Abstract
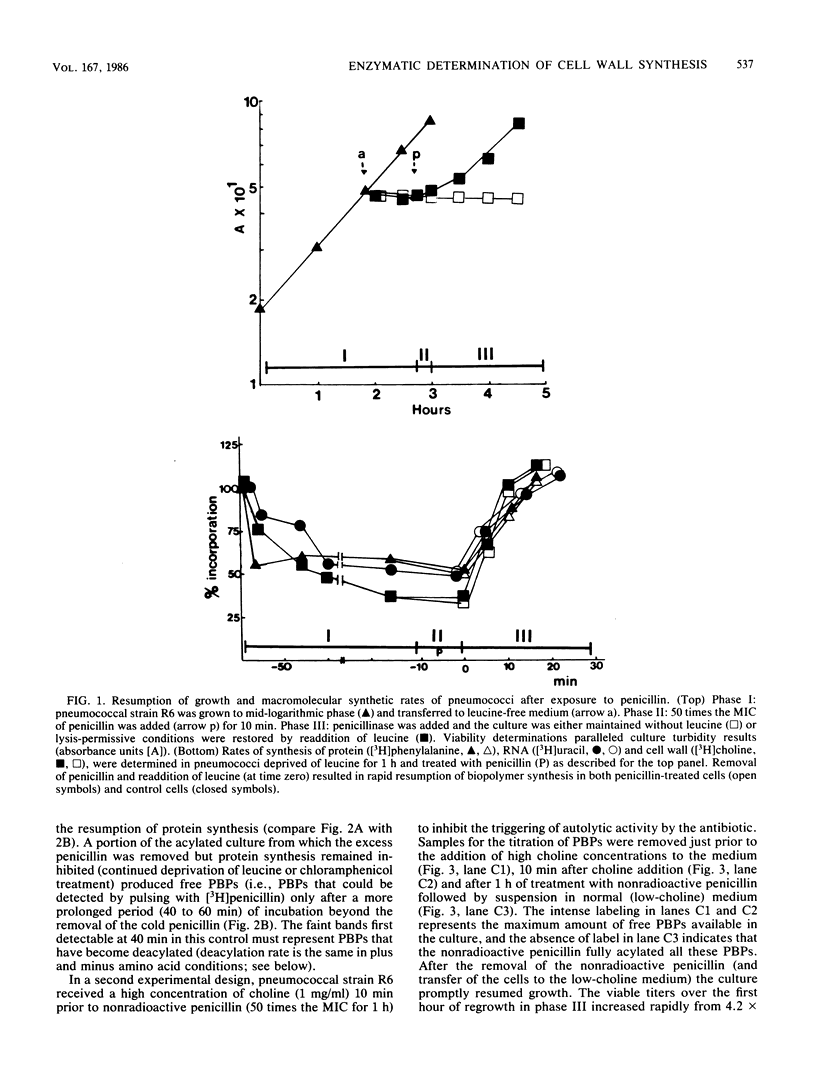
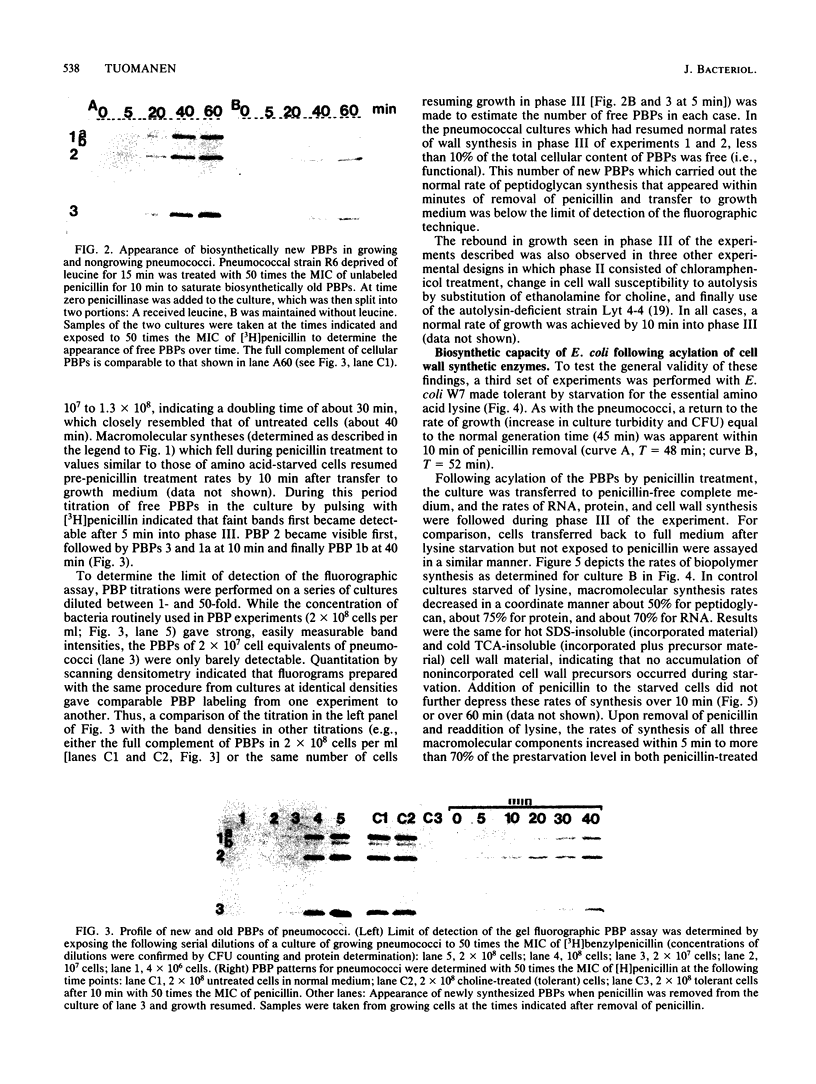
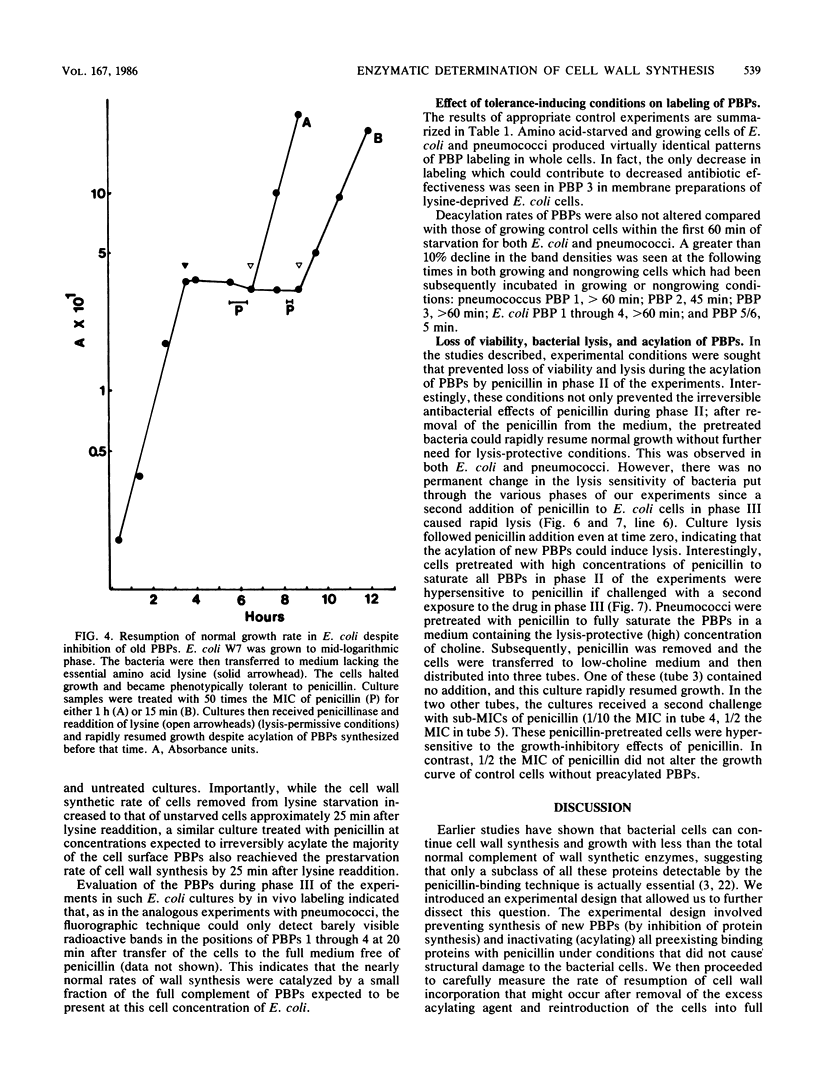
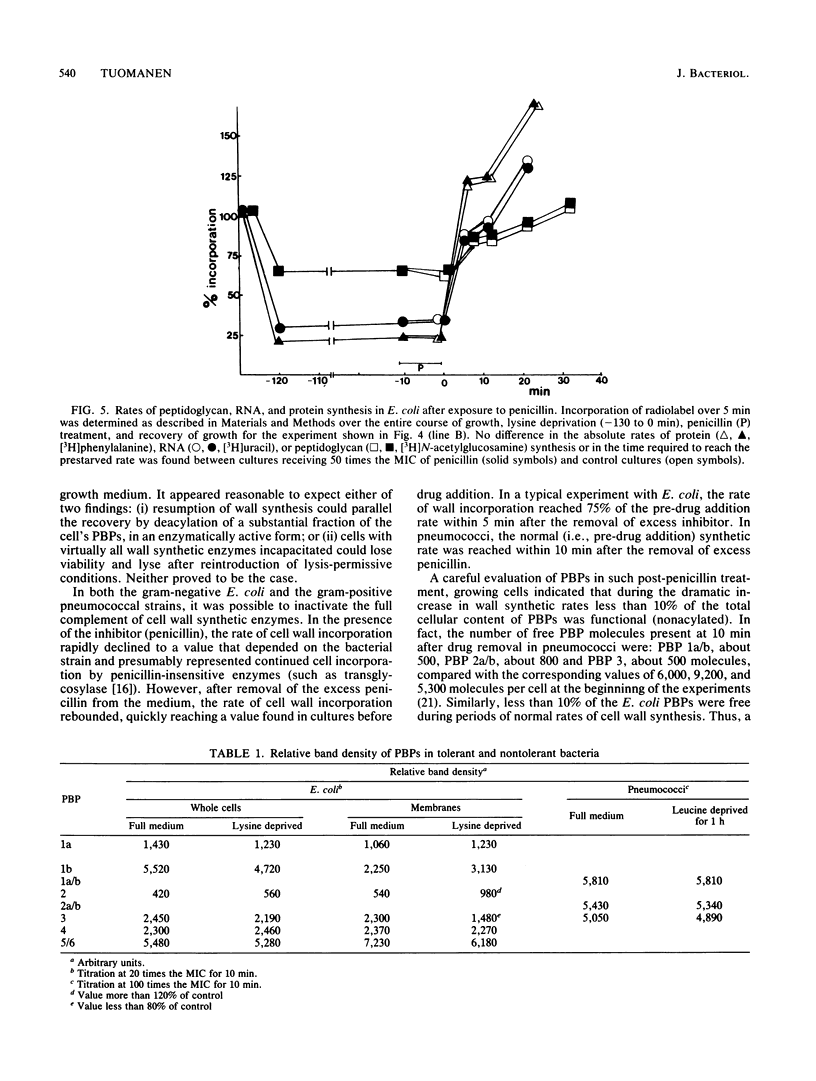
Cell wall synthesis can continue with less than the total complement of cell wall synthetic enzymes present in normal growing cells. A method was developed to investigate whether there exists an excess of cell wall-synthesizing enzymes (penicillin-binding proteins [PBPs]) which all remain functional or whether a mixed population of functional and nonfunctional enzymes characterize normal cells. Surprisingly, cells in which less than 10% of the PBPs were functional could grow at a normal rate, as evidenced by increases in viable counts, culture turbidity, and rates of peptidoglycan, protein, and RNA synthesis. This subset of functional enzymes was biosynthetically new. Penicillin-induced lysis occurred contingent on the acylation of this same small fraction of PBPs, the copy number and affinities of which were below the level of detection by current fluorographic assay techniques. We propose that PBPs have a short functional half-life and that cell wall synthesis and bacterial lysis reflect the activity of newly synthesized PBPs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbas J. A., Díaz J., Rodríguez-Tébar A., Vázquez D. Specific location of penicillin-binding proteins within the cell envelope of Escherichia coli. J Bacteriol. 1986 Jan;165(1):269–275. doi: 10.1128/jb.165.1.269-275.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Inactivation of D-alanine carboxypeptidase by penicillins and cephalosporins is not lethal in Bacillus subtilis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2814–2817. doi: 10.1073/pnas.68.11.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone E. J., Todd J. A., Ellar D. J., Sargent M. G., Wyke A. W. Membrane particles from Escherichia coli and Bacillus subtilis, containing penicillin-binding proteins and enriched for chromosomal-origin DNA. J Bacteriol. 1985 Oct;164(1):192–200. doi: 10.1128/jb.164.1.192-200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Radioautographic evidence for equatorial wall growth in a gram-positive bacterium. Segregation of choline-3H-labeled teichoic acid. J Cell Biol. 1970 Dec;47(3):786–790. doi: 10.1083/jcb.47.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome-Smith J. K., Spratt B. G. Deletion of the penicillin-binding protein 6 gene of Escherichia coli. J Bacteriol. 1982 Nov;152(2):904–906. doi: 10.1128/jb.152.2.904-906.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison M. T., Garland P. B. Immunochemical demonstration of zonal growth of the cell envelope of Escherichia coli. Eur J Biochem. 1983 Feb 15;130(3):589–597. doi: 10.1111/j.1432-1033.1983.tb07190.x. [DOI] [PubMed] [Google Scholar]
- Eagle H., Musselman A. D. THE SLOW RECOVERY OF BACTERIA FROM THE TOXIC EFFECTS OF PENICILLIN. J Bacteriol. 1949 Oct;58(4):475–490. doi: 10.1128/jb.58.4.475-490.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudicelli S., Tomasz A. Attachment of pneumococcal autolysin to wall teichoic acids, an essential step in enzymatic wall degradation. J Bacteriol. 1984 Jun;158(3):1188–1190. doi: 10.1128/jb.158.3.1188-1190.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmeiner J., Essig P., Martin H. H. Characterization of minor fragments after digestion of Escherichia coli murein with endo-N,O-diacetylmuramidase from Chalaropsis, and determination of glycan chain length. FEBS Lett. 1982 Feb 8;138(1):109–112. doi: 10.1016/0014-5793(82)80406-7. [DOI] [PubMed] [Google Scholar]
- Goodell E. W., Schwarz U. Enzymes synthesizing and hydrolyzing murein in Escherichia coli. Topographical distribution over the cell envelope. Eur J Biochem. 1977 Nov 15;81(1):205–210. doi: 10.1111/j.1432-1033.1977.tb11942.x. [DOI] [PubMed] [Google Scholar]
- Goodell W., Tomasz A. Alteration of Escherichia coli murein during amino acid starvation. J Bacteriol. 1980 Dec;144(3):1009–1016. doi: 10.1128/jb.144.3.1009-1016.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Role of the penicillin-sensitive transpeptidation reaction in attachment of newly synthesized peptidoglycan to cell walls of Micrococcus luteus. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3355–3359. doi: 10.1073/pnas.69.11.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Westphal M. Abnormal autolytic enzyme in a pneumococus with altered teichoic acid composition. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2627–2630. doi: 10.1073/pnas.68.11.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Täuber M. G., Zak O., Scheld W. M., Hengstler B., Sande M. A. The postantibiotic effect in the treatment of experimental meningitis caused by Streptococcus pneumoniae in rabbits. J Infect Dis. 1984 Apr;149(4):575–583. doi: 10.1093/infdis/149.4.575. [DOI] [PubMed] [Google Scholar]
- Williamson R., Tomasz A. Inhibition of cell wall synthesis and acylation of the penicillin binding proteins during prolonged exposure of growing Streptococcus pneumoniae to benzylpenicillin. Eur J Biochem. 1985 Sep 16;151(3):475–483. doi: 10.1111/j.1432-1033.1985.tb09126.x. [DOI] [PubMed] [Google Scholar]
- Zighelboim S., Tomasz A. Penicillin-binding proteins of multiply antibiotic-resistant South African strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):434–442. doi: 10.1128/aac.17.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]





