI] [PubMed] [Google Scholar]
- Pichel JG, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Sainio K, et al. Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development. 1997;124:4077–4087. doi: 10.1242/dev.124.20.4077. [DOI] [PubMed] [Google Scholar]
- Sanchez MP, et al. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, et al. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Schuchardt A. Renal agenesis and hypodysplasia in ret-k-mutant mice result from defects in ureteric bud development. Development. 1996;122:1919–1929. doi: 10.1242/dev.122.6.1919. [DOI] [PubMed] [Google Scholar]
- Shakya R, et al. The role of GDNF in patterning the excretory system. Dev Biol. 2005;283:70–84. doi: 10.1016/j.ydbio.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Srinivas S, et al. Expression of green fluorescent protein in the ureteric bud of transgenic mice: a new tool for the analysis of ureteric bud morphogenesis. Dev Genet. 1999;24:241–251. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<241::AID-DVG7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, et al. Enteric neuroblasts require the phosphatidylinositol 3-kinase/Akt/Forkhead pathway for GDNF-stimulated survival. Mol Cell Neurosci. 2005;29:107–119. doi: 10.1016/j.mcn.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Stambolic V, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Steck PA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- Suzuki A, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- Tamura M, et al. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- Tang MJ, et al. The RET-glial cell-derived neurotrophic factor (GDNF) pathway stimulates migration and chemoattraction of epithelial cells. J Cell Biol. 1998;142:1337–1345. doi: 10.1083/jcb.142.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MJ, et al. Ureteric bud outgrowth in response to RET activation is mediated by phosphatidylinositol 3-kinase. Dev Biol. 2002;243:128–136. doi: 10.1006/dbio.2001.0557. [DOI] [PubMed] [Google Scholar]
- Wong A, et al. Phosphotyrosine 1062 is critical for the in vivo activity of the Ret9 receptor tyrosine kinase isoform. Mol Cell Biol. 2005;25:9661–9673. doi: 10.1128/MCB.25.21.9661-9673.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, et al. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol. 2004;276:403–415. doi: 10.1016/j.ydbio.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


