Abstract
Background
Although reward processing is considered an important part of affective functioning, few studies have investigated reward-related decisions or responses in young people with affective disorders. Depression is postulated to involve decreased activity in reward-related affective systems.
Methods
Using functional MRI, we examined behavioral and neural responses to reward in young people with depressive disorders using a reward decision-making task. The task involved choices about possible rewards involving varying magnitude and probability of reward. The study design allowed the separation of decision/anticipation and outcome phases of reward processing. Participants were 9–17 years old and had diagnoses of Major Depressive Disorder (MDD), anxiety disorders, or no history of psychiatric disorder.
Results
Participants with MDD exhibited less neural response than control participants in reward-related brain areas during both phases of the task. Group differences did not appear to be a function of anxiety. Depressive and anxiety symptoms were associated with activation in reward-related brain areas.
Conclusions
Results suggest that depression involves altered reward processing and underscore the need for further investigation of relations among development, affective disorders, and reward processing.
Keywords: depression, reward, decision-making
Affective neuroscience provides new ways of understanding the substrates of developmental psychopathology, including those associated with mood disorders. In depression, a likely substrate involves the neural systems implicated in reward processing (Nestler & Carlezon, 2006). Conceptual models from several perspectives postulate that depression is accompanied by altered motivation to obtain reward, low frequency of pursuing rewarding experiences, and reduced enjoyment of rewarding outcome (Forbes & Dahl, 2005).
Functional brain imaging has investigated neural responses to rewarding events such as monetary wins (e.g., Delgado, Locke, Stenger, & Fiez, 2003) and simple feedback signals (Elliott, Sahakian, Michael, Paykel, & Dolan, 1998). Along with studies of non-human primates (Schultz, 2000), neuroimaging studies have revealed that reward processing occurs in regions including the dorsal and ventral striatum, amygdala, and orbitofrontal cortex (OFC). Functional abnormalities in all of these regions have been observed in depression (Drevets, 2001), although the specific nature of reward-processing disruptions in depression is still somewhat unclear.
One reason may be that there are many sub-processes of reward responding, including decisions about possible future rewards and response to rewarding outcomes (Rogers et al., 2004). Decision-making itself involves several phases, each of which relies on the activity of specific brain regions (Ernst & Paulus, 2005). The striatum, amygdala, and OFC are among the brain regions implicated in processing a decision's outcome. In decision-making and anticipation of reward, relevant regions include the anterior cingulate cortex (ACC), dorsolateral prefrontal cortex, medial and lateral OFC, and ventral striatum (Ernst et al., 2004).
Little is known about behavioral and neural responses to reward in child and adolescent depression. The only behavioral study of reward-related decisions to our knowledge reported that boys with current depressive disorders failed to distinguish low- and high-magnitude rewards under high-probability conditions (Forbes, Shaw, & Dahl, in press), and that reward-related decisions predicted depressive disorders and symptoms one year later. Neural response to reward processing has been studied in healthy young people (Bjork et al., 2004; Ernst et al., 2005; May et al., 2004) but not in those with affective disorders. Healthy adolescents exhibit neural responses that are generally similar to those in adults, but developmental effects have been reported, such as ventral but not dorsal activation in adolescents during a guessing task (May et al., 2004). During reward anticipation, adolescents exhibit ventral striatal activation (Bjork et al., 2004). During rewarding outcomes, adolescents exhibit activation of the amygdala (Ernst et al., 2005), ventral striatum (Ernst et al., 2005), lateral and medial OFC (May et al., 2004), and mesial prefrontal cortex (Bjork et al., 2004).
Adults with depression fail to display increased ventral striatal activation to pleasant visual stimuli (Lawrence et al., 2004), and anhedonia is inversely correlated with amygdala and ventral striatal activation (Keedwell, Andrew, Williams, Brammer, & Phillips, 2005b). Extending this work by examining reward processing in pediatric depression can elucidate the affective mechanisms that are disrupted in the disorder, provide details on developmental effects, and suggest possible targets for both pharmacologic and psychosocial treatments of the disorder.
The present study investigated how the neural correlates of reward-related decision-making in young people with Major Depressive Disorder (MDD) differ from those in typically developing control children. Using a functional magnetic resonance imaging (fMRI) reward paradigm, we attempted to examine group differences in two reward processes: decision-making/anticipation and outcome.
Based on conceptual models and empirical findings, we hypothesized that MDD would be associated with (1) reduced neural response to reward decision-making/anticipation in the ACC, medial and lateral OFC, and striatum, and (2) reduced neural response to reward outcome in the striatum, amygdala, and OFC. We hypothesized, based on previous findings (Forbes, Shaw, & Dahl, in press), that participants with depression would exhibit less reward-seeking behavior than would participants in the control group.
Secondarily, we examined the influence of anxiety disorders on neural response to reward. Analyses involving anxiety were exploratory because the anxiety-only group was small and because conceptual accounts provided little to guide our expectations. Anxiety disorders are thought to involve enhanced vigilance for threat (Mogg & Bradley, 1998; Vasey & Dadds, 2001), but some models propose that reward responding is not importantly disrupted in anxiety (Clark & Watson, 1991).
Methods
Participants
Participants were 31 youths age 9–17 years (M = 14.58, SD = 1.64; 21 female) from our larger longitudinal study. Participants had MDD (n=14; age: M(SD)=14.73(1.49); 11 female) or were controls with no history of psychiatric disorder (n=17, age: M(SD)= 14.45(1.79); 10 female). Comorbidities among MDD participants included Dysthymia (2), Generalized Anxiety Disorder (10), Social Phobia (5), Panic Disorder (1), and Separation Anxiety Disorder (1). Participants were free of psychotropic medications, cigarettes, and illicit drugs. Of 47 initial participants, 16 were excluded based on technical or administration problems (3 MDD, 12 control) or narcolepsy diagnosis (1 MDD). Participants were generally Caucasian (84%) and right-handed (90%), M(SD)IQ=116(13.3), M(SD)socio-economic status (SES) (Hollingshead, 1975)= 43.5(10.4). There were no significant group differences in age, IQ, or SES. MDD participants had higher scores on all symptom measures (Table 1).
Table 1. Mean (SD) of Self- and Parent-Reported Problems and Symptoms, by Group.
| Measure | CONT
(n=17) |
MDD
(n=14) |
|---|---|---|
| CBCL (T score) | ||
| externalizing | 47.71 (7.75) | 57.92 (5.65) |
| internalizing | 45.21 (7.69) | 64.83 (12.28) |
| total | 45.93 (8.11) | 65.08 (8.75) |
| SCARED (total score) | ||
| child | 7.00 (5.77) | 34.58 (15.13) |
| parent | 4.07 (5.18) | 29.83 (13.00) |
| CDI/BDI (z-score) | -0.64 (0.61) | 0.97 (0.90) |
| Choice behavior | ||
| High probability/high magnitude | .51 (.06) | .52 (.09) |
| High probability/low magnitude | .53 (.06) | .50 (.06) |
| Low probability/high magnitude | .51 (.07) | .51 (.10) |
| Low probability/low magnitude | .49 (.06) | .50 (.03) |
Note: MDD=Major Depressive Disorder; CONT=Control; CBCL=Child Behavior Checklist; SCARED=Screen for Childhood Anxiety and Related Disorders; CDI=Children's Depression Inventory; BDI=Beck Depression Inventory. For symptom variables, CONT<MDD, p<.01. Values for choice behavior conditions are proportion of trials in which risky game chosen.
Exploratory analyses examined whether results were likely driven by anxiety. We therefore included a small non-MDD group with Generalized Anxiety Disorder (n = 5, age: M=11.622; 2 female; Specific Phobia (2), Separation Anxiety Disorder (1), Panic Disorder (1), Dysthymic Disorder (3)). This group was younger than controls, but low power limited ability to account for age in analyses. Data for one participant were excluded from analyses because of clear outlier status.
Participants in the clinical groups were recruited from outpatient clinics at Western Psychiatric Institute and Clinic, Pittsburgh, PA. Controls were recruited through advertisements. Diagnoses were determined through administration of the Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL) (Kaufman et al., 1997). Participants in the anxiety-only and MDD groups were in a current episode based on DSM-III-R and DSM-IV criteria. Each participant and a parent (or guardian) were interviewed separately by a trained research specialist. Reliability for depression and anxiety diagnoses was above 90%; reliability was maintained through monthly diagnostic review meetings. Results of diagnostic interviews were presented at consensus case conferences with a child psychiatrist, who reviewed all findings and diagnoses.
Measures
Self- and parent-reported problems and symptoms
Participants and their parents or guardians completed he Child Behavior Checklist – parent form (CBCL) (Achenbach & Edelbrock, 1983); Screen for Childhood Anxiety and Related Disorders (SCARED) (Birmaher et al., 1997); Children's Depression Inventory (CDI) (Kovacs, 1985) (8-12-year-olds only); Beck Depression Inventory (BDI) (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) (13-18-year-olds only).
Reward task
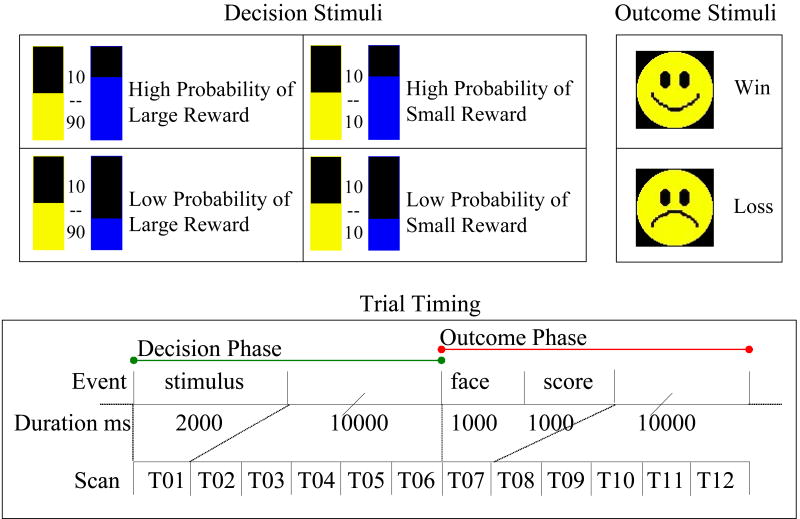
The reward task (adapted from Rogers et al, 2003) required participants to choose between options having constant or varying magnitude and probability of reward (Figure 1). The overall goal for the subject was to maximize point winnings; points were later converted to money. For every trial, the safe option maintained an equal probability (.50/.50) of winning or losing a small-magnitude reward (10 points). The risky option provided a .66 or .33 probability of winning 90 or 10 points. Thus, there were four conditions: high-probability/high-magnitude, high-probability/low-magnitude, low-probability/high-magnitude, and low-probability/low-magnitude. The probability of winning was represented by a color-filled bar, and point values for options were printed explicitly. Each point was worth 1.5¢; participants began with 1000 points to offset possible early losses. After each choice, subjects received feedback on whether they won or lost (an iconic smile or frown face) and saw their cumulative score.
Figure 1.
Design of the reward task. The upper left panel depicts decision the stimuli, showing each of the four possible trial types. The upper right panel depicts outcome stimuli. The lower panel depicts trial timing. The complete trial consisted of a decision phase and an outcome phase, each lasting 12 s. Imaging data were acquired at 6 time points during each phase.
The reward task used a two-phase trial design to separate hemodynamic responses linked to decision-making and anticipation (decision phase) from those linked to experiencing outcome (outcome phase). Trial length was 24 s: 2 s option presentation, 10 s inter-stimulus interval (ISI), 1 s outcome, 1 s presentation of updated score, and 10 s ISI. A total of 144 trials was acquired, with 36 trials per condition distributed evenly over 12 blocks. After the scanning session, participants were debriefed and paid $60.00 plus total winnings.
Post-task questionnaire
Administered immediately following the scan, this questionnaire contained 14 items designed to assess participants' task strategies; perception of risks and rewards on the task; and reasoning behind decisions. There were no group, age, or sex differences for any of the constructs.
Procedure
The University of Pittsburgh Institutional Review Board approved the study. Parents signed informed consent forms, participants younger than 14 provided verbal assent, and participants older than 14 provided written consent. Upon entry into the study, participants were admitted to the Child and Adolescent Sleep and Neurobehavioral Laboratory at Western Psychiatric Institute and Clinic for a three-day assessment that included a functional MRI scan. Participants received $150 for their 3-day stay in the lab and $75-90 for completing the scan, depending on task performance.
Before the scan was conducted, all participants practiced the task and experienced the scanning environment through a simulator. For task practice, a research staff member explained the task, showed participants examples of the stimuli, and asked questions to clarify understanding of the task. Participants then went through a series of mock trials on a computer. For the simulator experience, participants were exposed to an apparatus with similarly sized bore, sounds, and headcoil as those in the actual MR magnet to ensure their familiarity with the environment and to gauge their comfort and likely success. During structural scans, participants were shown movies.
fMRI Data Acquisition and Preprocessing
Functional and structural data were acquired using a 1.5-Tesla GE Signa 5x whole-body magnet (General Electric Medical Systems, Milwaukee Wisconsin) with a standard radio frequency (RF) headcoil. The structural volume consisted of thirty-seven contiguous, T1-weighted, double-oblique axial slices parallel to the anterior/posterior commissure (AC/PC) plane. A subset of 33 T2*-weighted images in the same plane (3.75 X 3.75 X 3.8 mm voxels) composed the functional volume. A ‘reversed’ spiral imaging sequence (Bornert, Aldefeld, & Eggers, 2000; Noll, Cohen, Meyer, & Schneider, 1995) was used to recover signal loss in regions affected by susceptibility artifacts (Haacke, Tkach, & Parrish, 1989) (echo time=25 ms, field of view=24 cm, flip angle=80°). A complete full-volume image was acquired every 2 s (TR=2000 ms), sampling each trial 12 times (6 time-points per decision phase, 6 per outcome phase).
Images were reconstructed using the NeuroImaging Software package (NIS; http://kraepelin.wpic.pitt.edu/nis/), and preprocessing employed NIS, Analysis of Functional NeuroImages (AFNI) (Cox, 1996), and AIR (Automated Image Registration) (Woods, Cherry, & Mazziotta, 1992) applications. Preprocessing steps were: slice-time correction via Afni-3dtshift, motion correction via a 6-parameter AIR algorithm, a high-pass filter via Afni-3dfourier (0.014 Hz), a within-block linear detrend, and a between-block baseline correction (NIS, outliers corrected to 3 standard deviations from per-voxel means). A 60-parameter warp function (Woods, Mazziotta, & Cherry, 1993) was used to cross-register each participant's structural data to a standard reference brain (Montreal Neurologic Institute (MNI), ftp://ftp.mrccbu.cam.ac.uk/pub/imaging/Colin/) that had been re-sampled to match the voxel resolution of the T1-weighted structural images (0.9375 X 0.09375 X 3.8 mm). To account for small anatomical differences not addressed in the cross-registration, a 6 mm full-width-half-maximum three-dimensional Gaussian filter was applied to smooth the functional data.
Data Analysis
Behavior
Behavioral analyses examined choice (proportion of trials in which risky option chosen) and reaction time (RT). Random-effects analyses of variance (ANOVAs) included Subject as a random factor, Group as a between-subjects factor, and Probability (high, low) and Magnitude (large, small) as within-subjects factors. RT analyses also included a within-subjects factor of Choice (fixed, risky).
fMRI
Separate fMRI data analyses were conducted for the decision and outcome phases; trials with no response were excluded. The following a priori regions were extracted from the Automatic Anatomical Labeling atlas (Tzourio-Mazoyer et al., 2002): ACC, amygdala, caudate, inferior OFC, middle OFC, superior OFC, and medial OFC. The ACC region included Brodmann's Areas 32 and 24, rostral to the anterior commissure, but not area 25, the subgenual cingulate. Tzourio-Mazoyer et al. (2002) describe the OFC regions. The average blood-oxygen-level-dependent (BOLD) response across all voxels in each region was computed as the mean percent change from the first scan in each trial for decision phase analyses, and from the sixth scan for outcome analyses. Extracted time-series were then subjected to a second level of outlier correction in which values over twice the interquartile range from the 25th or 75th percentiles were Windsorized to these limits. Mean responses in each condition for each participant were examined using separate mixed effects analyses for each task phase.
Mixed effects analyses were computed to allow the modeling of within-subject stability across scans and conditions of temporal autocorrelation among scans. For the decision phase, Subject was a random factor; Probability, Magnitude, and Time (scans 1-6 within a trial) were fixed repeated measures. Time was assumed to have an AR1 covariance structure, which accounted for temporal autocorrelation among scans. Group was a between-subjects fixed factor. Follow-up t-tests were used to decompose interactions. Similarly, for the outcome phase, Subject was a random factor, Outcome (large win, large loss, small win, small loss) and Time (scans 7-12 within trials) were fixed repeated measures; Time again was assumed to have an AR1 covariance structure. Follow-up F tests of the response patterns, with reward magnitude (small/large) and status (win/loss), and post-hoc t-tests were used to decompose interactions. Exploratory analyses with the anxiety group examined group effects in the regions for which depression effects had been detected. Effect sizes (ds) were formulated to allow equal weighting of variance despite unequal group ns.
Results
Behavioral Response
For choice behavior there was a significant group X probability X magnitude interaction, F(1,29)=4.40, p=.045, h2=.13. As shown in Table 1, all groups chose the safe option between 49% and 53% of the time in every condition, and no group-contrasts within either condition were significant. Rather, the interaction was driven by the difference of differences in the conditions. There were no significant condition- or group-related effects, or interactions of group and condition on choice reaction times. Decision times varied between groups and conditions from 1025ms to 1135ms. Reaction times to the outcome varied with the type of outcome, F(1,29)=30.3, p<.0005, h2=.51. Reaction times were faster following a win, M(SD)=772.4(853.3) milliseconds, than a loss, M(SD)=804.3(866.903) milliseconds. There were no main effects or interactions involving group.
Neural Response
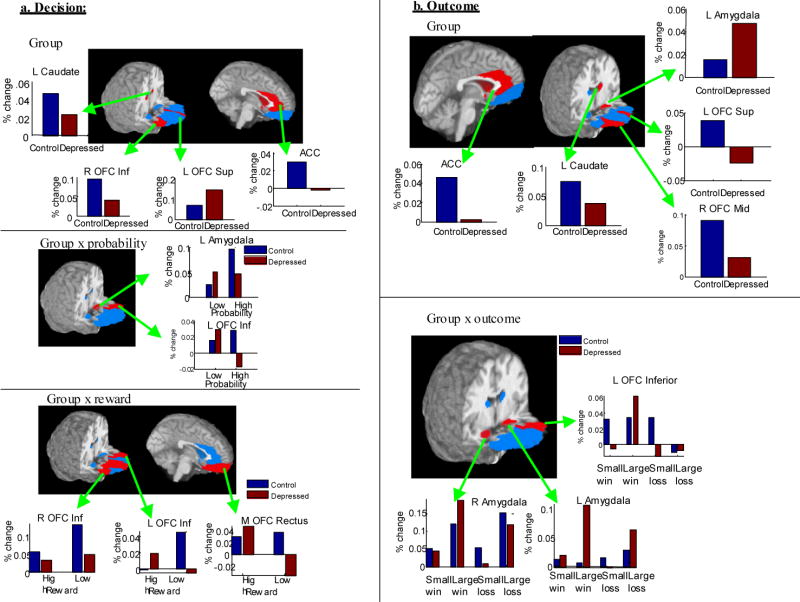
Table 2 lists the a priori regions that showed significant BOLD response differences. Effects for group differences are rendered on the brain's surface in each region of interest in Figure 2. In nearly all cases, relevant group- and condition-related differences did not interact with time, suggesting that differences reflected area under the curve rather than differently shaped time-courses as a function of condition or group. As shown in Table 2, in the decision phase, nearly all of the examined regions displayed differential activity as a function of probability, reward magnitude, or both. As shown in Table 2 and Figure 2, the amygdala, inferior, and superior OFC displayed differential relationships with magnitude and reward as a function of condition. Participants with depression displayed increased responses relative to controls in the left superior OFC, but decreased responses in the caudate bilaterally and the right inferior OFC. Similarly, in the outcome phase, nearly all of the examined regions displayed differential reactivity as a function of outcome, with ACC, amygdala bilaterally, right mid and superior OFC regions displaying decreased responses to small, compared with large, reward or loss irrespective of reward status. The caudate, left mid OFC, and medial rectus of the OFC were all sensitive to winning versus losing. Participants with depression displayed decreased responses compared with controls in the caudate and regions of the OFC, but interactions with the type of outcome in the ACC, amygdala, and left inferior OFC.
Table 2. Significant Findings for Neural Response during the Reward Task, by Task Phase.
| Effect | Region | F | p | ds | Direction of Effects |
|---|---|---|---|---|---|
| Group X Probability | L Amygdala | 7.35 | .007 | .19 | D>C (high probability), |
| -.34 | D<C (low probability) | ||||
| L OFC Inf | 6.63 | .010 | .10 | D>C (high probability), | |
| -.34 | D<C (low probability) | ||||
| Group X Magnitude | L OFC Inf | 15.81 | <.0005∼ | .13 | D>C (high reward), |
| -.41 | D<C (low reward) | ||||
| R OFC Inf | 11.74 | .001 | -.66 | D<<C (low reward) | |
| -.17 | D<C (high reward) | ||||
| R OFC Sup | 11.35 | .001 | -.13 | D<C (low reward) | |
| .32 | D>C (high reward) | ||||
| M OFC Rectus | 20.54 | <.0005 | -.42 | D<C (low reward) | |
| .13 | D>>C (high reward) | ||||
| Group | ACC | 4.04 | .047 | -.37 | D<C |
| L OFC Sup | 7.73 | .006 | .40 | D>C | |
| L Caudate | 4.12 | .044∼∼ | -.32 | D<C | |
| 4.49+ | .001 | ||||
| R Caudate | 6.82 | .000 | -.39 | D<C | |
| 3.27+ | .007 | ||||
| R OFC Inf | 8.00 | .005∼∼ | -.40 | D<C | |
| Probability x Magnitude | L Amygdala | 4.97 | .026 | High>Low Probability (high reward), | |
| High<Low Probability (low reward), | |||||
| L Caudate | 13.89 | <.0005 | High>Low Probability (high reward), | ||
| High<Low Probability (low reward), | |||||
| R Caudate | 7.94 | .005 | High<Low Probability (high reward), | ||
| High = Low Probability (Low reward), | |||||
| Probability | L Amygdala | 5.88 | .016 | Low>high | |
| R OFC Mid | 18.13 | <.0005 | Low>high | ||
| R OFC Sup | 7.62 | .006 | Low>high | ||
| Magnitude | ACC | 40.9 | <.0005 | Low>high | |
| L Amygdala | 11.711 | .001 | Low>high | ||
| R Amygdala | 13.21 | <.0005 | Low>high | ||
| L OFC Sup | 9.07 | .003 | Low>high | ||
| R OFC Sup | 5.79 | .017 | Low>high | ||
| R OFC Inf | 28.005 | <.0005 | Low>high | ||
| 26.26+ | .023 | Low>high especially at the middle scans | |||
| R OFC Mid | 26.11 | <.0005 | Low>high | ||
| M OFC Rectus | 14.25 | <.0005 | Low>high | ||
|
| |||||
| Group X Outcome | ACC | 1.907 | .127 | -.25 | D<C (reward) |
| -.45 | D<<C (loss) | ||||
| L Amygdala | 4.126 | .006 | .32 | D>C (Large) | |
| -.03 | D=C (Small) | ||||
| R Amygdala | 3.80 | .01 | .06 | D>C (Large Reward) | |
| .01 | D<=C (all other conditions) | ||||
| L OFC Inf | 3.10 | .026∼ | .08 | D>C (Large reward) | |
| -.13 | D<C (all other conditions) | ||||
| Group | ACC | 19.185 | .000 | -.37 | D<C |
| L Amygdala | 6.102 | .014 | .19 | D>C | |
| L Caudate | 16.12 | <.0005∼ | -.36 | D<C | |
| L OFC Mid | 25.18 | <.0005 | -.47 | D<C | |
| R OFC Mid | 9.26 | .003∼∼ | -.27 | D<C | |
| L OFC Sup | 6.25 | .013∼∼ | -.26 | D<C | |
| Outcome | ACC | 6.048 | .000∼∼ | Small<Large | |
| L Amygdala | 3.558 | .014 | Small<Large | ||
| R Amygdala | 21.95 | <.0005 | Small<Large | ||
| L Caudate | 1.754 | .155 | Small<Large (especially at scans 3-4) | ||
| R Caudate | 3.92 | .009 | Win>Loss | ||
| 3.60+ | <.0005 | Win>Loss (especially at scans 3-4) | |||
| L OFC Mid | 11.13 | <.0005∼∼ | Large Loss<Small Loss<Small | ||
| Reward<Large Reward | |||||
| R OFC Mid | 9.99 | <.0005∼∼ | Small<Large | ||
| OFC Med Rectus | 12.22 | <.0005 | Large>Small, Reward>Loss | ||
| R OFC Inf | 5.35 | .001∼∼ | Large Loss<Small Loss<Small | ||
| Reward<Large Reward | |||||
| R OFC Sup | 6.748 | <.0005 | Small<Large | ||
occurred in interaction with scan, so that time-course of effect varied above and beyond group or condition-related differences in area under the curve
qualified by interaction with first versus second half, p<.05
qualified by interaction with first versus second half, p<.01
Note: D=Depression group; A=Anxiety group; C=Control; ds=effect size, ; ACC=anterior cingulate cortex; OFC=orbitofrontal cortex; L=left; R=right; Inf=inferior; Sup=superior; M=medial. Italics ➔ p<.05, but not significant after controlling for type 1 error using conservative threshold of p<.01.
df: Decision Phase: Diagnosis (1,101.9-192.3), Probability (1,634.9-683.9), Reward (1, 294.8-453.6), Probability x Reward: (1, 542.4-652.1). Interactions with diagnosis did not change df for Reward and Probability effects.
Figure 2.
Images showing significant main effects or interactions with group (p<.05, in red) in (a) anticipation/decision and (b) outcome phases. Blue regions were examined but not significant.
Secondary and Sensitivity Analyses
Anxiety-only group
Anxious youth systematically displayed strong, and sometimes different, patterns of reactivity than depressed youth. For example, for the Group X Magnitude interaction in the left inferior OFC, whereas depressed adolescents displayed higher activity than controls for the high-magnitude condition (ds=.13), anxious youth displayed much higher activity than controls, ds=1.01, F(1,263.3)=11.304, p=0.001. In contrast, for right inferior OFC, whereas depressed participants displayed lower activity than controls in the high-magnitude condition (ds=-.66), anxious participants displayed higher activity than controls, ds=.59, F(1,261.4)=14.3, p<.0005. Effects of anxiety were also dramatic in the outcome phase. For example, like depressed participants, anxious participants had higher activity to high-magnitude conditions in the left amygdala (ds=.19, F(3,453.3)=4.8, p=.002) and smaller reactions in the ACC (ds=-.08, F(3. 452.6)=2.9, p=.03). Main effects were also present. For example, like depressed participants, anxious participants displayed decreased reactions in the left caudate compared to controls (ds=-.29, F(1,149)=8.8, p=.004) but differed from the depressed group in other regions. Anxious participants displayed decreased reactions compared to depressed participants in the left and right middle OFC (ds=-.71, F(1,116.1)=19.03, p<.004 and ds=-.42, F(1,115.8)=9.16, p=.003) and left Superior OFC (ds=-.48, F(1,72.7)=4.92, p=.03). While some observed depression effects may have been driven by anxiety, most were probably not.
Relations with symptoms
Continuously measured depressive and anxiety symptoms were entered into the same mixed effects models as for the initial analyses. As reported in Table 3, significant correlations were generally small to modest and were evident in a number of reward-related regions. In the decision phase, depression interacted with probability or magnitude in the left amygdala and right OFC inferior, while anxiety interacted with probability or magnitude in the right OFC superior and medial OFC rectus. In the outcome phase, depression interacted with outcome for the ACC, left amygdala, and bilateral caudate. Several depression-by-anxiety-by-reward characteristic interactions were also evident.
Table 3. Main Effects and Interactions with Continuous Depressive and Anxiety Symptoms.
| Region | Effect |
|---|---|
| Decision | |
| L Amygdala | Probability*Depression (high:.17, low: -.14) |
| R OFC Inf | Reward* Depression (high: -.04, low: -.16) |
| R OFC Sup | Depression (.07), Anxiety (.1), Reward*Anxiety, (high: .19, low: -.05),
Reward*Anxiety*Depression |
| M OFC Rectus | Reward*Anxiety (high: .13, low: -.07),
Probability*Depression*Anxiety |
|
| |
| Outcome | |
| ACC | Outcome*Depression (WS: -.23 WL: -.01 LS: .05 LL: .-.16),
Outcome*Anxiety (WS: -.10 WL: -.14 LS: -.35 LL: .-.24) |
| L Amygdala | Outcome*Depression (WS: .07 WL: .43 LS: -.17 LL: .17) |
| L OFC Inferior | Outcome*Depression*Anxiety |
| R OFC Superior | Depression*Anxiety (WS: -.07 WL: -.07 LS: -.11 LL: -.11),
Outcome*Depression*Anxiety |
| M OFC Rectus | Outcome*Anxiety (WS: .06 WL: -.18 LS: -.21 LL: .04),
Outcome*Anxiety*Depression |
| L Caudate | Outcome*Depression (WS: -.11 WL: -.08 LS: .02 LL: -.2),
Outcome*Anxiety (WS: -.07 WL: -.18 LS: -.30 LL: -.22) |
| R Caudate | Depression*Anxiety, Outcome*Depression (WS: -.07 WL: .19 LS: .09 LL: -.30), Outcome*Anxiety (WS: -.12 WL: .03 LS: -.4 LL: -.13) |
|
| |
| R OFC Mid | Outcome*Depression*Anxiety |
Note: All values represent Pearson product-moment correlations with p<.01. WL=win large, WS=win small, LL=lose large, LS=lose small, ACC = anterior cingulate cortex; OFC = orbitofrontal cortex; L = left; R = right; Inf = inferior; Sup = superior; M = medial. Depressive symptoms measured with CDI/BDI; anxiety symptoms measured with SCARED.
Task duration
The task was quite long (>1 hour), and it is possible that some observed effects were a function of task duration. To understand whether this was likely, the same primary mixed effects analyses were re-run with administration-half as a factor. Effects in Table 2 which were qualified by administration-half effects are marked. Relatively few of the decision-phase results were qualified by these interactions, but the majority of outcome-phase results were.
Discussion and Conclusion
The current study indicates that young people with diagnosed MDD, many with anxiety disorders, exhibit disrupted neural responses to rewarding events. Depression was associated with reduced activation in some reward-related brain areas and increased activity in others during two phases of reward processing: decision-making/anticipation and outcome. Reward-related brain activation was also related to depressive and anxiety symptoms. Consistent with previous data, the brain regions involved were similar to those observed during reward in healthy adults and adolescents (e.g., May et al., 2004).
As expected, and in line with theoretical accounts (e.g., Forbes & Dahl, 2005), neuroimaging findings (e.g., Keedwell, Andrew, Williams, Brammer, & Phillips, 2005a), models of reward function (Nestler & Carlezon, in press), and self-report findings (e.g., Lonigan, Phillips, & Hooe, 2003), young people with depression exhibited disrupted responding in both reward decision/anticipation and reward outcome. In the decision phase, those with depression exhibited blunted responses in the ACC, bilateral caudate, and inferior OFC bilaterally, especially during high-magnitude reward conditions. This pattern is consistent with decreased emotional reactivity to reward. In addition, those with depression exhibited increased response in the middle and superior OFC bilaterally, especially to low-magnitude reward, which is consistent with over-regulation. In the outcome phase, those with depression exhibited blunted response in the ACC, caudate, and OFC, particularly during loss and low-magnitude reward but increased response in the amygdala. After high-magnitude rewards, those with depression exhibited a greater response than control participants in the amygdala bilaterally and the inferior OFC. This pattern of results suggests that depression is associated with generally diminished responses, particularly in conditions of small-magnitude reward. Contrary to our hypotheses, and despite a group-by-probability-by magnitude interaction for reward choice, groups did not differ meaningfully in reward-related behavior.
The regions in which we detected effects for affective disorders are important to decision-making generally (Ernst & Paulus, 2005), suggesting that depression in youth could interfere with decision-making. The ACC is thought to have particular importance in monitoring error (Carter et al., 1998) and conflict (Kerns et al., 2004). During decision-making/anticipation, decreased ACC activation might reflect a lack of awareness or concern for outcomes – possibly associated with low motivation. During outcome, increased OFC and amygdala activation might reflect relief or surprise about winning or having made a “good” choice. Finally, it is important to note that reward processing involves a circuit, not simply a set of individual regions.
Although a secondary focus of the study, anxiety findings were intriguing for two reasons. First, because the effects in the anxiety-only group were not always in the same direction as for the MDD group, and because depressive and anxious symptoms independently explained variance in relevant brain activity, it is unlikely that our depression results were wholly a function of anxiety. Second, findings with anxiety point to a promising new direction for research on reward and affective disorders. Young people with anxiety disorders exhibited variable but more extreme responses to the anticipation and outcome of reward than did those with depression. The size of the anxiety-only group cautions against a strong interpretation of this finding, but anxiety disorders may interfere with reward anticipation and outcome. In addition to supporting models of striatal function in depression (Nestler & Carlezon, in press), the difference we found between anxiety and MDD groups is consistent with a recent report that young people with temperamental anxiety exhibit increased striatal function to reward (Guyer et al., 2006).
The current study has several limitations that qualify interpretations of its findings. The sample size was small, resulting in small clinical groups. The sex and age compositions of the groups were less than optimal. The groups had considerable symptom overlap, with dysthymia in several members of the anxiety-only group and externalizing symptoms in both clinical groups. This overlap may have confounded results. Although the reward task allowed us to separate decision and outcome components of reward processing, it did not allow the separation of decision-making from anticipation. As many outcome-phase effects were qualified by task-administration-half, it may be important to use shorter tasks, and to fully account for variation throughout the course of the tasks in future studies.
Despite its limitations, the study indicates that reward-related decision-making is a promising approach to investigating specific components of the neural aspects of reward processing. The application of decision paradigms to young people allows examination of the developmental progression in reward processing as well as the examination of differences in clinical populations. Given the changes in reward-related behavior that occur with brain development during adolescence (Spear, 2000; Steinberg, 2004), it will be important for future studies to consider factors such as pubertal status when examining reward processing in child and adolescent affective disorders. In the future, use of reward paradigms that separate the components of reward processing, consideration of developmental factors, and continued investigation of alterations that occur with affective disorders will allow a greater understanding of affective processes in these disorders.
Acknowledgments
This study was supported by a National Institute of Mental Health Program Project (P01 MH41712; Neal D. Ryan, PI) and a National Institute of Mental Health Research Network (R24 MH067346; Ronald E. Dahl, PI). We thank Mauri Cesare, the staff of the Child and Adolescent Sleep and Neurobehavioral Laboratory, and the families who participated.
Abbreviations
- ACC
- fMRI
- OFC
Footnotes
Cameron S. Carter is now at the Department of Psychiatry and Behavioral Medicine, University of California, Davis.
Contributor Information
Erika E. Forbes, Department of Psychiatry, University of Pittsburgh
J. Christopher May, Department of Psychology, University of Pittsburgh.
Greg J. Siegle, Department of Psychiatry, University of Pittsburgh
Cecile D. Ladouceur, Department of Psychiatry, University of Pittsburgh
Neal D. Ryan, Department of Psychiatry, University of Pittsburgh
Cameron S. Carter, Department of Psychiatry, University of Pittsburgh
Boris Birmaher, Department of Psychiatry, University of Pittsburgh.
David A. Axelson, Department of Psychiatry, University of Pittsburgh
Ronald E. Dahl, Department of Psychiatry, University of Pittsburgh
References
- Achenbach TM, Edelbrock C. Manual for the Child Behavior Checklist and Revised Child Behavior Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1983. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: Similarities and differences from young adults. Journal of Neuroscience. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornert P, Aldefeld B, Eggers H. Reversed spiral MR imaging. Magnetic Resonance Medicine. 2000;44:479–484. doi: 10.1002/1522-2594(200009)44:3<479::aid-mrm20>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cognitive, Affective, and Behavioral Neuroscience. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: Implications for the cognitive-emotional features of mood disorders. Current Opinion in Neurobiology. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Michael A, Paykel ES, Dolan RJ. Abnormal neural response to feedback on planning and guessing tasks in patients with unipolar depression. Psychological Medicine. 1998;28:559–571. doi: 10.1017/s0033291798006709. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, et al. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biological Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Neural systems of positive affect: Relevance to understanding child and adolescent depression? Development and Psychopathology. 2005;17:827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Shaw DS, Dahl RE. Alterations in reward-related decision making in boys with current and future internalizing disorders. Biological Psychiatry. doi: 10.1016/j.biopsych.2006.05.026. in press. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, Bjork JM, Henderson HA, Pine DS, Fox NA, Ernst M. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience. 2006;26 doi: 10.1523/JNEUROSCI.0666-06.2006. ** [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke EM, Tkach JA, Parrish TB. Reduction of T2* dephasing in gradient field-echo imaging. Radiology. 1989;170:457–462. doi: 10.1148/radiology.170.2.2911669. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University Sociology Department; 1975. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biological Psychiatry. 2005a;58:495–503. doi: 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry. 2005b;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The children's depression inventory. Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Lonigan CJ, Phillips BM, Hooe ES. Relations of positive and negative affectivity to anxiety and depression in children: Evidence from a latent variable longitudinal study. Journal of Consulting and Clinical Psychology. 2003;71:465–481. doi: 10.1037/0022-006x.71.3.465. [DOI] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, et al. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biological Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Nestler E, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biological Psychiatry. doi: 10.1016/j.biopsych.2005.09.018. in press. [DOI] [PubMed] [Google Scholar]
- Noll DC, Cohen JD, Meyer CH, Schneider W. Spiral K-space MR imaging of cortical activation. Journal of Magnetic Resonance Imaging. 1995;5:49–56. doi: 10.1002/jmri.1880050112. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, et al. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biological Psychiatry. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Tunbridge EM, Bhagwagar Z, Drevets WC, Sahakian BJ, Carter CS. Tryptophan depletion alters the decision-making of healthy volunteers through altered processing of reward cues. Neuropsychopharmacology. 2003;28:153–162. doi: 10.1038/sj.npp.1300001. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk-taking in adolescence: What changes, and why? Annals of the New York Academy of Sciences. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Dadds MR. Information-processing factors in childhood anxiety: A review and developmental perspective. In: Vasey MW, Dadds MR, editors. The developmental psychopathology of anxiety. New York: Oxford University; 2001. pp. 253–277. [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. Journal of Computer Assisted Tomography. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. Journal of Computer Assisted Tomography. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]