Abstract
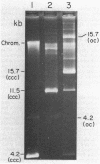
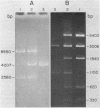
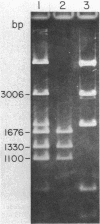
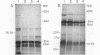
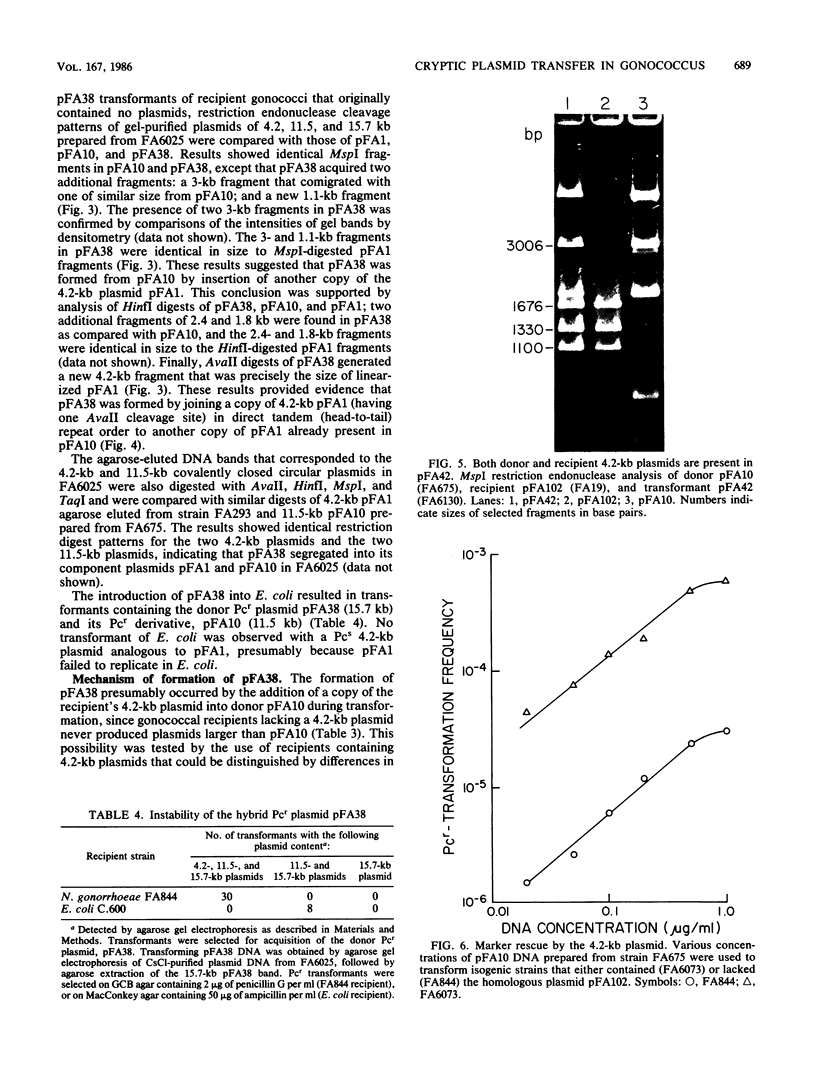
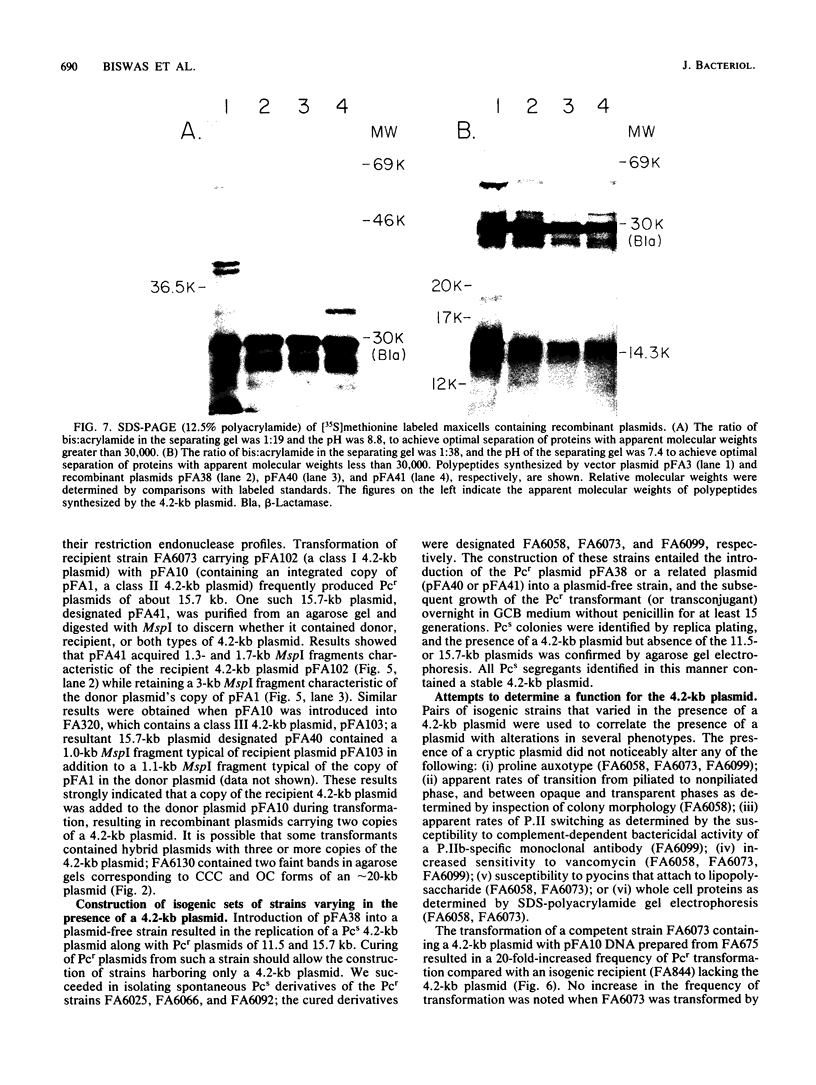
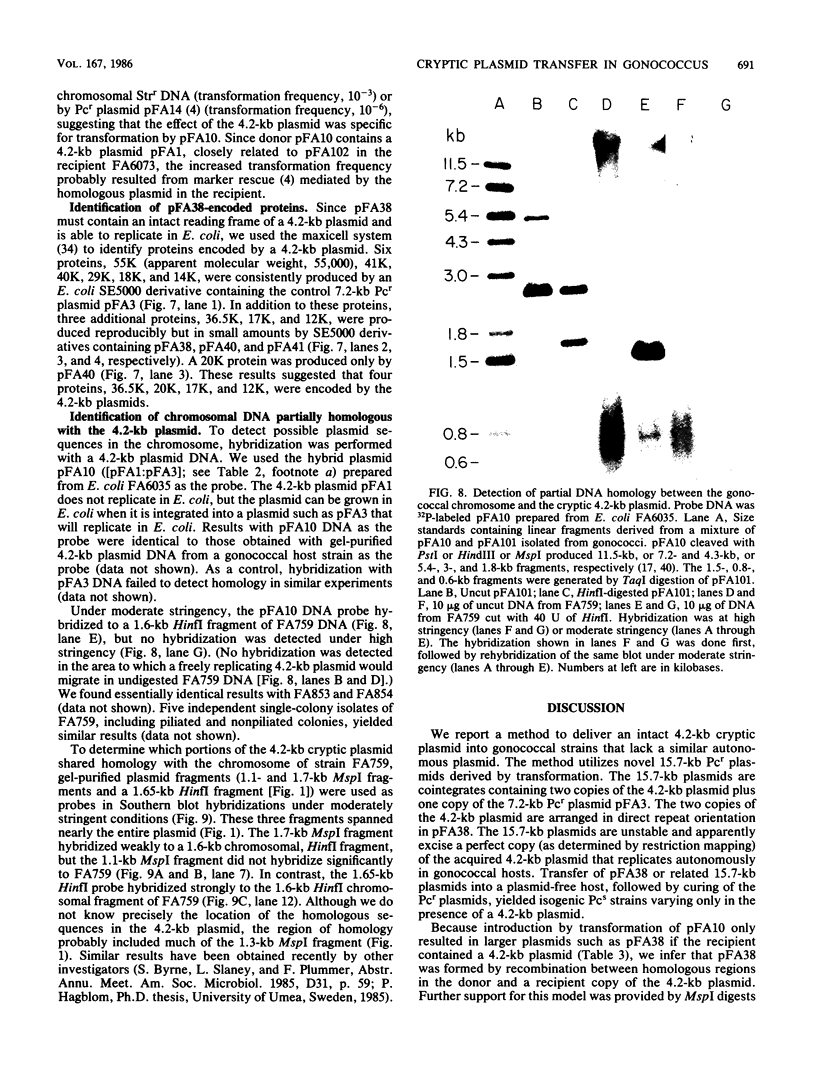
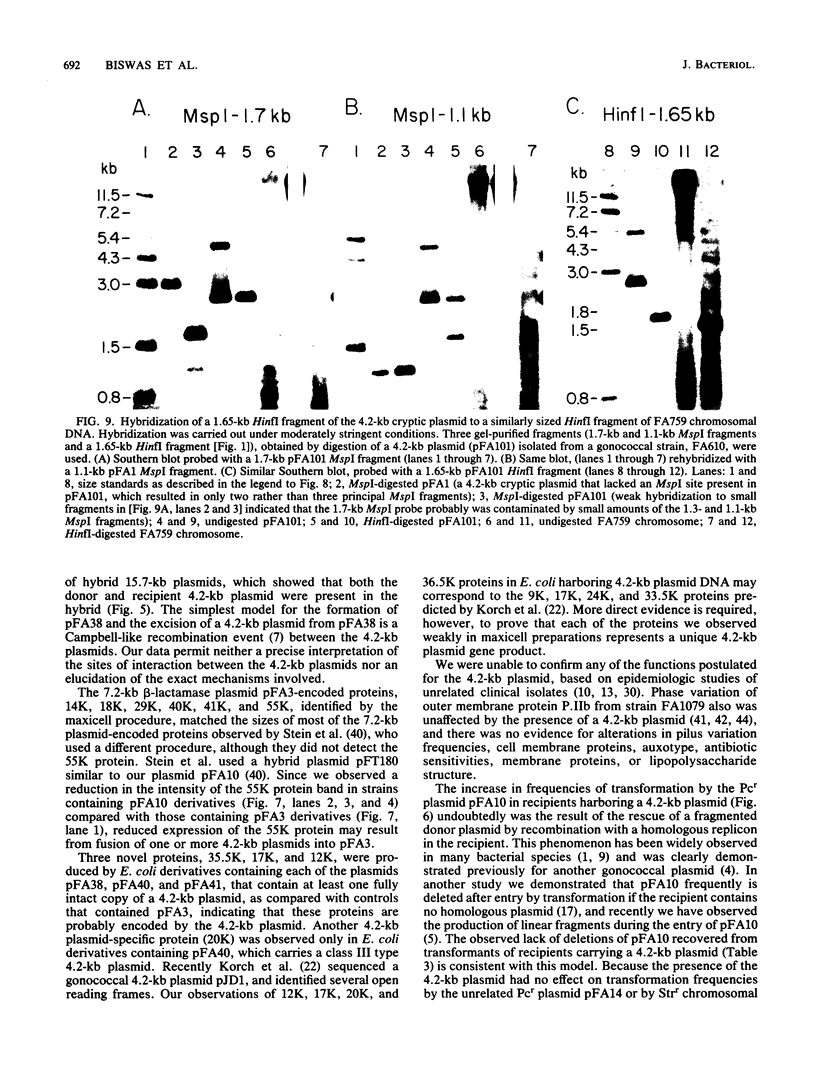
A 4.2-kilobase (kb) cryptic plasmid is present in 96% of isolates of Neisseria gonorrhoeae. An inability to construct isogenic derivatives which vary in the presence of the 4.2-kb plasmid has prevented the study of its function. We report a method to deliver an intact 4.2-kb plasmid into plasmidless gonococcal strains. The method involved transformation with novel 15.7-kb hybrid penicillinase-producing (Pcr) plasmids, which were cointegrates containing two copies of the 4.2-kb plasmid arranged in tandem direct repeat plus one copy of the 7.2-kb Pcr plasmid pFA3. When the 15.7-kb hybrid Pcr plasmids were introduced into a gonococcal recipient lacking evident plasmids, they dissociated at a relatively high frequency into plasmids identical to their parents: the 4.2-kb cryptic plasmid and pFA10 (a stable 11.5-kb plasmid containing one copy of each of the 7.2-kb Pcr plasmid pFA3 and the 4.2-kb cryptic plasmid pFA1). Curing strains of their Pcr plasmids resulted in isogenic strains which varied only in the presence of the 4.2-kb plasmid. The presence of the autonomously replicating 4.2-kb plasmid did not affect a number of tested phenotypes, including auxotype, antibiotic sensitivity, and frequencies of variation of outer membrane protein II. The interpretation of the functional significance of the 4.2-kb plasmid was complicated, however, by the additional finding that each of three tested plasmid-free strains contained a chromosomal fragment of about 1.6 kb that hybridized under moderate stringency with a 1.65-kb HinfI fragment of the 4.2-kb plasmid.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton W. L., Bendler J. W., Setlow J. K. Plasmid transformation in Haemophilus influenzae. J Bacteriol. 1981 Feb;145(2):1099–1101. doi: 10.1128/jb.145.2.1099-1101.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz G. A., Jacobs K. A., Eickbush T. H., Cherbas P. T., Kafatos F. C. Isolation of multigene families and determination of homologies by filter hybridization methods. Methods Enzymol. 1983;100:266–285. doi: 10.1016/0076-6879(83)00061-0. [DOI] [PubMed] [Google Scholar]
- Biswas G. D., Graves J. F., Sox T. E., Tenover F. C., Sparling P. F. Marker rescue by a homologous recipient plasmid during transformation of gonococci by a hybrid Pcr plasmid. J Bacteriol. 1982 Jul;151(1):77–82. doi: 10.1128/jb.151.1.77-82.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G., Comer S., Sparling P. F. Chromosomal location of antibiotic resistance genes in Neisseria gonorrhoeae. J Bacteriol. 1976 Mar;125(3):1207–1210. doi: 10.1128/jb.125.3.1207-1210.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black W. J., Schwalbe R. S., Nachamkin I., Cannon J. G. Characterization of Neisseria gonorrhoeae protein II phase variation by use of monoclonal antibodies. Infect Immun. 1984 Aug;45(2):453–457. doi: 10.1128/iai.45.2.453-457.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Contente S., Dubnau D. Marker rescue transformation by linear plasmid DNA in Bacillus subtilis. Plasmid. 1979 Oct;2(4):555–571. doi: 10.1016/0147-619x(79)90054-4. [DOI] [PubMed] [Google Scholar]
- Copley C. G., Egglestone I. Gonococci without plasmids. Lancet. 1982 May 15;1(8281):1133–1133. doi: 10.1016/s0140-6736(82)92324-8. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
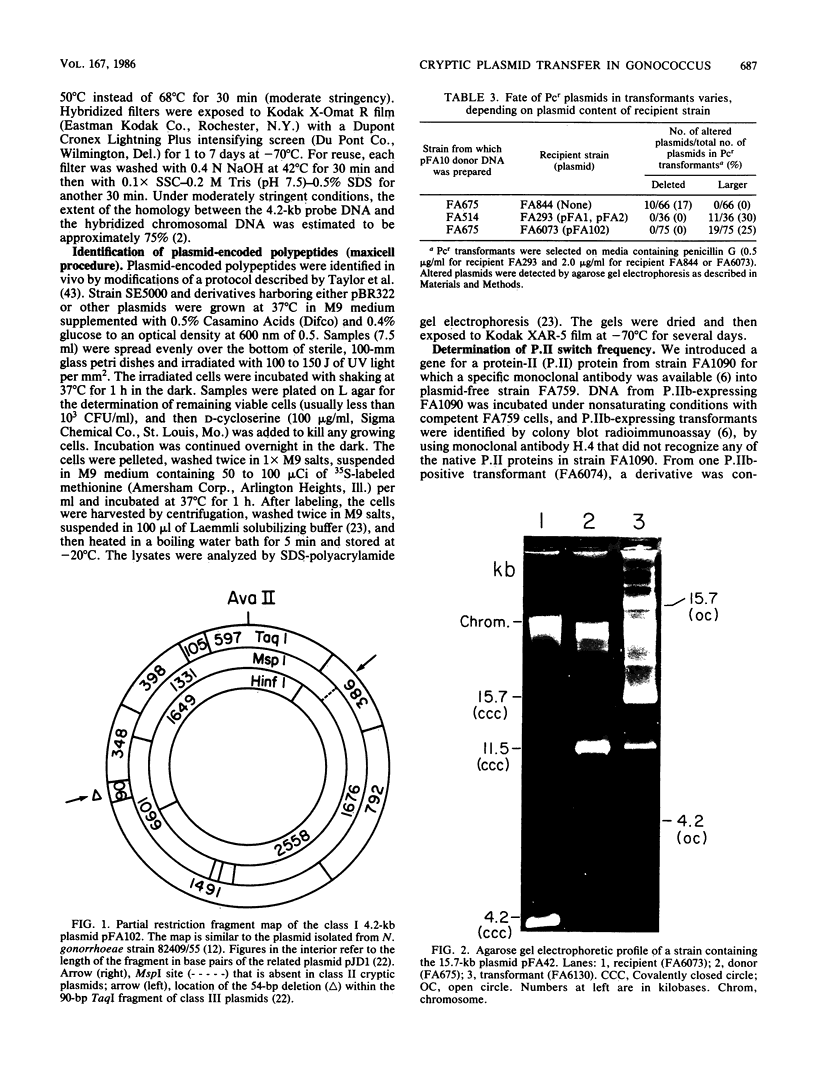
- Davies J. K., Normark S. A relationship between plasmid structure, structural lability, and sensitivity to site-specific endonucleases in Neisseria gonorrhoeae. Mol Gen Genet. 1980 Jan;177(2):251–260. doi: 10.1007/BF00267436. [DOI] [PubMed] [Google Scholar]
- Dillon J. R., Pauzé M. Relationship between plasmid content and auxotype in Neisseria gonorrhoeae isolates. Infect Immun. 1981 Aug;33(2):625–628. doi: 10.1128/iai.33.2.625-628.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
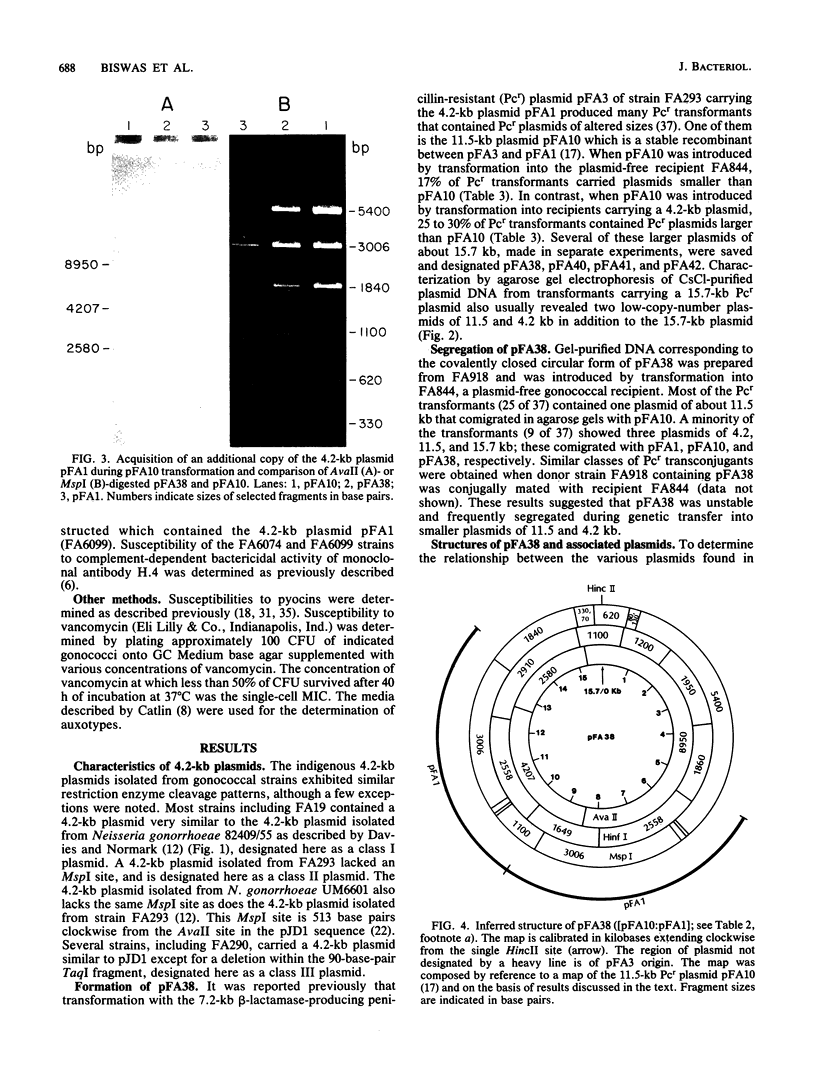
- Eisenstein B. I., Sox T., Biswas G., Blackman E., Sparling P. F. Conjugal transfer of the gonococcal penicillinase plasmid. Science. 1977 Mar 11;195(4282):998–1000. doi: 10.1126/science.402693. [DOI] [PubMed] [Google Scholar]
- Finkelstein M., Rownd T. H. A rapid method for extracting DNA from agarose gels. Plasmid. 1978 Sep;1(4):557–562. doi: 10.1016/0147-619x(78)90012-4. [DOI] [PubMed] [Google Scholar]
- Foster R. S., Foster G. C. Electrophoretic comparison of endonuclease-digested plasmids from Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1297–1304. doi: 10.1128/jb.126.3.1297-1304.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J. F., Biswas G. D., Sparling P. F. Sequence-specific DNA uptake in transformation of Neisseria gonorrhoeae. J Bacteriol. 1982 Dec;152(3):1071–1077. doi: 10.1128/jb.152.3.1071-1077.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymon L. F., Esser M., Shafer W. M. Pyocin-resistant lipopolysaccharide mutans of Neisseria gonorrhoeae: alterations in sensitivity to normal human serum and polymyxin B. Infect Immun. 1982 May;36(2):541–547. doi: 10.1128/iai.36.2.541-547.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., Stang H. D., Browne J. K., Martin M. O., Huot M., Lipeles J., Salser W. Human ribosomal RNA gene spacer sequences are found interspersed elsewhere in the genome. Gene. 1981 Nov;15(2-3):177–186. doi: 10.1016/0378-1119(81)90127-x. [DOI] [PubMed] [Google Scholar]
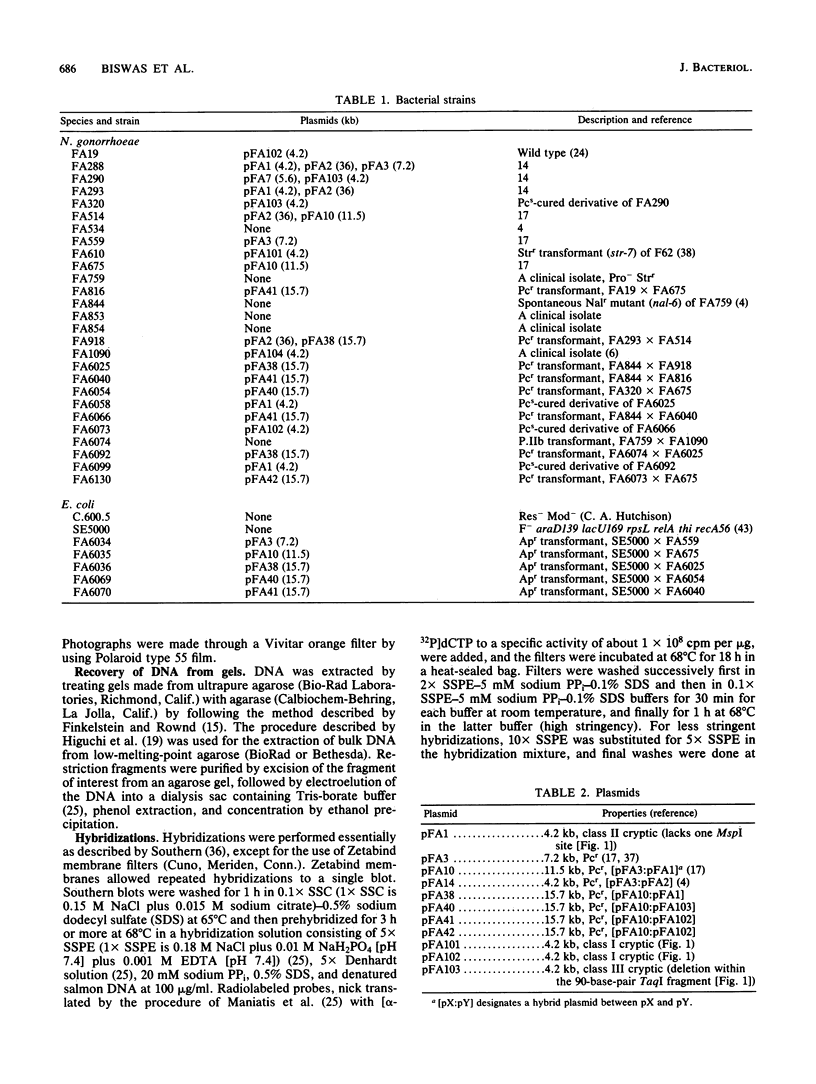
- Johnson S. R., Anderson B. E., Biddle J. W., Perkins G. H., DeWitt W. E. Characterization of concatemeric plasmids of Neisseria gonorrhoeae. Infect Immun. 1983 May;40(2):843–846. doi: 10.1128/iai.40.2.843-846.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomey J. M., Gill R. E., Falkow S. Genetic and biochemical analysis of gonococcal IgA1 protease: cloning in Escherichia coli and construction of mutants of gonococci that fail to produce the activity. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7881–7885. doi: 10.1073/pnas.79.24.7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korch C., Hagblom P., Ohman H., Göransson M., Normark S. Cryptic plasmid of Neisseria gonorrhoeae: complete nucleotide sequence and genetic organization. J Bacteriol. 1985 Aug;163(2):430–438. doi: 10.1128/jb.163.2.430-438.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maness M. J., Sparling P. F. Multiple antibiotic resistance due to a single mutation in Neisseria gonorrhoeae. J Infect Dis. 1973 Sep;128(3):321–330. doi: 10.1093/infdis/128.3.321. [DOI] [PubMed] [Google Scholar]
- Mayer L. W., Holmes K. K., Falkow S. Characterization of plasmid deoxyribonucleic acid from Neisseria gonorrhoeae. Infect Immun. 1974 Oct;10(4):712–717. doi: 10.1128/iai.10.4.712-717.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelsen P. A., Blackman E., Sparling P. F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from lactoferrin. Infect Immun. 1982 Mar;35(3):915–920. doi: 10.1128/iai.35.3.915-920.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. A., Anderson P., Parker J. W., Rohrer H. H. Inhibition of Neisseria gonorrhoeae isolates by Martin-Lewis medium. Epidemiology, susceptibility profile, and plasma analysis. Br J Vener Dis. 1982 Apr;58(2):96–100. doi: 10.1136/sti.58.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Piot P., Falkow S. The ecology of gonococcal plasmids. J Gen Microbiol. 1979 Oct;114(2):491–494. doi: 10.1099/00221287-114-2-491. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidberry H. D., Sadoff J. C. Pyocin sensitivity of Neisseria gonorrhoeae and its feasibility as an epidemiological tool. Infect Immun. 1977 Feb;15(2):628–637. doi: 10.1128/iai.15.2.628-637.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sox T. E., Mohammed W., Sparling P. F. Transformation-derived Neisseria gonorrhoeae plasmids with altered structure and function. J Bacteriol. 1979 May;138(2):510–518. doi: 10.1128/jb.138.2.510-518.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J Bacteriol. 1966 Nov;92(5):1364–1371. doi: 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. C., Young F. E., Tenover F. C., Clark V. L. Characterization of a chimeric beta-lactamase plasmid of Neisseria gonorrhoeae which can function in Escherichia coli. Mol Gen Genet. 1983;189(1):77–84. doi: 10.1007/BF00326058. [DOI] [PubMed] [Google Scholar]
- Stern A., Nickel P., Meyer T. F., So M. Opacity determinants of Neisseria gonorrhoeae: gene expression and chromosomal linkage to the gonococcal pilus gene. Cell. 1984 Jun;37(2):447–456. doi: 10.1016/0092-8674(84)90375-1. [DOI] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Hall M. N., Enquist L., Silhavy T. J. Identification of OmpR: a positive regulatory protein controlling expression of the major outer membrane matrix porin proteins of Escherichia coli K-12. J Bacteriol. 1981 Jul;147(1):255–258. doi: 10.1128/jb.147.1.255-258.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walstad D. L., Guymon L. F., Sparling P. F. Altered outer membrane protein in different colonial types of Neisseria gonorrhoeae. J Bacteriol. 1977 Mar;129(3):1623–1627. doi: 10.1128/jb.129.3.1623-1627.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]