Abstract
Pax transactivation-domain interacting protein (PTIP) is a widely expressed nuclear protein that is essential for early embryonic development. PTIP was first identified on the basis of its interactions with the developmental regulator Pax2 but can also bind to other nuclear transcription factors. The Pax2 protein is essential for development of the renal epithelia and for regulating the response of mature collecting ducts to hyperosmotic stress. For determination of whether PTIP also functions in more differentiated cell types, the Cre-LoxP system was used to delete the ptip gene in the renal collecting ducts using Ksp-Cre driver mice. Collecting duct–specific ptip knockout mice were viable with little discernible phenotype under normal physiologic conditions. However, collecting duct–specific ptip mutants were unable to concentrate urine after the treatment of desamino-cis, D-arginine vasopressin, an antidiuretic hormone. Furthermore, aquaporin-2 (AQP2) expression in the inner medulla of the ptip knockout mice was decreased approximately 10-fold compared with that of wild-type littermates. Expression level of tonicity responsive enhancer binding protein, a transcription factor of AQP2, is not altered in the mutant mice, but its nuclear localization in the inner medulla is unresponsive after treatment with vasopressin agonists. This was due, at least in part, to decreased expression of the arginine vasopressin receptor 2 in ptip mutants. Furthermore, ptip null inner medullary collecting duct cells were sensitive to hyperosmolality in vitro. Thus, ptip is required for the urine concentration mechanism by modulating arginine vasopressin receptor 2 and AQP2 expression in the inner medulla. The data suggest an essential role for ptip in regulating urine concentration and in controlling survival of collecting duct epithelial cells in high osmolality.
The ptip gene encodes a 1056–amino acid nuclear protein that is expressed in most cell types examined (1). Pax transactivation-domain interacting protein (PTIP) was first identified in a two-hybrid screen using the activation domain of the Pax2 transcription factor as a bait (1). Full-length PTIP encompasses two amino-terminal BRCT domains, a glutamine (Q)-rich region, and four carboxyl-terminal BRCT domains. The BRCT domain proteins have been implicated in a variety of nuclear processes, including DNA repair, cell-cycle checkpoints in response to DNA damage, and transcription activation (2). PTIP is expressed in all cell types and can interact with the activation domains of other Pax proteins and potentially other transcription factors. A ptip null allele is homozygous lethal in development around the time of gastrulation. The ptip null mouse embryos are disorganized by embryonic day 7.5 and show increased free DNA ends and decreased cell proliferation (3). Thus, ptip is essential for the proper progression of the embryo from the epiblasts to the gastrula. However, the potential role of ptip in more terminally differentiated cells has not been investigated.
Pax2 is a transcription factor that is essential for the development of the kidney, eye, ear, and hindbrain. In the developing kidney, Pax2 is expressed in the mesonephric duct, the ureteric bud, and the condensing metanephric mesenchyme and is downregulated as the tubular epithelium forms (4,5). However, a recent study showed three findings on the osmoregulatory activity of Pax2 (5). First, the low level of Pax2 expression persists into adulthood in the inner medullary collecting ducts (IMCD). Second, this adult Pax2 expression is further increased when cells of the IMCD are placed in a high-osmolality environment, suggesting that Pax2 may control the expression of osmoregulated genes in vivo. Third, administration of [desamino-cis, D-arginine]-vasopressin (dDAVP) in rats stimulates urine concentration, increases the IMCD osmolality, and increases medullary Pax2 protein expression. The increase in Pax2 expression seems protective because Pax2 is required for cells to survive in high-salt conditions in vitro (5).
In this study, we asked whether deletion of ptip in the more differentiated cells of the IMCD affected the physiologic function of these cells, particularly with respect to the role of Pax2 given that PTIP is a potential adaptor protein of Pax2. A conditional ptip allele was generated by flanking exon 1 with Cre recombinase recognition (LoxP) sites. We used the kidney-specific Cadherin-16 promoter (Ksp-Cre) to express Cre and delete ptip in the renal inner medulla, where Pax2 is expressed and required for osmotic tolerance. Mice that lacked PTIP in the IMCD were viable under normal physiologic conditions. However, after administration of dDAVP, mice that lacked PTIP in the IMCD were unable to concentrate urine, most likely as a result of failure to express aquaporin-2 (AQP2) at the apical plasma membrane. Primary IMCD cells that lacked PTIP were also hypersensitive to high osmolality. The data suggest a crucial role for PTIP in regulating the physiology of the IMCD with respect to urine concentration and Pax2 function.
Materials and Methods
Targeting Construct
The targeting vector, pFloxP-Flp-Neo reverse, was provided by the Transgenic Core, University of Michigan. This plasmid contains two loxP sites and a phosphoglycerol kinase-neomycin resistance gene cassette (PGK-neoR) flanked by FRT sites. pFloxP-PTIP–targeting construct was created (Figure 1A) by insertion of a 5′ NheI-BamH1 PTIP genomic fragment (2.8 kb) upstream of PGK-neoR gene. A BamH1-NruI ptip genomic fragment (1.2 kb) that contained 5′ untranslated region and exon 1 was then inserted between the two loxP sites. Finally, an NruI-XmnI ptip genomic fragment from intron 1 (4.8 kb) was inserted downstream of the second loxP site.
Figure 1.
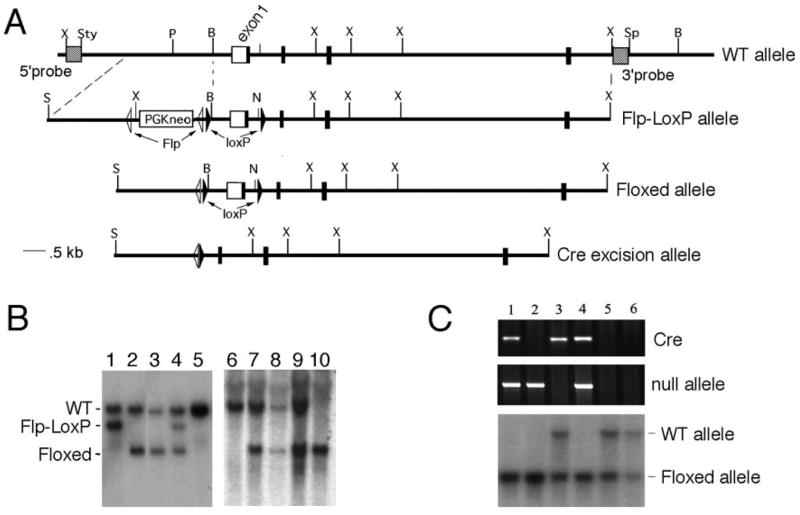
Generation of a conditional ptip null allele. (A) Schematic of the targeting vector and resulting alleles that were generated by homologous recombination into the mouse germ line. (B) Genotyping of weanlings using 5′ probes outside the targeting vector to indicate excision of phosphoglycerol kinase-neomycin resistance gene cassette (PGK-neo) by crossing to Flp recombinase–expressing strains (lanes 2 and 3). Note homozygous Floxed allele (10) in an seemingly normal weanling. (C) PCR genotyping of tail DNA with Cre primers, null allele primers, and Southern blot using Pax transactivation-domain interacting protein (PTIP) internal probe (bottom). Lane 1, −/fl;Ksp-Cre; lane 2, −/fl; lane 3, +/fl;Ksp-Cre; lane 4, −/fl;Ksp-Cre; lanes 5 and 6, +/fl.
Gene Targeting, Blastocyst Injections, and Animal Breeding
A total of 60 μg of the targeting vector was linearized with SspI and then electroporated into 2.4 × 107 embryonic stem R1 cells that were grown in DMEM supplemented with 15% FCS, 0.1 mM β-mercapto-ethanol, 4 mM glutamine, and 103 U/ml recombinant leukemia inhibitory factor (rLIF) (Chemicon, Temecula, CA). Cells were plated onto NeoR mouse embryonic fibroblast feeders and selected with the same medium that contained 0.3 mg/ml G418. The G418-resistant clones were identified by Southern blot, and the chromosome analysis was performed to ensure the euploidy. Three targeted embryonic stem cell clones were microinjected into donor blastocysts from C57BL/6 × (C57BL/6 × DBA/2)F1, and the blastocysts were transferred into pseudopregnant female recipients. Male chimeras were mated with C57BL/6 females, and germline transmission was identified by Southern blot of tail DNA. Mice that possessed the entire targeting vector (PTIPFlp·fl) were mated with Flippase transgenic mice to excise PGK-neo cassette from the targeting vector, resulting in mice that carried a ptip allele flanked by LoxP (ptipfl). Mice with ptipfl gene were mated with either Ksp-Cre mice (6) or with mice that carried a ptip+/− null allele so that eventually ptipfl/− that carried Ksp-Cre mice (PTIPfl/−; Ksp-Cre) were produced and used as ptip conditional mutant mice (PTIP−), and other littermates, such as ptip+/fl, ptip−/fl and ptip+/fl; Ksp-Cre were used as controls (PTIP+) for this study.
Genotyping
Tail DNA was prepared by a standard method and amplified with PCR for the Ksp-Cre transgene using Cre primers 5′-TGATGAGGTTCGCAAGAACC-3′ and 5′-CCATGAGTGAACGAACCTGG-3′. Detection of null allele was done with neo forward and PTIP reverse primers 5′-GCTTCCTCGTGCTTTACGGTATC-3′ and 5′-ATTTCCCTCCCCAACCAAGGTC-3′, respectively.
Primary Inner Medullary Epithelial Cell Culture
Two- to 3-mo-old mice were killed, and their kidneys were quickly removed. The inner medulla was dissected, minced, and transferred to DMEM-Ham’s F-12 medium that contained 10% FBS and collagenase. Then, the inner medulla was digested for 20 min at 37°C under continuous agitation in a humidified incubator (5% CO2, 95% O2). After centrifugation, the cellular pellet was washed three times in the medium, and then the cells were resuspended in the medium and cultured for 1 wk until they were confluent. The cells were split at 1:3 and incubated for approximately 3 to 4 d until they reached confluence. These first-passage cells were split again and seeded at 4 × 105/ml onto six-well plates, then used after they were confluent.
Animal Experiments
PTIP+ and PTIP− mice (n = 5 for each group) were placed in cages and given food and water ad libitum 1 d before experiment. Saline or a single dose of 1 μg/kg AVP V2 receptor agonist desmopressin (dDAVP; Sigma, St. Louis, MO) in saline was given subcutaneously to the mice. Five hours after injection, urine was collected by spontaneous voiding, and its osmolality was measured using Westcor 5100C Pressure Vapor Osmometer (Westcor, Logan, UT). Mice were killed, and their kidneys were removed for protein, RNA, and immunohistochemistry.
Protein Determination
Inner medullas were isolated and homogenized in isolation buffer (10 mM triethanolamine and 250 mM sucrose [pH 7.6]) with protease inhibitor cocktail (Roche, Indianapolis, IN). Cells that were cultured in vitro were lysed in PK lysis buffer (7). SDS-PAGE, transfer of membrane, and visualization by chemiluminescence were previously described (7). Antibodies that were used in this study were anti-PTIP, anti-cleaved caspase-3 (Cell Signaling, Danvers, MA), anti-AQP2 (Alomone Labs, Jerusalem, Israel), anti-nuclear factor of activated T cells 5 (anti-NFAT5; AbCam, Cambridge, MA) anti–arginine vasopres-sin receptor 2 (anti-AVPR2; AbCam, ab13149), and anti–β-tubulin (Sigma).
Immunohistochemistry
Kidneys were fixed in 4% paraformaldehyde-PBS at 4°C, dehydrated in graded ethanol, and embedded in paraffin. Sections were cut at 5 μm. After dewaxing and rehydration, sections were boiled for 12 min in a pressure cooker with a 1:100 dilution of antigen unmasking solution (Vector Laboratories, Burlingame, CA). Sections were washed in PBS and incubated with antibodies against PTIP, AQP2, NFAT5, and AVPR2 in a buffer that consisted of PBS, 0.1% Tween 20, and 2% goat serum. After incubation for 1 h at room temperature, sections were washed two times for 10 min, then incubated with FITC-conjugated secondary antibody for 1 h. Sections were washed two times and covered with Gelvatol that contained 0.2% 1,4-diazabicyclo(2,2,2)octane. Controls included second antibodies only to ensure specificity. Images were captured on a Nikon ES800 fluorescence scope with a digital camera. A Histostain-Plus kit (Zymed, Carlsbad, CA) was used for an additional AQP2 immunolabeling according to the manufacturer’s directions.
Southern Blot Analysis
PTIP gene targeting and its deletion in kidneys were confirmed with Southern hybridization in which 5 μg of genomic DNA was digested with HpaI, electrophoresed, transferred to a Genescreen Plus hybridization transfer membrane (Perkin Elmer, Waltham, MA), and hybridized with a 1.2-kb genomic fragment indicated in Figure 1A.
Real-Time Reverse Transcription–PCR
Total RNA extraction and reverse transcription–PCR analysis were performed as described previously (8). For real-time PCR, primer pairs were as follows: AQP1, GGGTAGGCACCTTATCTCCA and CAGGGACAATTCCAAGGTCT; AQP2, GGTCTGTGGTTAGTGCATCTATTTTAT and TCTCTGCACGTGAGGAAAGAAA; AQP3, GCCCTCCAGAATTTCTATGAACTCT and TTTGCTATCCTACCTTGGCTTAAAG; AVPR2, CACCCTTCTTCCTTGTGCAG and CTACGCAACTCCGAGGAGAC; Pax2, GGCATCTGCGATAATGACACA and GGTGGAAAGGCTGCTGAACTT; PTIP, CCGAAGTTCCAGAGGAGCTA and GTCCCCATCCTCGAAATGA; glyceralde-hyde-3-phosphate dehydrogenase, TCCGCCCCTTCTGCCGATG and CACGGAAGGCCATGCCAGTGA.
Results
Generation of PTIP Conditional Knockout Mouse Model
To address the functions of ptip in more terminally differentiated kidney cells, we designed a conditional targeting construct using the Flp-FRT and Cre-LoxP systems (Figure 1A). Flp recombinase sites flank the PGK-neo cassette, and the 5′ promoter region of ptip was inserted into the 5′ flank of the cassette. The LoxP sites flank exon 1 and will excise the translation start site and much of the potential promoter region once exposed to Cre recombinase. After excision of the PGK-neo cassette by crossing to Flp-expressing mice, the floxed allele has a single Flp and loxP site in the 5′ promoter region and a second loxP site in intron 1 (Figure 1B). Upon exposure to Cre recombinase, the excised allele removes approximately 1.2 kb of sequence that contains the 5′ untranslated region and transcription start site and the translation start site. Mice that carried one floxed allele were intercrossed to generate homozygous ptipfl/fl mice that are viable and fertile. Collecting duct–specific ptip knockout mice (PTIP−) were produced after crossing ptipfl/fl with ptip+/−;Ksp-Cre (6,9). PCR with both Cre and null allele primers and Southern blot were performed to identify genotypes of mice (Figure 1C). Embryos that carried Ksp-Cre, the null allele, and the floxed allele should be tissue-specific nulls (PTIP−). Littermates without Cre were either ptipfl/− or ptipfl/+and expressed PTIP protein (PTIP+). Table 1 represents body weight, kidney weight, and serum electrolyte profile in PTIP+ and PTIP− mice. Gross body weight, kidney weight, and serum electrolytes tested did not show any significant difference in the two groups. Therefore, PTIP− mice exhibited apparently normal phenotype.
Table 1.
Kidney weights and serum electrolyte profile of 3-mo-old mice
| Genotype | Body Weight (g)a | Kidney Weight (g)a | Na (mmol/L) | K (mmol/L) | Cl (mmol/L) |
|---|---|---|---|---|---|
| PTIP+ | 25.9 ± 0.63 | 0.298 ± 0.013 | 160.7 ± 3.7 | 6.6 ± 0.61 | 119.7 ± 2.0 |
| PTIP− | 25.8 ± 0.058 | 0.292 ± 0.012 | 161.0 ± 2.1 | 6.5 ± 0.29 | 117.7 ± 2.33 |
Means ± 1 SD; n = 5 for weights; n = 3 for electrolytes.
Ability of Urine Concentration in PTIP− Mice
PTIP− mice exhibited no gross abnormalities over time. Given the potential role for Pax2 in regulating the response to hyperosmolality (7), we examined changes in the gene expression in the inner medulla of PTIP− mice under hyperosmolar condition after the administration of dDAVP, a vasopressin agonist. Two- to 3-mo-old mice were administered a subcutaneous injection of either saline or dDAVP. Five hours after injection, urine was collected and measured for its osmolality (Figure 2A). In PTIP+ mice, osmolality of urine before treatment was 1891.2 ± 608 mosmol/kg, and that of urine after dDAVP treatment was 3484.8 ± 547 mosmol/kg, an approximately two-fold significant increase after treatment (P < 0.005). However, in PTIP− mice, the osmolality of urine after dDAVP treatment was 1913.4 ± 624 mosmol/kg, statistically insignificant with the osmolality of urine before treatment (1725.6 ± 650 mosmol/kg; P > 0.5). These results suggest that PTIP is required for activation of the urine-concentrating mechanism in response to vasopressin receptor activation.
Figure 2.
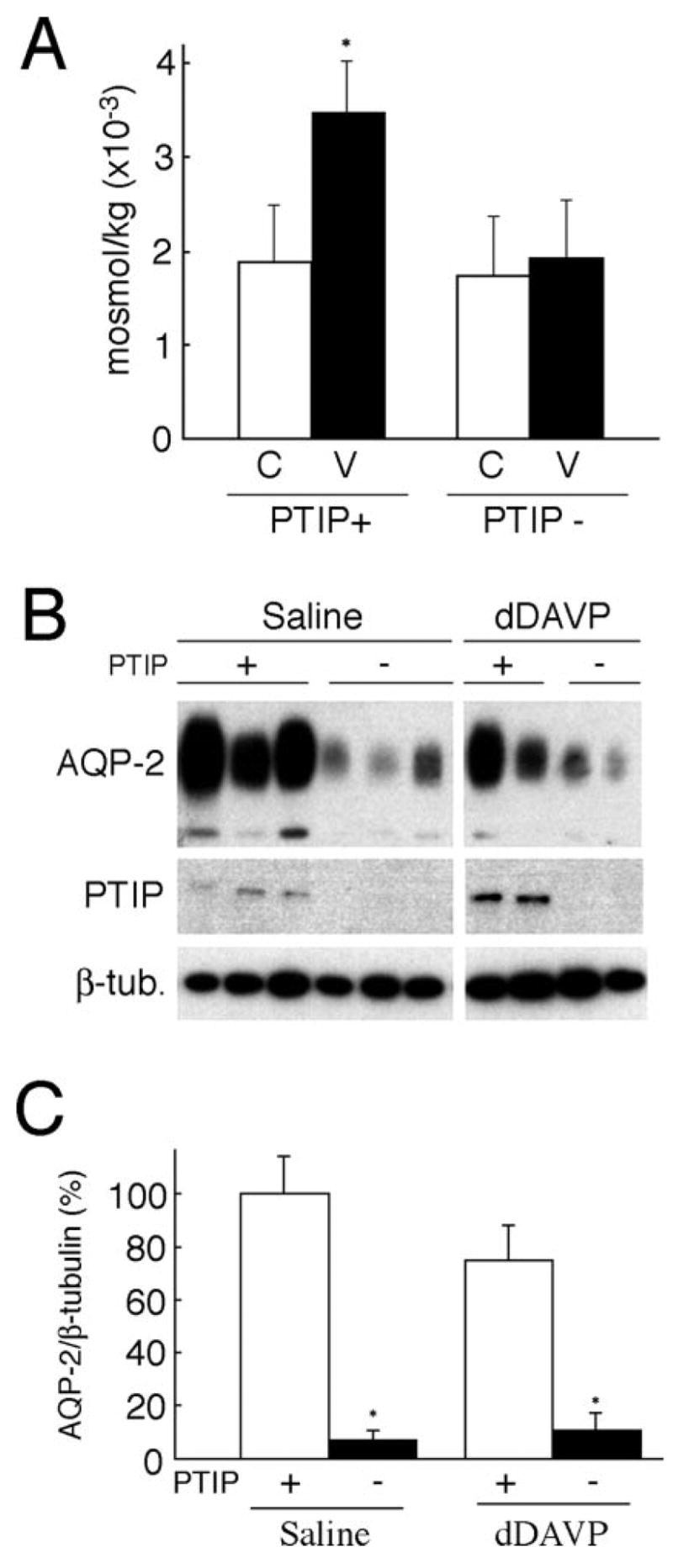
Analysis of PTIP kidney–specific knockout mice. (A) Osmolality in urine from mice 5 h after desamino-cis, D-arginine (dDAVP) injection. C, background urine; V, urine from dDAVP-injected mice. *P < 0.005 versus background urine of PTIP+. (B) Western blotting for aquaporin-2 (AQP2), PTIP, and β-tubulin expression in control mice. Each lane represents the protein expression in the inner medulla from the individual mouse. (C) Densitometric analysis of AQP2 normalized to β-tubulin. Data are percentage of means ± SD from five mice each group. *P < 0.001.
Expression of AQP2 in PTIP- Mice
AQP2 is one of the key water channels for the water reabsorption in the kidney. To determine whether the loss of the urine-concentrating ability in the PTIP− mice was due to the abnormal expression of AQP2, we assessed its protein expression in the inner medulla by Western blot (Figure 2, B and C). AQP2 protein expression was reduced in PTIP− compared with PTIP+ mice. When AQP2 protein expression was normalized to β-tubulin, PTIP− mice expressed only 7.4 ± 2.8% of the amount found in PTIP+ mice (P < 0.001), where PTIP+ AQP2 expression was set at 100%. These data demonstrate that AQP2 protein expression is significantly reduced in PTIP− mice compared with PTIP+ mice, suggesting that PTIP may be required for the expression of AQP2 in renal medullary collecting duct cells. A Western blot was performed with cortex samples as well. However, no AQP2 signal was detected at the exposure when its signal was very strong in the inner medulla (data not shown).
Immunohistochemical Detection of AQP2
In the collecting ducts, water reabsorption is regulated by the shuttling of AQP2 to the apical plasma membrane after stimulation of the AVPR2 by vasopressin. To investigate the localization of AQP2 in the inner medulla, we performed immunohistochemistry in PTIP+ and PTIP− mice (Figure 3). With the use of peroxidase-conjugated secondary antibodies and diaminobenzidine staining, the intensity of AQP2 staining was significantly less in the renal papillae of PTIP− mice (Figure 3F) than that of PTIP+ mice (Figure 3C). To localize better AQP2 protein in the cells, we also used a fluorescence-conjugated secondary antibody (Figure 3, G through J). In PTIP+ mice, AQP2 signal was distributed throughout the cytosol in the control, and its expression had a clear apical location after dDAVP injection (Figure 3, G and I). In PTIP− mice, AQP2 signal was also found in the cytosol in the control, but after dDAVP injection, lower AQP2 was observed on the apical membranes (Figure 3, H and J). To confirm that these mice had reduced PTIP expression in the inner medulla, we probed similar sections with anti-PTIP (Figure 3, K and L) to show a reduction of PTIP in the nuclear staining.
Figure 3.
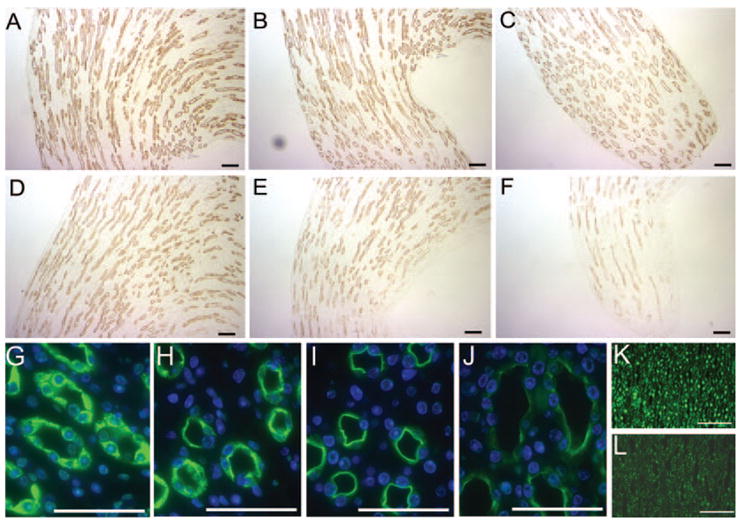
Immunohistochemistry for AQP2 in the inner medulla and renal papilla. A through F are immunoperoxidase labeled (brown); G through L are immunofluorescence staining (green). (A through C) Control PTIP+ outer and inner medullary zone. (D through F) Control PTIP− outer and inner medullary zone. (G) Control PTIP+ renal papilla; AQP2 is green, and nuclei are blue. (H) Control PTIP− papilla; AQP2 is green, and nuclei are blue. An area with strong AQP2 signal was chosen for the comparison with control PTIP+. (I) PTIP+ papilla after dDAVP injection. Note shuttling of AQP2 (green) to apical plasma membrane. (J) PTIP− papilla after dDAVP injection. Note significant decrease in AQP2 signals (green). (K and L) Immunostaining for PTIP in a PTIP+ (K) and PTIP− (L) papilla, respectively. Bars = 100 μm. All exposure times were set manually and were equivalent for PTIP+ and PTIP− micrographs.
Expression of AVPR2
The inability of PTIP− mice to absorb water in response to vasopressin agonists could be due to alterations in the receptor. To test this possibility, we examined AVPR2 localization in the inner medulla in PTIP+ and PTIP− kidneys after saline or dDAVP treatment (Figure 4). By immunostaining, receptor levels were significantly lower in PTIP− kidneys with or without dDAVP treatment. In PTIP+ mice, strong immunostaining was observed in individual cells along the inner medullary collecting ducts. This staining became even stronger after dDAVP treatment. Therefore, it is likely that the inability to respond to vasopressin in PTIP− kidneys is due to low levels of AVPR2. In response to AVPR2 activation in our wild-type mice, the transcription factor tonicity responsive enhancer binding protein (TonEBP) shuttles from the cytoplasm to the nucleus, consistent with published reports (10,11). In PTIP− collecting duct cells, we did not see significantly more nuclear TonEBP after dDAVP treatment when compared with saline controls (data not shown). These observations are also consistent with the reduced AVPR2 levels in the PTIP− mice.
Figure 4.
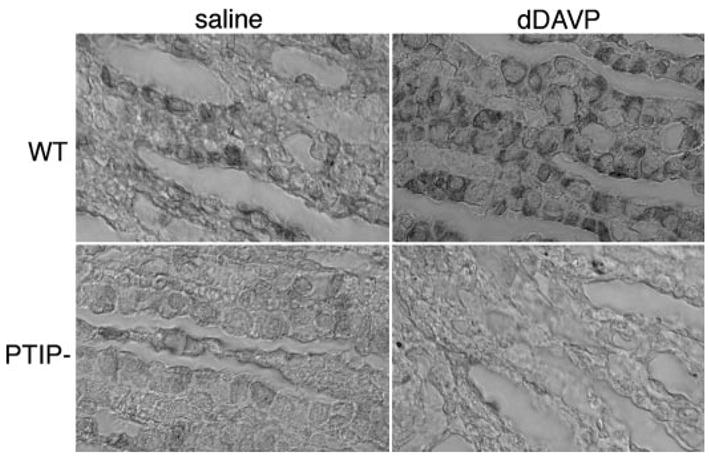
Immunohistochemical analysis of arginine vasopressin receptor 2 (AVPR2). Sections through the inner medulla were stained with an antibody against AVPR2. Wild-type (WT; PTIP+) and PTIP− mice were administered an injection of saline or dDAVP as indicated. Note that AVPR2 is easily detected in WT and increases in dDAVP-treated kidneys. PTIP− kidneys show low levels of AVPR2 that do not increase with dDAVP treatment.
mRNA Expression in the Inner Medulla
Real-time reverse transcription–PCR was performed using total RNA that was isolated from the inner medulla to find any changes in the gene expression (Figure 5). AQP2 mRNA expression in the PTIP− control mice was reduced to 75% compared with that in the PTIP+ control mice (P < 0.01). AQP3 and Pax2 expression level was unchanged both in the control and in the dDAVP injection. AQP1 expression in PTIP+ was slightly increased after dDAVP injection. However, AQP1 expression in PTIP− is slightly decreased. PTIP− kidneys also showed reduced AVPR2 mRNA levels, particularly after dDAVP injection.
Figure 5.
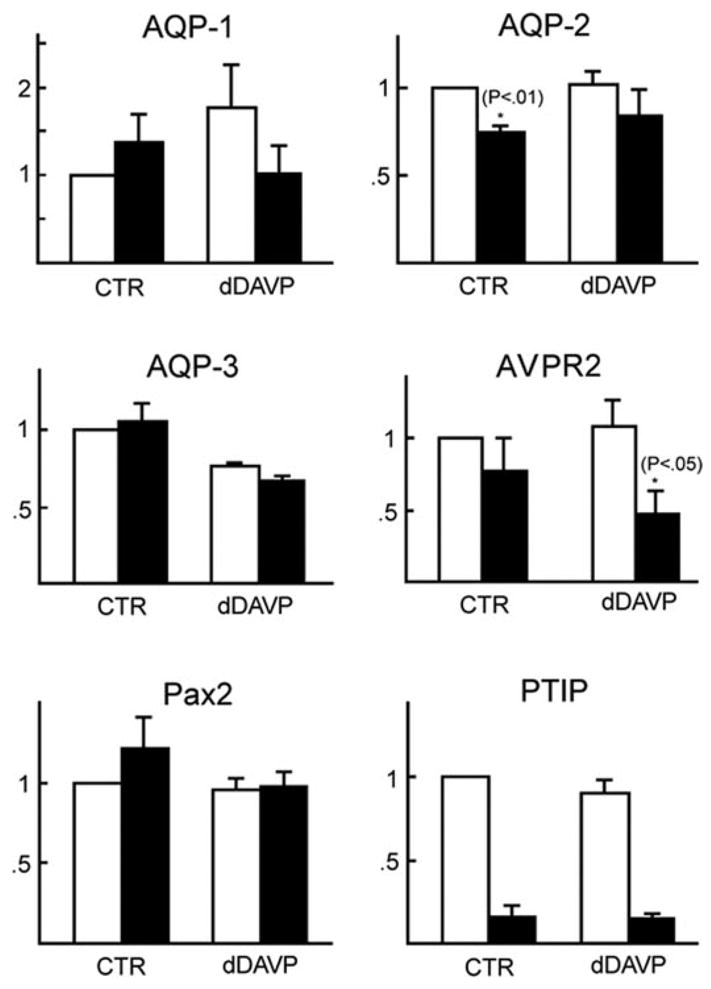
Quantitative reverse transcription–PCR analysis of mRNA expression in the inner medulla isolated from mice. □, PTIP+; ■, PTIP−.
Primary IME Cells and Sensitivity to Hyperosmolality
It was reported that confluent passage 2 primary IME (p2mIME) cells are tolerant to high NaCl levels (12). In p2mIME cells, only 10% apoptosis is observed in 900 mosmol/kg, whereas 60% apoptosis is seen in an mIMCD3 cell line at the same salt concentration. This resistance to high osmolality was Pax2 dependent. To determine whether PTIP expression is necessary for osmotolerance, we made primary cells from the inner medullary region of PTIP+ and PTIP− kidneys. NaCl was added to the confluent cells to 600 and 900 mosmol/kg for 8 h. At normal physiologic salt concentrations (300 mosmol/kg), both PTIP+ and PTIP− cells appeared similar (Figure 6A). However, at 600 mosmol/kg, PTIP− cell monolayer began to detached from the culture dish. At 900 mosmol/kg, the PTIP− cell monolayer showed increasing evidence of detachment and cell death, whereas PTIP+ cells became smaller and more rounded but remained attached to the dish. To confirm that the primary cell lines were indeed PTIP−, isolated DNA was subjected to Southern blotting (Figure 6B). DNA from p2mIME cells of PTIP− mice shows almost complete knockout of the floxed PTIP allele, whereas that of PTIP+ mice exhibits the floxed allele, which is similar to wild type. By Western blotting, the PTIP− p2mIME cells show no detectable levels of the 130-kD PTIP protein (Figure 6C). Cell lysates were also examined for activated caspase 3, a marker of apoptosis (Figure 6D). Very low levels of activated caspase-3 were found in PTIP− cells at 600 mosmol/kg, whereas no signal was found in PTIP+ cells at the same osmolality. At 900 mosmol/kg, a very high level of cleaved caspase-3 expression was seen in PTIP− cells, compared with PTIP+ cells. Therefore, the IME cells from PTIP− kidneys are more susceptible to hyperosmolality in cell culture. To rule out nonspecific sensitivity to toxic agents, we examined the resistance of IME cells to cadmium (Cd; Figure 6E). Various concentrations (0 to 500 μM) of Cd were added into IME culture media, and cell numbers were counted after 24 h. No significant difference in the two groups was found in each concentration of Cd, suggesting that IME cells from PTIP− mice are not more susceptible to Cd toxicity.
Figure 6.
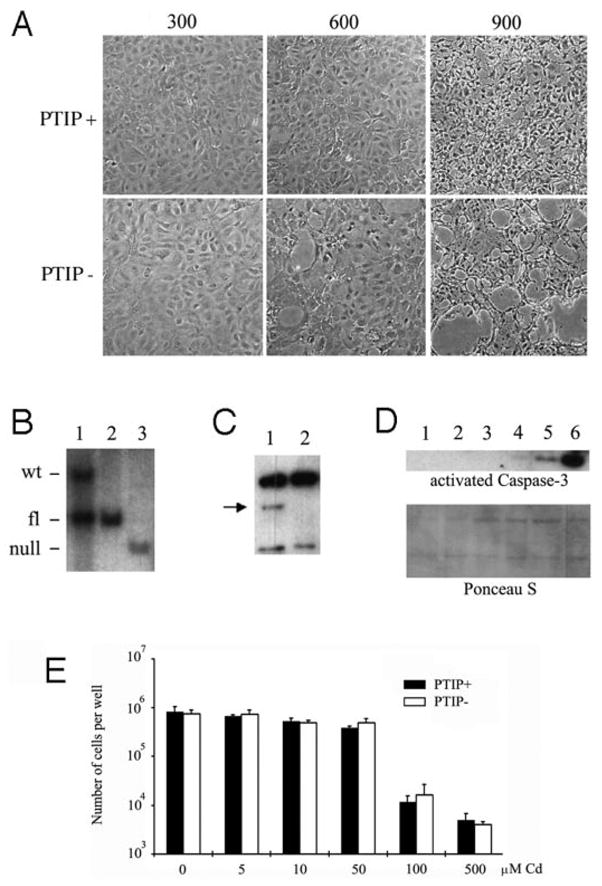
Primary inner medullary epithelial (IME) cell culture from PTIP+ and PTIP− mice. (A) Cell morphology in response to increased osmolality. Three concentrations of NaCl were added to the medium and cultured for 8 h. (B) Southern blotting of IME cells in the various mouse genotypes. Lane 1, PTIP+/fl; lane 2, PTIPfl/fl; lane 3, PTIP−/fl:Ksp-Cre. Note that PTIP−/fl; Ksp-Cre has a single band at the deleted position. (C) Detection of the 150-kD PTIP by Western blotting. Lane 1, PTIP+; lane 2, PTIP−. (D) Activated, cleaved caspase-3 expression as detected by Western blotting lysates from PTIP+ cells (lanes 1, 3, and 5) and PTIP− cells (lanes 2, 4, and 6) cultured in 300 (lanes 1 and 2), 600 (lanes 3 and 4), and 900 (lanes 5 and 6) mosmol/kg. Equal amounts of protein were loaded as indicated by Ponceau-S staining of membranes. (E) Cell numbers per well after 24 h of incubation with increasing concentrations of cadmium as indicated.
Discussion
In this study, we generated tissue-specific ptip knockout mice using the Cre-LoxP system to understand the function of ptip in the differentiated cells of the inner renal medulla. Whereas ptip is essential for early patterning and growth at the time of gastrulation, its function in more differentiated cells remained obscure. The PTIP was identified on the basis of its interaction with the transcription factor Pax2 but may interact equally well with other nuclear factors, such as Smad2 (13). Whereas Pax2 is highly expressed in early kidney progenitor cells and is essential for early renal development (14), the expression of Pax2 in differentiated collecting duct epithelia is necessary for osmotolerance and potentially for regulating water reabsorption (7). Indeed, we have found that ptip loss of function in collecting ducts leads to defects in the urine-concentrating mechanisms and severely limits the ability of epithelial cells to survive in high-salt conditions in vitro.
Under normal physiologic conditions, the loss of ptip in collecting ducts had little discernible effect, because no detectable pathology was evident on histologic sections and the mice survived well into adulthood. To find a tissue-specific function of ptip in vivo, we administered an AVPR2-specific agonist, dDAVP, to induce hyperosmolality in the inner medulla, where ptip is deleted by Ksp-Cre recombinase. Surprising, urine from the PTIP− mice was not concentrated properly after dDAVP treatment. This was most likely due to a decrease in AVPR2, which abrogated the response to vasopressin. This resulted in low levels of AQP2 expression and poor localization to the apical plasma membrane. AQP2 is a pore-forming water channel that is expressed in collecting duct principal cells in the kidney (15). Under basal conditions, AQP2 resides in intracellular vesicles in the cells. However, if AVP binds its receptor, AVPR2, on the basolateral membrane of the collecting duct cells, then intracellular cAMP increases and binds to the regulatory subunit of protein kinase A so that free catalytic subunit of protein kinase A incorporates into the vesicles and phosphorylates AQP2 at serine 256 of the C-terminal tail, resulting in the localization to the plasma membrane (16,17). The failure to express AQP2 at the apical cell membrane in PTIP− collecting ducts may be due to more protein turnover than any transcriptional regulation, because mRNA levels were not grossly affected.
Because PTIP was known as an adaptor protein of Pax2, a transcription factor, it is plausible that PTIP cooperates with other transcription factors. An essential transcription factor of AQP2 expression in renal collecting duct principal cells is TonEBP (11). TonEBP is thought to induce the expression of genes that leads to the accumulation of organic osmolytes to protect cells against a hypertonic stress. Our histologic analysis shows that some TonEBP still remains in the cytosol after induction of hyperosmolality by dDAVP treatment, like the case of saline. However, this is likely to be due to low levels of AVPR and not an inherent problem with TonEBP.
The LoxP insertions in a part of the 5′ untranslated region and intron 1 did not affect ptip gene expression. Because Pax2 is known to have an antiapoptotic activity, PTIP might have concerted activity with Pax2. Our primary cell culture with high salt demonstrated that the p2IME cells from PTIP− mice were highly sensitive to hyperosmolality. It is possible that Pax2 cannot function in the absence of the adaptor protein PTIP. However, PTIP may also work with other transcription factors or proteins that are involved in antiapoptotic activity against hypertonicity. Currently, we are investigating to which proteins PTIP binds to concert its antiapoptotic activity.
Recently, an aqp2fl/fl conditional knockout mice was generated by crossing to tamoxifen-inducible Cre-expressing mice (18). Before tamoxifen administration, there was no significant difference in urine osmolality between wild-type and mutant mice in this system. However, after tamoxifen treatment, urine osmolality was decreased from 2000 to 500 by approximately 4 to 5 d. Lack of AQP2 by this inducible aqp2 knockout mouse model reflects nephrogenic diabetes insipidus, which is characterized by inability to concentrate the urine, resulting in polyuria and polydipsia. A study on families with congenital nephrogenic diabetes insipidus showed that mutations have been identified in the aqp2 gene (19). The inducible aqp2 knockout mice showed a five-fold increase in AQP3 protein expression (18). Another report demonstrated that AQP2 and AQP4 expression was decreased in renal collecting duct in aqp3 null mice (20). Downregulation of one of the aquaporin family may compensate for changes of expression in other aquaporins. However, there was no significant change in AQP3 expression in PTIP− mice in this study.
Urea transporter (UT-A) is also crucial for the water reabsorption in the kidney (21). When both UT-A1 and UT-A3 were genetically deleted from the IMCD UT-A1/3−/−, the mice showed the urine-concentrating defect that is caused by a failure of urea transport from the IMCD lumen to the inner medullary interstitium, resulting in osmotic diuresis. Mouse UT-A1 expression is controlled under UT-Aα promoter, which contains tonicity-responsive enhancer (TonE) (22). A putative TonEBP consensus sequence is also present in the promoter region of aqp2 gene (23). In our preliminary study, UT-A1 mRNA expression was significantly reduced in the PTIP− mice after dDAVP administration (data not shown), suggesting that PTIP affects the expression of both aqp2 and UT-A1, although again this may be a more indirect result of low AVPR2 levels. The suppression of AVPR2 decouples the translocation of TonEBP from dDAVP administration in the PTIP− mice and therefore may not lead to the upregulation of tonicity response genes such as UT-A1 and aqp2. The end result is an inability to adsorb water in the collecting ducts of the renal papilla.
Conclusion
Mice with ptip knockout in the collecting duct of kidney are unable to concentrate urine. They exhibited low levels of AVPR2 and less activated AQP2 protein on the cell surface of inner medulla after dDAVP treatment. In vitro, PTIP− collecting duct cells are hypersensitive to high osmolality, compared with their wild-type counterparts. Therefore, in addition to its necessity in the developing early embryo, the PTIP is involved in the genetic regulation of the urine-concentration machinery in the inner medulla.
Acknowledgments
D.K. was supported in part by a fellowship from the American Foundation for Urological Disease. This work was supported in part by National Institutes of Health grants DK054740 and DK062914 to G.R.D.
We thank E. Hughes and T. Saunders of the University of Michigan Transgenic Animal Model Core Facility for help with the gene targeting, P. Igarashi for the Ksp-Cre mice, J. Sands for sharing the AQP2 antibody, and H.M. Kwon for the TonEBP antibody.
Footnotes
Disclosures
None.
References
- 1.Lechner MS, Levitan I, Dressler GR. PTIP, a novel BRCT domain-containing protein interacts with Pax2 and is associated with active chromatin. Nucleic Acids Res. 2000;28:2741–2751. doi: 10.1093/nar/28.14.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bork P, Hofmann K, Bucher P, Neuwald AF, Altschul SF, Koonin EV. A superfamily of conserved domains in DNA damage-responsive cell cycle checkpoint proteins. FASEB J. 1997;11:68–76. [PubMed] [Google Scholar]
- 3.Cho EA, Prindle MJ, Dressler GR. BRCT domain-containing protein PTIP is essential for progression through mitosis. Mol Cell Biol. 2003;23:1666–1673. doi: 10.1128/MCB.23.5.1666-1673.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P. Pax2, a new murine paired-box containing gene and its expression in the developing excretory system. Development. 1990;109:787–795. doi: 10.1242/dev.109.4.787. [DOI] [PubMed] [Google Scholar]
- 5.Eccles MR, Wallis LJ, Fidler AE, Spur NK, Goodfellow PJ, Reeve AE. Expression of the Pax2 gene in human fetal kidney and Wilms’ tumor. Cell Growth Differ. 1992;3:279–289. [PubMed] [Google Scholar]
- 6.Shao X, Somlo S, Igarashi P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol. 2002;13:1837–1846. doi: 10.1097/01.asn.0000016444.90348.50. [DOI] [PubMed] [Google Scholar]
- 7.Cai Q, Dmitrieva NI, Ferraris JD, Brooks HL, van Balkom BW, Burg M. Pax2 expression occurs in renal medullary epithelial cells in vivo and in cell culture, is osmoregulated, and promotes osmotic tolerance. Proc Natl Acad Sci U S A. 2005;102:503–508. doi: 10.1073/pnas.0408840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim D, Dressler GR. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol. 2005;16:3527–3534. doi: 10.1681/ASN.2005050544. [DOI] [PubMed] [Google Scholar]
- 9.Shao X, Johnson JE, Richardson JA, Hiesberger T, Igarashi P. A minimal ksp-cadherin promoter linked to a green fluorescent protein reporter gene exhibits tissue-specific expression in the developing kidney and genitourinary tract. J Am Soc Nephrol. 2002;13:1824–1836. doi: 10.1097/01.asn.0000016443.50138.cd. [DOI] [PubMed] [Google Scholar]
- 10.Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci U S A. 1999;96:2538–2542. doi: 10.1073/pnas.96.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasler U, Jeon US, Kim JA, Mordasini D, Kwon HM, Feraille E, Martin PY. Tonicity-responsive enhancer binding protein is an essential regulator of aquaporin-2 expression in renal collecting duct principal cells. J Am Soc Nephrol. 2006;17:1521–1531. doi: 10.1681/ASN.2005121317. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Cai Q, Michea L, Dmitrieva NI, Andrews P, Burg MB. Proliferation and osmotic tolerance of renal inner medullary epithelial cells in vivo and in cell culture. Am J Physiol Renal Physiol. 2002;283:F302–F308. doi: 10.1152/ajprenal.00038.2002. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu K, Bourillot PY, Nielsen SJ, Zorn AM, Gurdon JB. Swift is a novel BRCT domain coactivator of Smad2 in transforming growth factor beta signaling. Mol Cell Biol. 2001;21:3901–3912. doi: 10.1128/MCB.21.12.3901-3912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995;121:4057–4065. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- 15.Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature. 1993;361:549–552. doi: 10.1038/361549a0. [DOI] [PubMed] [Google Scholar]
- 16.Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem. 1997;272:14800–14804. doi: 10.1074/jbc.272.23.14800. [DOI] [PubMed] [Google Scholar]
- 17.Kuwahara M, Asai T, Terada Y, Sasaki S. The C-terminal tail of aquaporin-2 determines apical trafficking. Kidney Int. 2005;68:1999–2009. doi: 10.1111/j.1523-1755.2005.00654.x. [DOI] [PubMed] [Google Scholar]
- 18.Yang B, Zhao D, Qian L, Verkman AS. Mouse model of inducible nephrogenic diabetes insipidus produced by floxed aquaporin-2 gene deletion. Am J Physiol Renal Physiol. 2006;291:F465–F472. doi: 10.1152/ajprenal.00494.2005. [DOI] [PubMed] [Google Scholar]
- 19.Bichet DG. Nephrogenic diabetes insipidus. Adv Chronic Kidney Dis. 2006;13:96–104. doi: 10.1053/j.ackd.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Kim SW, Gresz V, Rojek A, Wang W, Verkman AS, Frokiaer J, Nielsen S. Decreased expression of AQP2 and AQP4 water channels and Na,K-ATPase in kidney collecting duct in AQP3 null mice. Biol Cell. 2005;97:765–778. doi: 10.1042/BC20040148. [DOI] [PubMed] [Google Scholar]
- 21.Fenton RA, Flynn A, Shodeinde A, Smith CP, Schnermann J, Knepper MA. Renal phenotype of UT-A urea transporter knockout mice. J Am Soc Nephrol. 2005;16:1583–1592. doi: 10.1681/ASN.2005010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fenton RA, Cottingham CA, Stewart GS, Howorth A, Hewitt JA, Smith CP. Structure and characterization of the mouse UT-A gene (Slc14a2) Am J Physiol Renal Physiol. 2002;282:F630–F638. doi: 10.1152/ajprenal.00264.2001. [DOI] [PubMed] [Google Scholar]
- 23.Storm R, Klussmann E, Geelhaar A, Rosenthal W, Maric K. Osmolality and solute composition are strong regulators of AQP2 expression in renal principal cells. Am J Physiol Renal Physiol. 2003;284:F189–F198. doi: 10.1152/ajprenal.00245.2002. [DOI] [PubMed] [Google Scholar]


