Abstract
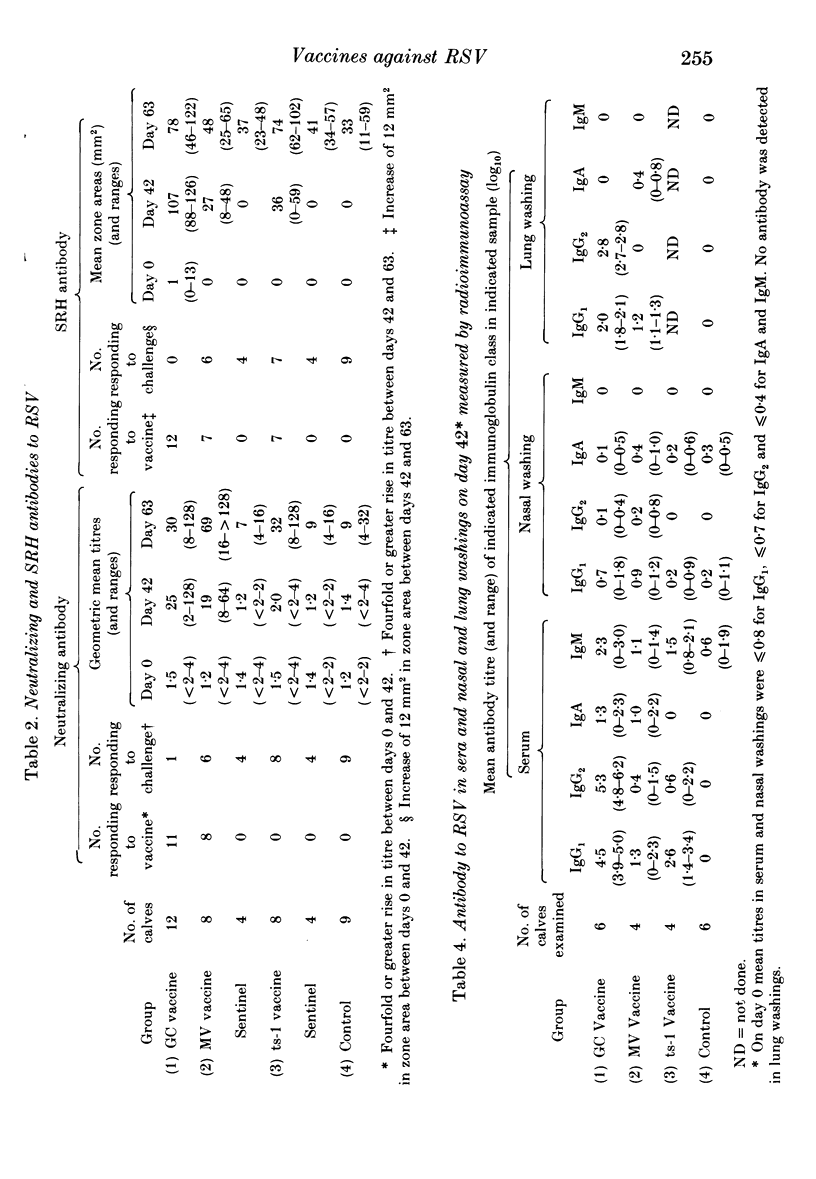
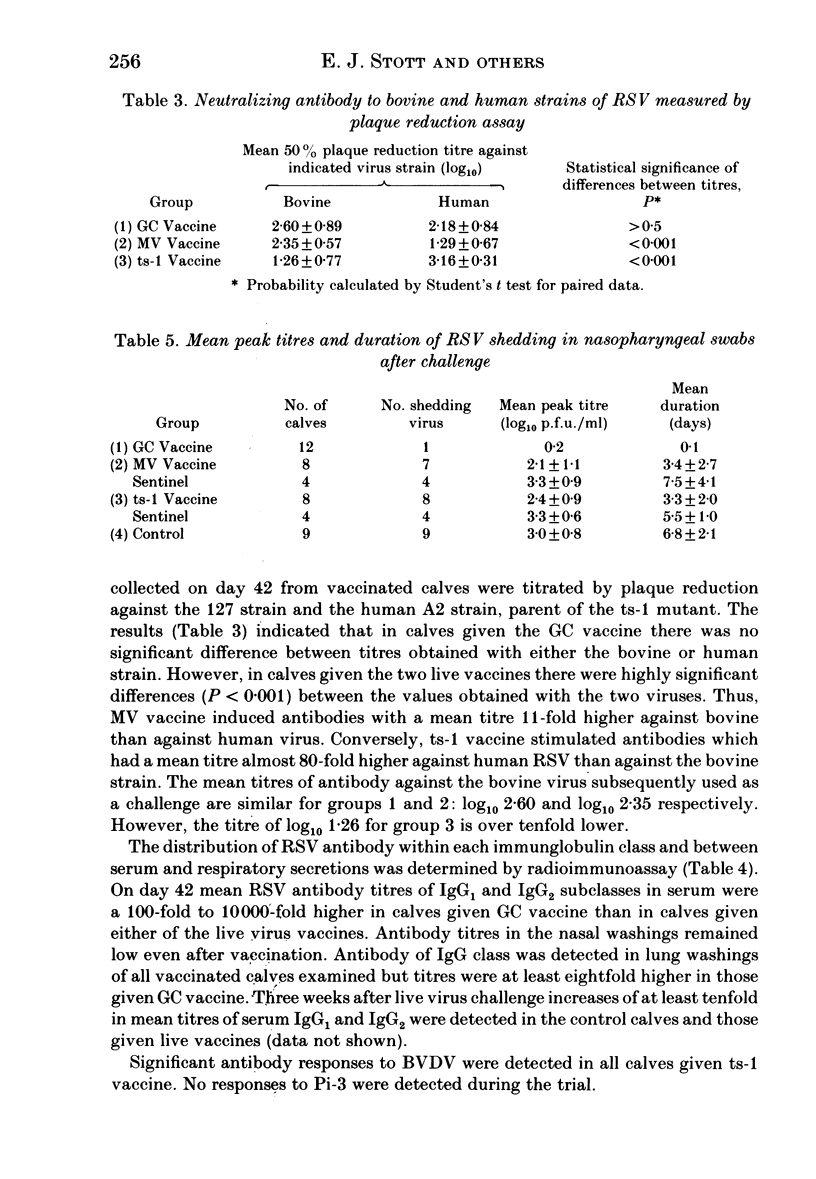
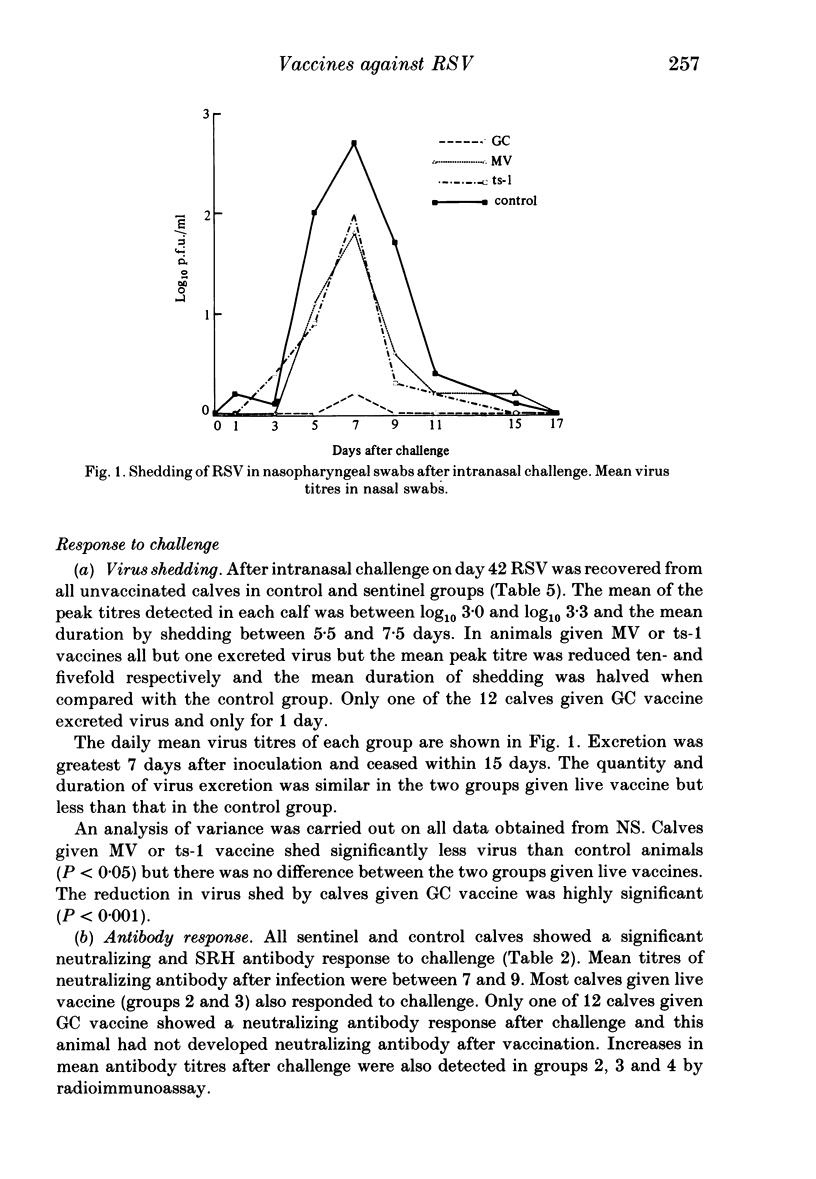
An inactivated vaccine against respiratory syncytial virus (RSV) was compared with two live vaccines. The inactivated (GC) vaccine consisted of glutaraldehyde-fixed bovine nasal mucosa cells persistently infected with RSV and emulsified with oil adjuvant. The live vaccines were a modified virus (MV) derived from a bovine strain of RSV and a temperature-sensitive mutant (ts-1) derived from a human strain. The GC vaccine was inoculated subcutaneously into 12 calves and the live vaccines intramuscularly into eight calves each. Nine unvaccinated calves acted as controls. The vaccines were administered in two doses 3 weeks apart and all calves were challenged intranasally with 2 X 10(7) p.f.u. of bovine RSV 3 weeks after the second dose. At the time of challenge calves given GC, MV and ts-1 vaccines had mean serum neutralizing antibody titres of 25, 19 and 2 respectively; mean titres of IgG1 antibody by radioimmunoassay were log10 4.5, 1.3 and 2.6 respectively and mean zone areas by single radial haemolysis (SRH) were 107, 27 and 36 mm2 respectively. Eleven of 12 calves given GC vaccine were completely protected against challenge but all control animals and those given the two live vaccines were infected. The mean peak titre of virus in nasal swabs of control calves was 3.0 log10 p.f.u./ml and the mean duration of virus shedding was 6.8 days. Both these parameters were significantly reduced in animals given MV and ts-1 vaccines: mean peak titres were 2.1 and 2.4 log10 p.f.u./ml and mean duration of shedding was 3.4 and 3.3 days respectively. Thus, protection correlated better with RSV antibody detected by radioimmunoassay and SRH than with neutralizing antibody. These results are discussed in relation to the possible mechanism by which protection was mediated.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beem M. Repeated infections with respiratory syncytial virus. J Immunol. 1967 Jun;98(6):1115–1122. [PubMed] [Google Scholar]
- Belshe R. B., Van Voris L. P., Mufson M. A. Parenteral administration of live respiratory syncytial virus vaccine: results of a field trial. J Infect Dis. 1982 Mar;145(3):311–319. doi: 10.1093/infdis/145.3.311. [DOI] [PubMed] [Google Scholar]
- Buynak E. B., Weibel R. E., McLean A. A., Hilleman M. R. Live respiratory syncytial virus vaccine administered parenterally. Proc Soc Exp Biol Med. 1978 Apr;157(4):636–642. doi: 10.3181/00379727-157-40112. [DOI] [PubMed] [Google Scholar]
- CHANOCK R. M., PARROTT R. H. ACUTE RESPIRATORY DISEASE IN INFANCY AND CHILDHOOD: PRESENT UNDERSTANDING AND PROSPECTS FOR PREVENTION. Pediatrics. 1965 Jul;36:21–39. [PubMed] [Google Scholar]
- Fernie B. F., Gerin J. L. Immunochemical identification of viral and nonviral proteins of the respiratory syncytial virus virion. Infect Immun. 1982 Jul;37(1):243–249. doi: 10.1128/iai.37.1.243-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharpure M. A., Wright P. F., Chanock R. M. Temperature-sensitive mutants of respiratory syncytial virus. J Virol. 1969 Apr;3(4):414–421. doi: 10.1128/jvi.3.4.414-421.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grist N. R., Ross C. A., Stott E. J. Influenza, respiratory syncytial virus, and pneumonia in Glasgow, 1962-5. Br Med J. 1967 Feb 25;1(5538):456–457. doi: 10.1136/bmj.1.5538.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. B. Prevention of infections with respiratory syncytial virus: the hopes and hurdles ahead. Rev Infect Dis. 1980 May-Jun;2(3):384–392. doi: 10.1093/clinids/2.3.384. [DOI] [PubMed] [Google Scholar]
- Henderson F. W., Collier A. M., Clyde W. A., Jr, Denny F. W. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979 Mar 8;300(10):530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- Holzhauer C., Van Nieuwstadt A. P. De Etiologische Rol Van Het Bovine Respiratory Syncytial Virus Bij Pinkengriep. Voorlopige mededeling. Tijdschr Diergeneeskd. 1976 Sep 15;101(18):1023–1031. [PubMed] [Google Scholar]
- Kapikian A. Z., Mitchell R. H., Chanock R. M., Shvedoff R. A., Stewart C. E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969 Apr;89(4):405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- McClelland A. J. Vaccination against paramyxoviruses. Nature. 1980 Apr 3;284(5755):404–404. doi: 10.1038/284404a0. [DOI] [PubMed] [Google Scholar]
- McNulty M. S., Bryson D. G., Allan G. M. Experimental respiratory syncytial virus pneumonia in young calves: microbiologic and immunofluorescent findings. Am J Vet Res. 1983 Sep;44(9):1656–1659. [PubMed] [Google Scholar]
- Mohanty S. B., Lillie M. G., Ingling A. L. Effect of serum and nasal neutralizing antibodies on bovine respiratory syncytial virus infection in calves. J Infect Dis. 1976 Oct;134(4):409–413. doi: 10.1093/infdis/134.4.409. [DOI] [PubMed] [Google Scholar]
- Nuttall P. A., Luther P. D., Stott E. J. Viral contamination of bovine foetal serum and cell cultures. Nature. 1977 Apr 28;266(5605):835–837. doi: 10.1038/266835a0. [DOI] [PubMed] [Google Scholar]
- Nuttall P. A., Stott E. J., Thomas L. H. Experimental infection of calves with two strains of bovine virus diarrhoea virus: virus recovery and clinical reactions. Res Vet Sci. 1980 Jan;28(1):91–95. [PubMed] [Google Scholar]
- Powell P. C. Immunity to Marek's disease induced by glutaraldehyde-treated cells of Marek's disease lymphoblastoid cell lines. Nature. 1975 Oct 23;257(5528):684–685. doi: 10.1038/257684a0. [DOI] [PubMed] [Google Scholar]
- Prince G. A., Potash L., Horswood R. L., Camargo E., Suffin S. C., Johnson R. A., Chanock R. M. Intramuscular inoculation of live respiratory syncytial virus induces immunity in cotton rats. Infect Immun. 1979 Mar;23(3):723–728. doi: 10.1128/iai.23.3.723-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probert M., Russell S. M. Measurement of parainfluenza-3 virus antibody by the single radial hemolysis technique. J Clin Microbiol. 1975 Sep;2(3):157–161. doi: 10.1128/jcm.2.3.157-161.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott E. J., Thomas L. H., Collins A. P., Crouch S., Jebbett J., Smith G. S., Luther P. D., Caswell R. A survey of virus infections of the respiratory tract of cattle and their association with disease. J Hyg (Lond) 1980 Oct;85(2):257–270. doi: 10.1017/s0022172400063294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. Solid-phase micro-radioimmunoassay to measure immunoglobulin class-specific antibody to Mycoplasma pulmonis. Infect Immun. 1979 Jun;24(3):701–706. doi: 10.1128/iai.24.3.701-706.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Bew M., Fernie B. F., Cote P. J., Collins A. P., Hughes M., Jebbett J. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology. 1984 May;52(1):137–142. [PMC free article] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Bew M., Fernie B. F., Cote P. J. Monoclonal antibodies protect against respiratory syncytial virus. Lancet. 1983 Oct 22;2(8356):976–976. doi: 10.1016/s0140-6736(83)90499-3. [DOI] [PubMed] [Google Scholar]
- Thomas L. H., Slott E. J., Collins A. P., Jebbett J. Experimental pneumonia in gnotobiotic calves produced by respiratory syncytial virus. Br J Exp Pathol. 1984 Feb;65(1):19–28. [PMC free article] [PubMed] [Google Scholar]
- Thomas L. H., Stott E. J., Collins A. P., Jebbett N. J., Stark A. J. Evaluation of respiratory disease in calves: comparison of disease response to different viruses. Res Vet Sci. 1977 Sep;23(2):157–164. [PubMed] [Google Scholar]
- Tyeryar F. J. Report of a workshop on respiratory syncytial virus and parainfluenza viruses. J Infect Dis. 1983 Sep;148(3):588–598. doi: 10.1093/infdis/148.3.588. [DOI] [PubMed] [Google Scholar]
- Wright P. F., v Mills J., Chanock R. M. Evaluation of a temperature-sensitive mutant of respiratory syncytial virus in adults. J Infect Dis. 1971 Nov;124(5):505–511. doi: 10.1093/infdis/124.5.505. [DOI] [PubMed] [Google Scholar]
- Zaia J. A., Oxman M. N. Antibody to varicella-zoster virus-induced membrane antigen: immunofluorescence assay using monodisperse glutaraldehyde-fixed target cells. J Infect Dis. 1977 Oct;136(4):519–530. doi: 10.1093/infdis/136.4.519. [DOI] [PubMed] [Google Scholar]
- Zygraich N., Wellemans G. Immunological markers of an attenuated bovine respiratory syncytial (BRS) virus vaccine. Zentralbl Veterinarmed B. 1981;28(5):355–362. doi: 10.1111/j.1439-0450.1981.tb01924.x. [DOI] [PubMed] [Google Scholar]


