Abstract
Human declarative memory involves a systematic organization of information that supports generalizations and inferences from acquired knowledge. This kind of memory depends on the hippocampal region in humans, but the extent to which animals also have declarative memory, and whether inferential expression of memory depends on the hippocampus in animals, remains a major challenge in cognitive neuroscience. To examine these issues, we used a test of transitive inference pioneered by Piaget to assess capacities for systematic organization of knowledge and logical inference in children. In our adaptation of the test, rats were trained on a set of four overlapping odor discrimination problems that could be encoded either separately or as a single representation of orderly relations among the odor stimuli. Normal rats learned the problems and demonstrated the relational memory organization through appropriate transitive inferences about items not presented together during training. By contrast, after disconnection of the hippocampus from either its cortical or subcortical pathway, rats succeeded in acquiring the separate discrimination problems but did not demonstrate transitive inference, indicating that they had failed to develop or could not inferentially express the orderly organization of the stimulus elements. These findings strongly support the view that the hippocampus mediates a general declarative memory capacity in animals, as it does in humans.
Keywords: rats, entorhinal and perirhinal cortices, relational representation, declarative memory
Over a century ago, William James (1) characterized our capacity for conscious recollection as dependent on weaving experiences into systematic relations with each other, thus elaborating the access paths to memories beyond the route through repetition of the learning events. Modern efforts in cognitive neuroscience have shown that conscious recollection, observed in the capacity for “declarative” or explicit memory expression, is dependent on the hippocampal region in humans (2, 3). Progress in developing a valid animal model of conscious recollection is essential to identifying its neural mechanisms, but declarative or other explicit forms of memory expression are not directly observable in animals. To circumvent this obstacle, Cohen and Eichenbaum (4) suggested that studies on animals focus on two key properties of declarative memory that might be observable across species, specifically the ability to encode relations among to-be-remembered items and the capacity to express memories flexibly through inferences about items that are only indirectly related. Evidence to date concerning these capabilities in animals comes mainly from studies of spatial learning. Tolman’s (5) pioneering studies showed that rats form cognitive maps based on geometric relations among salient environment cues and that such representations support flexible, inferential expression in navigation by short cuts and roundabout routes. Subsequently, O’Keefe and Nadel (6) and others (7–10) identified a critical role for the hippocampus in spatial learning and memory. Furthermore Eichenbaum, Stewart, and Morris (11) showed that rats rely on hippocampal function for inferential expression of cognitive maps by navigation via novel routes. The observation that spatial learning and navigational inference depend on the hippocampus is consistent with the relational and inferential properties of declarative memory but leaves open the question of whether hippocampal function in animals is limited to spatial memory (12) or mediates these capacities across domains of information, as it is does in humans.
A method that can be used to directly address this issue is the test of transitive inference, previously used to assess human cognitive development. The term “transitive inference” signifies the ability to infer a relationship between items that have not been presented together, based on previous learning of a set of overlapping premises. For example, if presented with the premises “the blue rod is longer than the red rod” and “the red rod is longer than the green rod,” one can infer that the blue rod is longer than the green rod. That appropriate inferential judgment in this test is interpreted as prima facie evidence of the representation of orderly relations. The capacity for transitive inference is acquired in children by the age of 7 according to Piaget (13) and up to 3 years earlier if the ability to remember the premises has developed (14).
More recently, tests of transitive inference have been used to determine whether animals are capable of relational representation and inferential judgment (15). Subjects are first trained on a series of two-item discriminations called premise pairs (A > B, B > C, C > D, D > E; where each letter stands for a stimulus element and “>” describes the relationship “should be selected over”; Table 1). Each of the discriminations could be learned individually or represented as an orderly hierarchy that includes all five items (A > B > C > D > E). To examine which of the representations is actually used, animals are then given probe tests derived from pairs of nonadjacent elements, specifically B vs. D and A vs. E. An appropriate choice between the two nonadjacent and nonend elements, B and D, provides unambiguous evidence for transitive inference. Conversely, the choice between end elements A and E can be entirely guided by the independent reinforcement histories of these elements because choices of A during premise training are always rewarded and choices of E are never rewarded. Thus, the combination of the probe tests B vs. D and A. vs. E provide the strongest assessment of capacities for making novel judgments guided by inferential expression of the orderly organization or by reward history of the individual elements, respectively.
Table 1.
Stages of training and probe tests
| Premise pair training |
| A > B |
| B > C |
| C > D |
| D > E |
| Ordered representation |
| A > B > C > D > E |
| Probe tests |
| B vs. D: test of transitivity |
| A vs. E: nontransitive novel pairing |
Anatomical Structures Important for Declarative Memory.
Another area of controversy concerns which structures within the hippocampal region, that is, the hippocampus itself plus adjacent perirhinal and entorhinal (PRER) cortices, are critical to memory. Recent studies on simple recognition memory have shown that selective damage to the hippocampus itself or to its subcortical connections via the fornix results in little or no memory deficit whereas severe memory deficits are observed when the damage includes the cortical regions adjacent to the hippocampus in both rats (16, 17) and monkeys (18–20). These findings have led some to suggest that the hippocampus itself plays either a relatively unimportant role in memory (20) or a role limited to spatial memory (6, 10) whereas the adjacent parahippocampal cortex plays a broader role, including nonspatial declarative memory. Interpreting these findings differently, we have suggested that the importance of the hippocampus itself in nonspatial memory can be demonstrated in tests in which the memory demands exceed that of simple recognition or acquisition of biases toward individual stimuli and instead require memory for stimulus relations and flexible, inferential memory expression (11, 21). Such a demonstration would show that damage limited to the hippocampus results in a broad scope of memory impairment in animals, as it does in humans (22). In the present experiment, we compared the performance of normal rats on an odor-guided transitive inference test with rats that had a transection of the fornix or removal of the adjacent PRER cortices, which mediate hippocampal connections with the neocortex. The hypothesis that the parahippocampal cortex is more important to nonspatial memory would be supported if the deficit was substantially greater after perirhinal plus entorhinal ablation than fornix transection. Conversely, severe and equivalent deficits after either type of lesion would suggest that disconnection of the hippocampus itself is sufficient to eliminate the relational processing functions of this system (23).
METHODS
Subjects.
Subjects were 26 male Long–Evans hooded rats (Charles River Laboratories) that were individually housed in an environmentally controlled room in which a 12:12 h light-to-dark schedule was maintained. Throughout the course of the experiment, water was available to the subjects ad libitum with access to food limited to rewards received during the experiment and a daily ration of 19–25 g of rat chow.
Surgical Procedures.
For ablations of the perirhinal and entorhinal cortices (PRER group), seven subjects were anesthetized and placed in a custom-designed head holder that allowed unobstructed access to the temporal surface of the skull. After craniotomy, the underlying PRERs were aspirated using a blunt, curved 19-gauge needle. For transections of the fornix (FX group), nine other subjects were anesthetized, a small craniotomy was made over the parietal cortex, and an electrode was lowered to the following coordinates relative to bregma bilaterally: AP = −0.3, ML = 0.7, DV = 4.2, 4.0, AP = −0.8, ML = 1.7, DV = 4.4, 4.0, plus a single oblique penetration (10° to medial) at AP = −0.3, ML = 0.7, DV = 4.4, 4.2. Radiofrequency lesions were made by raising the temperature of the electrode tip to 70°C for 60 s using the RFG4-A lesion maker (Radionics, Burlington, MA). In addition, five subjects had the lateral craniotomy but no aspiration, two had the midline craniotomy but no electrode penetration, and three were unoperated. Because there were no significant performance differences among these subgroups, their data were combined in the analyses below and they were designated as the control group.
Apparatus and Shaping.
During initial shaping, rats were provided with a 4-oz Nalgene plastic cup (6.4 cm high × 6.2 cm diameter) filled with 110 g of sand and mounted onto a Plexiglas base (6.5 × 3.5 in). A cup was baited with several food rewards (Froot Loops, Kellogg’s, Battle Creek, MI) that were partially buried in and on top of the sand, placed in one end of the rat’s home cage, and then removed when no rewards were visible. Subsequently, subjects were presented with unscented sand in two cups mounted onto the Plexiglas base, and only one cup contained food rewards. Pretraining continued with a simple odor discrimination task in which the discriminative stimuli consisted of common food spices mixed into the sand. On each of two 10-trial sessions, subjects were presented with two cups of sand; one containing celery was baited with a single buried reward, and the other containing thyme was unbaited. A choice response was defined as the first cup in which a subject began to dig although the rat was permitted to explore both cups until the reward was collected. To prevent the adoption of spatial biases, the relative left–right positions of the cups and the placement of the pair of cups among three locations within the home cage were randomized.
Stimuli.
After the preliminary odor discrimination experience, subjects were fully trained on a new set of overlapping odor discriminations that formed the premises for subsequent transitive inference testing. Each odor stimulus was comprised of 1 g of a common household spice mixed with 110 g of clean sand that was presented in a 4-oz plastic cup. On every trial, two cups with different odors were mounted 1–2 cm apart onto a Plexiglas base. The stimulus assignments were identical for all subjects and are identified here by alphabetic characters used henceforth: A, paprika; B, coffee; C, basil; D, cumin; and E, cocoa. The specific stimulus combinations presented were: AB, BC, CD, and DE, where the first of each pairing designates the rewarded stimulus in that premise pair.
Premise Training.
Initial daily training sessions included presentation of blocks of trials on each premise pair, with the trial blocks presented in serial order AB, BC, CD, and then DE. During phase 1, a 10-trial block of the AB problem was presented, followed by 10 BC trials, then 10 CD trials, and then 10 DE trials. This sequence was repeated on each daily session until subjects responded correctly on 8 of the 10 trials (80%) on each premise pair or until they failed to reach the criterion in 10 sessions. Training phases 2–4 involved progressive decreases in the number of trials per block on the same problem. In phase 2, five trials were presented on each block of AB, BC, CD, and DE problems, and then the sequence was repeated once each day until the same criterion was met. In phase 3, three trials were presented in each block, and the sequence was repeated three times, for a total of nine daily presentations of each premise pair, and the criterion was seven correct responses on each pair (78%) for this and the next phase. In phase 4, each premise pair was presented for only one trial per block, and the entire ordered sequence was repeated nine times daily. Finally, in phase 5, the four premise pairs were presented in pseudorandom order, and the criterion was 14 (78%) correct responses of 18 repetitions of each premise pair across two consecutive sessions.
A few animals in each group (three FX, one PRER, two control) failed to attain the performance criterion during one of the training phases within the 10-session limit. In each case, performance could be characterized either as an intractable preference for a particular odor or as the inability to simultaneously reach the criterion on all of the premise pairs. Training of these subjects was discontinued, and their data were excluded from all statistical analyses.
Probe Tests.
Transitive inference was tested with 10 presentations of BD. In addition, to assess the selectivity of the impairment on required transitive judgments, 10 AE trials also were presented during the same test period. To maintain vigorous performance on BD and AE probe tests, rewards were assigned to B and A, respectively, consistent with the hierarchical stimulus ordering. To minimize new learning of these reward assignments during the course of repetitions, probe trials were spaced widely; on each of five daily 40-trial sessions, the BD pair was presented on trials 8 and 26 and the AE pair on trials 16 and 34 or vice versa, with nine presentations of each premise pair randomly intermixed among the intervening trials. As an additional control for the possibility of new learning, a second set of five test sessions involved the identical protocol except that two new odor pairs (WX and YZ, where W = ginger, X = garlic, Y = anise, and Z = cinnamon) were substituted for BD and AE, respectively. These sessions provided an objective measure of the rate of acquisition of novel odor associations presented in highly spaced trials.
Histological Analysis.
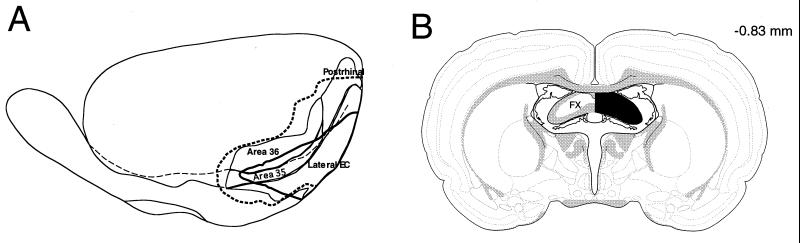
After completion of the behavioral testing, rats were perfused with a 0.9% saline solution followed by 10% formalin. In PRER subjects, the extent of the damage visible on the cortical surface was reconstructed onto templates based on the designations of Burwell et al. (ref. 24; Fig. 1A). In all six PRER rats, there was nearly complete bilateral ablation of the lateral entorhinal cortex (mean removal = 92.8%), substantial damage to the perirhinal cortex (mean removal = 71.2%), and minimal damage to the postrhinal cortex (mean removal = 14.8%). In addition, each brain then was sectioned (50 μm) in the coronal plane, and every fifth section was stained with thionin. Analysis of these sections confirmed nearly complete ablations of the lateral entorhinal and perirhinal regions, severe bilateral damage to the caudal portions of the medial entorhinal cortex in all but one rat who had some unilateral sparing, and moderate-to-severe bilateral damage in postrhinal cortex. In some subjects, there was variable damage in ventral CA1 and subiculum, adjacent neocortical regions, and the amygdala, but there was no relationship between the extent of this extraneous damage and transitive inference performance.
Figure 1.
(A) Lateral reconstruction of the smallest (solid line) and largest (dashed line) of the PRER ablations; areas 35 and 36 collectively constitute the perirhinal cortex. This figure is based on coordinates used by Burwell, Amaral and Witter (24). (B) Coronal reconstruction of the FX transection (right hemisphere) and intact brain region (left hemisphere). (Figure based on ref. 25.)
Brains of FX rats were sectioned coronally (30 μm), and adjacent sections were stained with thionin and for acetylcholinesterase (AChE) activity. In four rats, the FX transection was complete (ref. 25; Fig. 1B), and reduced AChE staining was observed throughout the hippocampus. In two other FX subjects, the transection was incomplete, and positive staining for AChE was observed throughout the hippocampus. These subjects were removed from the FX group, and their behavioral data are considered separately below.
RESULTS
Preliminary Odor Discrimination.
Animals from all three groups readily acquired the preliminary odor discrimination. On the second day of training, control rats made an average of 8.5 correct choices of 10, and the PRER and FX subjects made averages of 9.7 and 9.6 correct responses, respectively. ANOVA revealed no significant group differences [F(2, 15) = 2.003; P > 0.10].
Premise Pair Training.
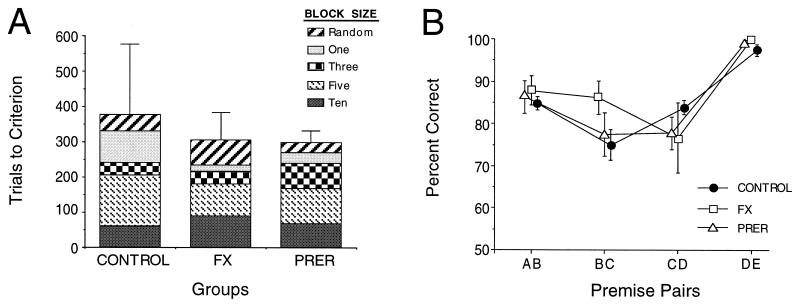
Control subjects, as well as PRER and FX rats, achieved criterion performance on each training phase very rapidly (Fig. 2A). In addition, control as well as PRER and FX rats readily reached criterion with randomly presented premise pairs (phase 5), and an ANOVA revealed no significant group difference [F(2, 15) = 0.760; P > 0.10] on this phase. A repeated measures ANOVA for all training phases revealed no significant group differences [F(2, 15) = 0.622; P > 0.10] nor differences in performance across phases [F(4, 60) = 2.304; P > 0.06] nor any significant difference among group performance across phases (group × phase interaction: F(8, 60) = 0.664; P > 0.10).
Figure 2.
(A) The mean number of trials required to reach the criterion for each phase of premise training. Error bars represent SE above the mean. (B) Mean response accuracy (±SE) on each of the four premise pairs during the test sessions.
Probe Testing.
Controls as well as PRER and FX rats continued to perform well on the premise pairs during the test sessions (Fig. 2B). All groups demonstrated a serial position curve such that performance was best on pairs that included one of the end items (26). Repeated measures ANOVA indicated a significant difference in the performance across premise pairs [F(3, 45) = 31.730; P < 0.0001], but no significant differences among groups [F(2, 15) = 0.283 P > 0.10], and no differences in group performance across premise pairs [group × pair interaction: F(6, 45) = 1.761; P > 0.10)]. Post hoc (Scheffé) comparisons confirmed that overall performance was more accurate on pairs that included an end item (AB and DE) than on pairs created entirely from inner items (BC and CD) (all Ps < 0.05). A more detailed examination of separate post hoc comparisons for each group indicated that performance on DE was consistently superior to that on all other premise pairs (all Ps < 0.05), except in FX rats, in whom superiority on DE over CD was only marginally significant (P = 0.07). However, performance on AB did not differ significantly from that on CD in any group (all Ps > 0.1) nor from that on BC (Ps > 0.1), except in controls, in whom superiority of AB over BC was marginal (P = 0.053).
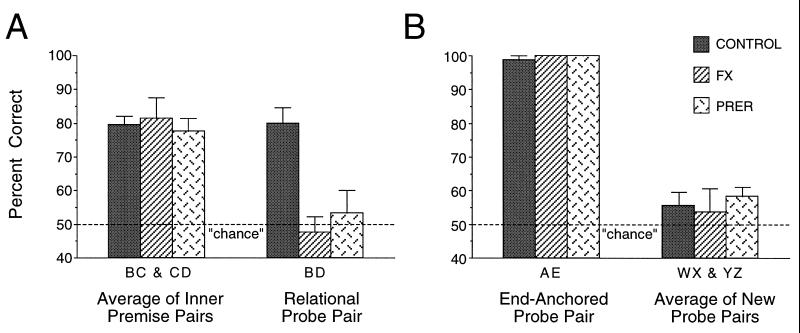
On the critical BD probe test, control subjects demonstrated robust transitive inference. Their performance on BD trials significantly exceeded chance level [t(7) = 6.481; P < 0.001)] and was not different from their performance on premise pairs that included items B and D (P > 0.10) (see Fig. 3A). In striking contrast, the FX and PRER groups did not perform better than chance on the BD probe (Ps > 0.10). Likewise, the performance of the FX and PRER groups on the BD pair was significantly lower than their performance on BC and CD pairs (Ps < 0.01). ANOVA confirmed a significant group difference in performance [F(2, 15) = 10.20; P < 0.001] on BD trials. Post hoc comparisons indicated that control performance was better than that of both the PRER (P < 0.01) and FX (P < 0.01) groups, which did not significantly differ from each other (P > 0.10). In addition, by contrast to the performance of other FX subjects, the two subjects removed from the FX group because of ineffective lesions performed within the range of control performance on BD (70 and 80% correct).
Figure 3.
(A) Mean response accuracy (+SE) for the average performance on premise pairs BC and CD and for the critical test pair BD during the test sessions. (B) Response accuracy (+SE) for control probe pair AE and the average response accuracy for the new control pairs (WX and YZ).
A further analysis of transitivity examined performance on the very first presentation of the BD pair, which may be considered a “pure” test of inferential responding uncontaminated by reinforcement on repeated probe trials. Of control subjects, 88% choose correctly on the first BD presentation (binomial P < 0.05) whereas only 50% of the FX and PRER subjects were successful on the initial BD judgment (binomial P > 0.10).
Analyses of performance on other types of probe trials demonstrated the selectivity of the deficit in transitive inference in rats with hippocampal region damage. All rats performed extremely well on the AE trials, which can be solved without a transitive judgment (Fig. 3B), and there was no significant group difference in performance on this problem [ANOVA: F(2, 15) = 0.595; P > 0.10]. Conversely, all groups showed minimal evidence of learning during presentations of the new odor pairs (WX and YZ). Combining the performance across both pairs, performance did not rise above chance levels in control subjects (P > 0.10) or FX rats (P > 0.10). PRER subjects performed significantly [t(5) = 2.988; P < 0.05], albeit slightly, better than chance (Fig. 3B). ANOVA confirmed that there were no significant group differences on new probe performance [F(2, 15) = 0.221; P > 0.10]. Furthermore, controls performed significantly better on BD trials than on new odor pairs [t(7) = 3.529; P < 0.01] whereas PRER and FX rats did not (Ps > 0.10). The contrast between robust performance on BD over new odor pairs in control rats strongly indicates that their judgments on the BD pairs reflected inferential capacity. Conversely, the absence of a significant difference on this comparison, combined with intact performance on the AE pair, emphasizes the selective loss of the capacity for transitive inference in FX and PRER rats.
DISCUSSION
Previous studies have demonstrated that several animal species can learn an overlapping series of discrimination problems and demonstrate a capacity for transitive inference (15, 26–32). The present results identify the hippocampal region as critical to transitive inference and indicate that the hippocampus plays a critical role in the development or flexible expression of a representation of orderly relations among stimulus items. Conversely, the finding that animals with hippocampal damage could still succeed in acquiring the overlapping odor discriminations indicates that nonrelational strategies can support learning a set of conditional reward contingencies, e.g., B is rewarded only in the presence of C, albeit without development of a single orderly representation of all the items. Conditional reward assignments could be mediated by “configural” associations that represent each stimulus pairing as a unique compound cue with an associated reward contingency (33, 34). However, this type of representation would not be expected to support accurate responding on probe stimulus configurations that had no reward history, consistent with the failure of rats with hippocampal region damage in the transitive inference test. In contrast, by virtue of their capacity for transitive inference, it can be concluded that normal rats relied on their hippocampus to form or express an orderly, relational organization that incorporated all of the odor stimuli.
Couvillon and Bitterman (35), in interpreting Fersen and colleagues’ (30) earlier demonstration of transitive capacity in intact animals, suggested that the selection of B over D could be supported simply by unequal cumulative reward associations for these stimuli. In that study, as in the present experiment, B and D received equal increments in reward strength on AB and CD trials, respectively, because animals were rewarded for an eventually correct response on every trial. However, in the Fersen et al. (30) study, performance on AB trials during testing was better than that on CD trials, so the reward strength for B would be decremented unequally by fewer nonrewarded responses to B (on AB error trials) than that to D (on CD error trials). This could have led to a greater cumulative reward strength for B than for D that could mediate the preference without need for a relational representation. However, unlike the Fersen et al. (30) study, in the present experiment performance on AB trials during testing did not significantly differ from that on CD trials (Fig. 2B), indicating that reward–association strengths would be equal for B and D, and so transitive performance in normal animals cannot have arisen secondary to differential reinforcement histories. Superior performances by intact animals on DE trials (Fig. 2B), as well as on AE probes (Fig. 3B), may well be attributed to a very low reward strength for E and a high reward strength for A, showing that influences of reward association can be observed in this protocol, and indeed these effects are fully present in rats with hippocampal damage in tests that do not require a transitive judgment. Taken together, the pattern of findings across premise and probe trials is consistent with our conclusion that normal rats develop and can flexibly express a representation of orderly relations among odor memories. Conversely, rats with hippocampal damage base their success in acquiring the premise pairs, as well as performing AE, on cumulative reinforcement histories for responses to each premise item or specific pairing, a strategy that does not support appropriate BD judgments in this study.
The present finding that focuses on expression of memory for orderly stimulus relations extends the domain of hippocampal function in animals beyond simple “associations” between stimuli, as recently demonstrated by Bunsey and Eichenbaum (21). In their study, rats initially learned two sets of odor-paired associates that shared common elements (e.g., A-B and X-Y then B-C and Y-Z) and were then tested for the capacity to infer indirect relationships between items not explicitly paired (A-C and X-Z). Normal rats, as well as rats with selective hippocampal lesions, readily acquired the paired associates, and normal subjects demonstrated the associative inference between indirectly related items. However, rats with hippocampal lesions showed no inferential capacity, implicating the hippocampus in mediating representations that link indirectly associated elements in memory and in the expression of these associations through inferential judgments. The present observations allow us to extend this hippocampal-dependent capacity to the representation of orderly relationships among stimulus elements within a larger memory network and to the expression of associative inferences based on logical relations in the network organization. This view of hippocampal function is entirely consistent with the characteristics of cognitive mapping described by Tolman (5) and identified with hippocampal function by O’Keefe and Nadel (6). Furthermore, the present data extend the properties of cognitive mapping and their mediation by hippocampal mechanisms to nonspatial dimensions of memory organization in animals, indicating that the role of the hippocampal region in declarative memory expression is global in rats as it is in humans (36). In addition, just as our tests of transitive inference have provided a bridge between general relational and specific spatial memory functions of the hippocampus in rats, tests of flexible and inferential memory capacity in humans could offer the best connection between relational processing capacity in animals and conventional assessments of declarative memory in humans (37).
Finally, the present findings also address the question of which components of the hippocampal region are critical to memory. Here we observed a deficit in inferential memory expression that was total after either the FX transection, which disconnects hippocampal–subcortical pathways, or the ablation of the parahippocampal region, which disconnects hippocampal–cortical pathways. This pattern of findings implicates the hippocampus itself as critical to transitive inference although, because some connections of the parahippocampal region pass through the FX, it is possible that our FX lesions compromised memory functions primarily mediated by the parahippocampal region. However, this account would not be consistent with other functional dissociations, indicating that parahippocampal damage, but not FX or hippocampal damage, results in a deficit on recognition memory performance (16–20). By contrast, the combination of these and other findings is consistent with a suggested parcellation of functions mediated by hippocampal region structures (23). Although the parahippocampal region may play a unique role in recognition memory, the hippocampus itself is critical to the memory processing that underlies relational organization and declarative memory expression.
Acknowledgments
We thank J. Neidermaier for histological assistance, P. Rapp for use of imaging equipment, and R. Burwell for assistance with lateral characterization of the perirhinal and entorhinal ablations. This work was supported by grants from the National Institutes of Mental Health and the Office for Naval Research (HE). The current study was submitted to the Department of Psychology at the State University of New York-Stony Brook in partial fulfillment of the Doctor of Philosophy degree (JAD).
ABBREVIATIONS
- PRER
perirhinal and entorhinal cortices
- FX
fornix
- A
paprika
- B
coffee
- C
basil
- D
cumin
- E
cocoa
- W
ginger
- X
garlic
- Y
anise
- Z
cinnamon
References
- 1.James W. The Principles of Psychology. New York, Holt: Holt; 1890. , 1918 Ed. [Google Scholar]
- 2.Cohen N J, Squire L R. Science. 1980;141:826–827. [Google Scholar]
- 3.Schacter D L. J Exp Psychol Learn Mem Cognit. 1987;13:501–508. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- 4.Cohen N J, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- 5.Tolman E C. Psychol Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- 6.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Oxford Univ. Press; 1978. [Google Scholar]
- 7.Olton D S, Samelson R J. J Exp Psychol Anim Behav Processes. 1976;2:97–116. [Google Scholar]
- 8.Morris R G M, Garrud P, Rawlins J N P, O’Keefe J. Nature (London) 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 9.Kesner R P. Hippocampus. 1991;1:279–282. doi: 10.1002/hipo.450010316. [DOI] [PubMed] [Google Scholar]
- 10.Jarrard L E. Behav Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- 11.Eichenbaum H, Stewart C, Morris R G M. J Neurosci. 1990;10:2531–2542. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadel L. Hippocampus. 1991;1:221–229. doi: 10.1002/hipo.450010302. [DOI] [PubMed] [Google Scholar]
- 13.Piaget J. Judgment and Reasoning in the Child. Paul, Trench, and Trubner, London: Kegan; 1928. [Google Scholar]
- 14.Bryant P, Trabasso T. Nature (London) 1971;232:456–458. doi: 10.1038/232456a0. [DOI] [PubMed] [Google Scholar]
- 15.McGonigle B O, Chalmers M. Nature (London) 1977;267:694–696. doi: 10.1038/267694a0. [DOI] [PubMed] [Google Scholar]
- 16.Mumby D G, Wood E R, Pinel J P J. Psychobiology. 1992;20:18–27. [Google Scholar]
- 17.Otto T, Eichenbaum H. Hippocampus. 1992;2:323–334.40. doi: 10.1002/hipo.450020310. [DOI] [PubMed] [Google Scholar]
- 18.Meunier M, Bachevalier J, Mishkin M, Murray E A. J Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaffan D. Exp Brain Res. 1994;99:411–422. doi: 10.1007/BF00228977. [DOI] [PubMed] [Google Scholar]
- 20.Zola-Morgan S, Squire L R, Ramus S J. Hippocampus. 1994;4:483–495. doi: 10.1002/hipo.450040410. [DOI] [PubMed] [Google Scholar]
- 21.Bunsey M, Eichenbaum H. Nature (London) 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- 22.Zola-Morgan S, Squire L R, Amaral D G. J Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eichenbaum H, Otto T, Cohen N J. Behav Brain Sci. 1994;17:449–517. [Google Scholar]
- 24.Burwell R D, Witter M P, Amaral D G. Hippocampus. 1995;5:390–408. doi: 10.1002/hipo.450050503. [DOI] [PubMed] [Google Scholar]
- 25.Swanson L W. Brain Maps: Structures of the Rat Brain. New York: Elsevier; 1992. [Google Scholar]
- 26.McGonigle B O, Chalmers M. Q J Exp Psychol Comp Physiol Psychol B. 1992;45:189–228. [Google Scholar]
- 27.Rapp P R, Kansky M T, Eichenbaum H. Behav Neurosci. 1996;110:887–897. doi: 10.1037//0735-7044.110.5.887. [DOI] [PubMed] [Google Scholar]
- 28.Gillan D J. J Exp Psychol Anim Behav Processes. 1981;7:150–164. [Google Scholar]
- 29.Boysen S T, Berntson G G, Shreyer T A, Quigley K S. J Comp Psychol. 1993;107:208–215. doi: 10.1037/0735-7036.107.2.208. [DOI] [PubMed] [Google Scholar]
- 30.Fersen L V, Wynne C D, Delius J D, Staddon J E. J Exp Psychol Anim Behav Processes. 1991;17:334–341. doi: 10.1037//0097-7403.17.3.281. [DOI] [PubMed] [Google Scholar]
- 31.Davis H. J Comp Psychol. 1992;106:342–349. doi: 10.1037/0735-7036.106.4.342. [DOI] [PubMed] [Google Scholar]
- 32.Roberts W A, Phelps M T. Psychological Sci. 1994;5:368–374. [Google Scholar]
- 33.Rescorla R A. J Comp Physiol Psychol. 1972;85:307–317. doi: 10.1037/h0032553. [DOI] [PubMed] [Google Scholar]
- 34.Sutherland R J, Rudy J W. Psychobiology. 1989;17:129–144. [Google Scholar]
- 35.Couvillon P A, Bitterman M E. J Exp Psychol. 1992;18:308–310. [Google Scholar]
- 36.Scoville W B, Milner B. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reber P J, Knowlton B J, Squire L R. Behav Neurosci. 1996;110:861–871. doi: 10.1037//0735-7044.110.5.861. [DOI] [PubMed] [Google Scholar]