Abstract
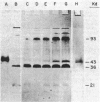
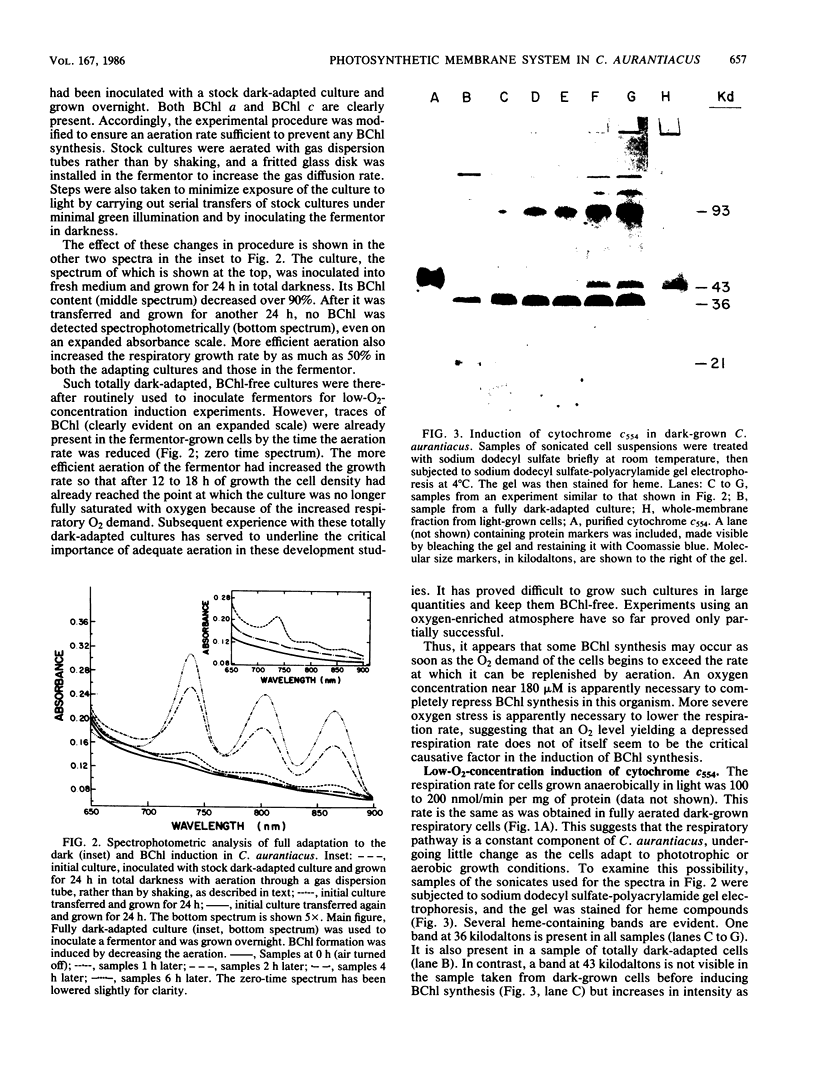
Oxygen levels which control induction of the assembly of the pigment-protein photosynthetic polypeptides in dark-grown Chloroflexus aurantiacus were determined. The induction signal by low-oxygen tension is not directly related to the respiratory competence of these photosynthetic cells. Cytochrome c554, the primary electron donor to P865+ of the reaction center, is not present in dark-grown respiratory cells but is induced in parallel with bacteriochlorophylls a and c and at similar oxygen partial pressure. The development of these components of the photosynthetic apparatus and its electron transport chain is completely independent of the presence of any detectable light or bacteriochlorophyll c or a pigments in C. aurantiacus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blankenship R. E., Feick R., Bruce B. D., Kirmaier C., Holten D., Fuller R. C. Primary photochemistry in the facultative green photosynthetic bacterium Chloroflexus aurantiacus. J Cell Biochem. 1983;22(4):251–261. doi: 10.1002/jcb.240220407. [DOI] [PubMed] [Google Scholar]
- Bruce B. D., Fuller R. C., Blankenship R. E. Primary photochemistry in the facultatively aerobic green photosynthetic bacterium Chloroflexus aurantiacus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6532–6536. doi: 10.1073/pnas.79.21.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J., Donohue T. J., Varga A. R., Staehelin L. A., Kaplan S. Induction of the photosynthetic membranes of Rhodopseudomonas sphaeroides: biochemical and morphological studies. J Bacteriol. 1984 Aug;159(2):540–554. doi: 10.1128/jb.159.2.540-554.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. G., Davidson E., Marrs B. L. Variation of levels of mRNA coding for antenna and reaction center polypeptides in Rhodopseudomonas capsulata in response to changes in oxygen concentration. J Bacteriol. 1984 Mar;157(3):945–948. doi: 10.1128/jb.157.3.945-948.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feick R. G., Fitzpatrick M., Fuller R. C. Isolation and characterization of cytoplasmic membranes and chlorosomes from the green bacterium Chloroflexus aurantiacus. J Bacteriol. 1982 May;150(2):905–915. doi: 10.1128/jb.150.2.905-915.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Saunders V. A., Jones O. T. Properties of the cytochrome a-like material developed in the photosynthetic bacterium Rhodopseudomonas spheroides when grown aerobically. Biochim Biophys Acta. 1974 Mar 26;333(3):439–445. doi: 10.1016/0005-2728(74)90128-5. [DOI] [PubMed] [Google Scholar]
- Sprague S. G., Staehelin L. A., DiBartolomeis M. J., Fuller R. C. Isolation and development of chlorosomes in the green bacterium Chloroflexus aurantiacus. J Bacteriol. 1981 Sep;147(3):1021–1031. doi: 10.1128/jb.147.3.1021-1031.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. E., Ryan D., Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976 Sep;75(1):168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]