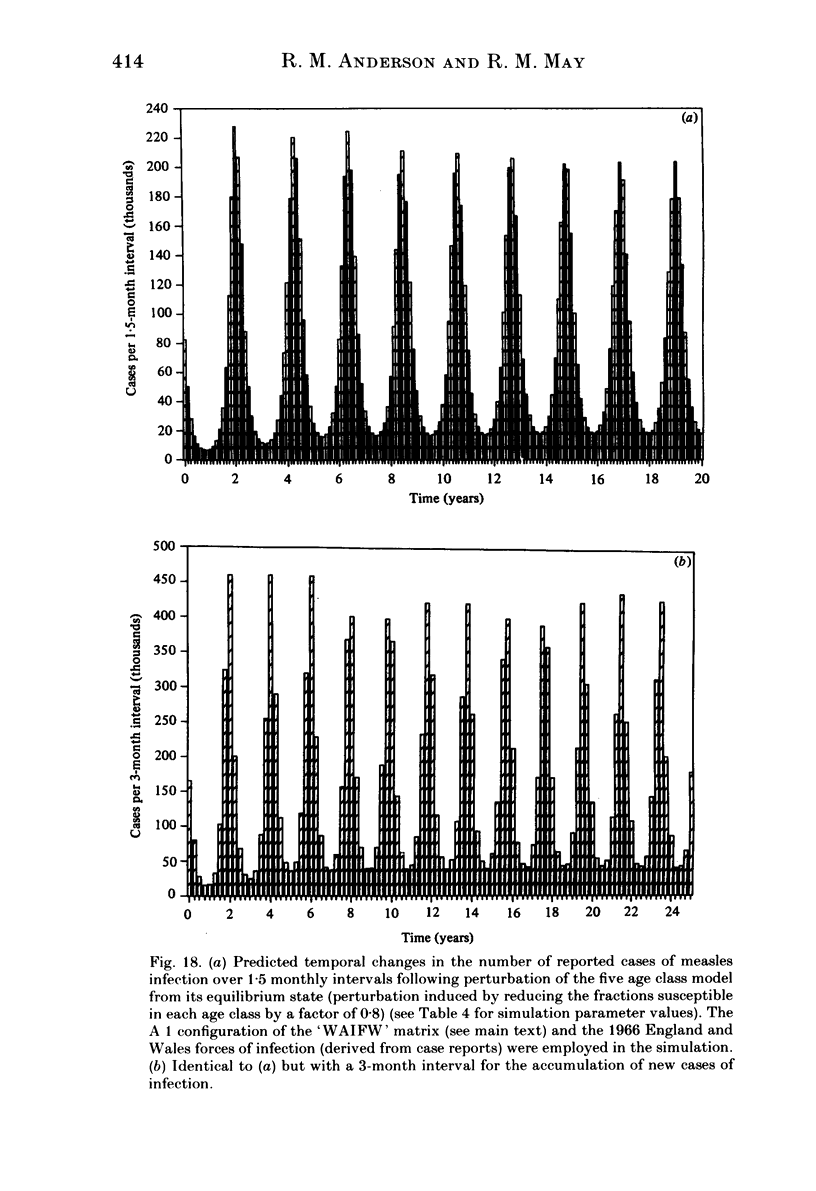
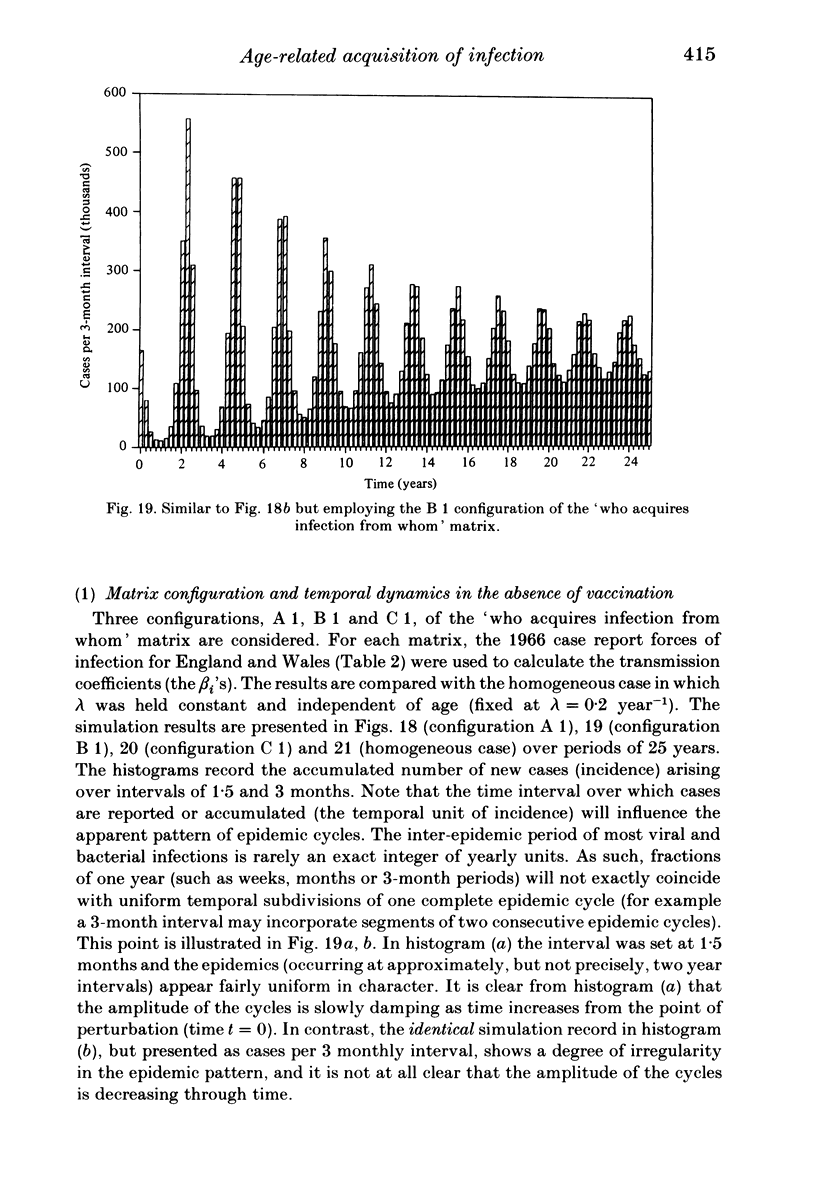
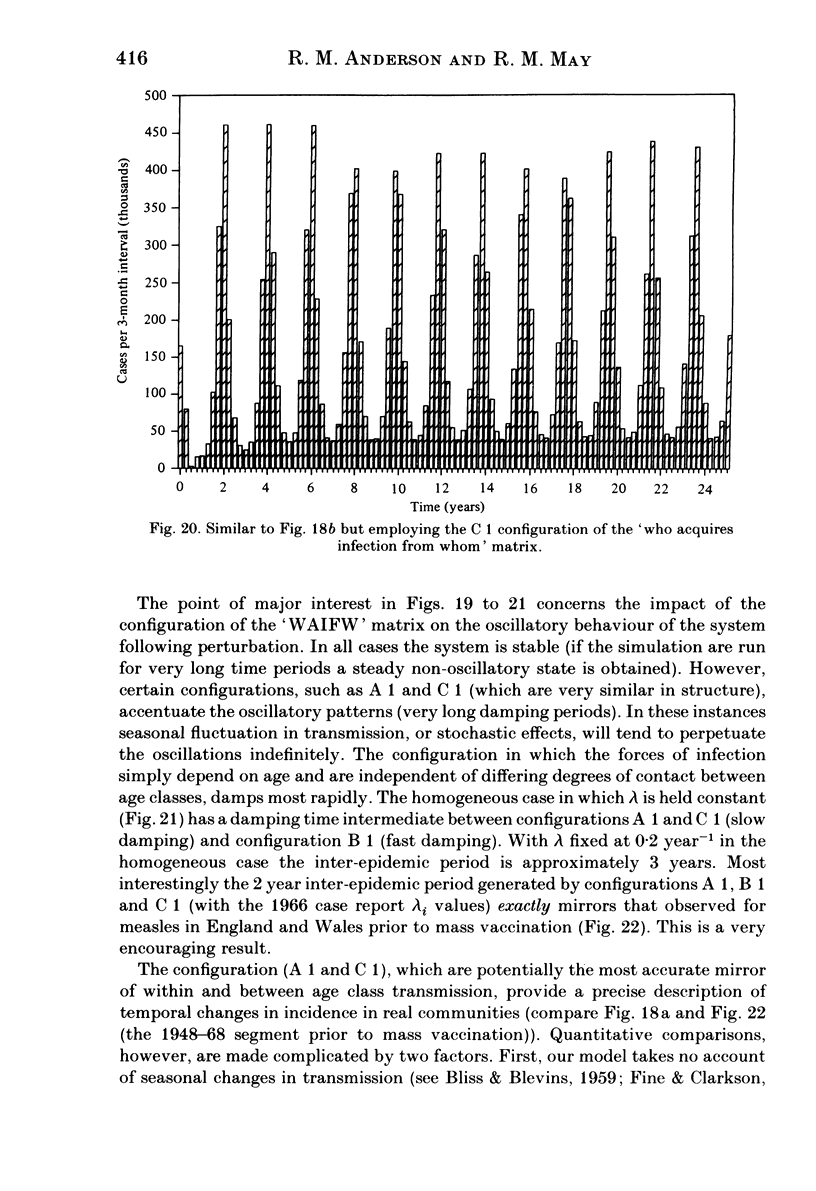
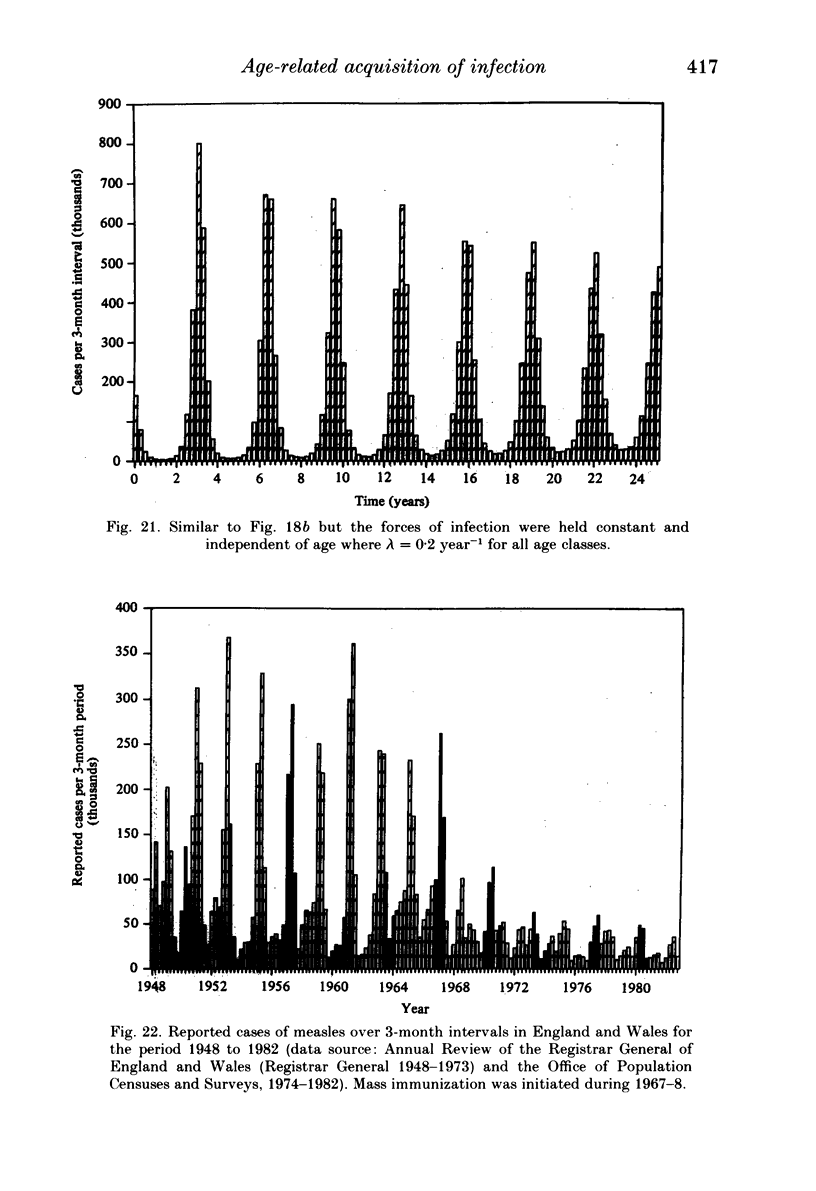
Abstract
Mathematical models are developed to aid in the investigation of the implications of heterogeneity in contact with infection within a community, on the design of mass vaccination programmes for the control of childhood viral and bacterial infections in developed countries. Analyses are focused on age-dependency in the rate at which individuals acquire infection, the question of 'who acquires infection from whom', and the implications of genetic variability in susceptibility to infection. Throughout, theoretical predictions are based on parameter estimates obtained from epidemiological studies and are compared with observed temporal trends in disease incidence and age-stratified serological profiles. Analysis of case notification records and serological data suggest that the rate at which individuals acquire many common infections changes from medium to high and then to low levels in the infant, child and teenage plus adult age groups respectively. Such apparent age-dependency in attack rate acts to reduce slightly the predicted levels of herd immunity required for the eradication of infections such as measles, when compared with the predictions of models based on age-independent transmission. The action of maternally derived immunity in prohibiting vaccination in infants, and the broad span of age classes over which vaccination currently takes place in the U.K., however, argue that levels of herd immunity of between 90 and 94% would be required to eliminate measles. Problems surrounding the interpretation of apparent age-related trends in the acquisition of infection and their relevance to the design of vaccination programmes, are discussed in relation to the possible role of genetically based variation in susceptibility to infection and observations on epidemics in 'virgin' populations. Heterogeneous mixing models provide predictions of changes in serology and disease incidence under the impact of mass vaccination which well mirror observed trends in England and Wales.
Full text
PDF

































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. M., Grenfell B. T., May R. M. Oscillatory fluctuations in the incidence of infectious disease and the impact of vaccination: time series analysis. J Hyg (Lond) 1984 Dec;93(3):587–608. doi: 10.1017/s0022172400065177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. M., May R. M. Directly transmitted infections diseases: control by vaccination. Science. 1982 Feb 26;215(4536):1053–1060. doi: 10.1126/science.7063839. [DOI] [PubMed] [Google Scholar]
- Anderson R. M., May R. M. Population biology of infectious diseases: Part I. Nature. 1979 Aug 2;280(5721):361–367. doi: 10.1038/280361a0. [DOI] [PubMed] [Google Scholar]
- Anderson R. M., May R. M. Vaccination against rubella and measles: quantitative investigations of different policies. J Hyg (Lond) 1983 Apr;90(2):259–325. doi: 10.1017/s002217240002893x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK F. L. Measles antibodies in the population of New Haven, Connecticut. J Immunol. 1959 Jul;83(1):74–82. [PubMed] [Google Scholar]
- BLISS C. I., BLEVINS D. L. The analysis of seasonal variation in measles. Am J Hyg. 1959 Nov;70:328–334. doi: 10.1093/oxfordjournals.aje.a120081. [DOI] [PubMed] [Google Scholar]
- Butler W. Measles. Proc R Soc Med. 1913;6(SECT):120–137. doi: 10.1177/003591571300601406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTENSEN P. E., SCHMIDT H., BANG H. O., ANDERSEN V., JORDAL B., JENSEN O. Measles in virgin soil, Greenland 1951. Dan Med Bull. 1954 Mar;1(1):2–6. [PubMed] [Google Scholar]
- Fine P. E., Clarkson J. A. Distribution of immunity to pertussis in the population of England and Wales. J Hyg (Lond) 1984 Feb;92(1):21–36. doi: 10.1017/s0022172400063993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine P. E., Clarkson J. A. Measles in England and Wales--I: An analysis of factors underlying seasonal patterns. Int J Epidemiol. 1982 Mar;11(1):5–14. doi: 10.1093/ije/11.1.5. [DOI] [PubMed] [Google Scholar]
- Fine P. E., Clarkson J. A. Measles in England and Wales--II: The impact of the measles vaccination programme on the distribution of immunity in the population. Int J Epidemiol. 1982 Mar;11(1):15–25. doi: 10.1093/ije/11.1.15. [DOI] [PubMed] [Google Scholar]
- Henderson E. C. A CENSUS OF THE CONTAGIOUS DISEASES OF 8,786 CHILDREN. Am J Public Health (N Y) 1916 Sep;6(9):971–981. doi: 10.2105/ajph.6.9.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman A. R., Brandling-Bennet A. D., Bernier R. H., Kirby C. D., Eddins D. L. Current features of measles in the United States: feasibility of measles elimination. Epidemiol Rev. 1980;2:153–170. doi: 10.1093/oxfordjournals.epirev.a036220. [DOI] [PubMed] [Google Scholar]
- Horwitz O., Grünfeld K., Lysgaard-Hansen B., Kjeldsen K. The epidemiology and natural history of measles in Denmark. Am J Epidemiol. 1974 Aug;100(2):136–149. doi: 10.1093/oxfordjournals.aje.a112016. [DOI] [PubMed] [Google Scholar]
- Knox E. G. Strategy for rubella vaccination. Int J Epidemiol. 1980 Mar;9(1):13–23. doi: 10.1093/ije/9.1.13. [DOI] [PubMed] [Google Scholar]
- MILLER D. L. FREQUENCY OF COMPLICATIONS OF MEASLES, 1963. REPORT ON A NATIONAL INQUIRY BY THE PUBLIC HEALTH LABORATORY SERVICE IN COLLABORATION WITH THE SOCIETY OF MEDICAL OFFICERS OF HEALTH. Br Med J. 1964 Jul 11;2(5401):75–78. doi: 10.1136/bmj.2.5401.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macgregor J. D., MacDonald J., Ingram E. A., McDonnell M., Marshall B. Epidemic measles in Shetland during 1977 and 1978. Br Med J (Clin Res Ed) 1981 Feb 7;282(6262):434–436. doi: 10.1136/bmj.282.6262.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J. S., Halpin T. J., Orenstein W. A. Measles vaccine efficacy in children previously vaccinated at 12 months of age. Pediatrics. 1978 Dec;62(6):955–960. [PubMed] [Google Scholar]
- Rand K. H., Emmons R. W., Merigan T. C. Measles in adults. An unforeseen consequence of immunization? JAMA. 1976 Aug 30;236(9):1028–1031. doi: 10.1001/jama.236.9.1028. [DOI] [PubMed] [Google Scholar]
- SEVER J. L., BRODY J. A., SCHIFF G. M., MCALISTER R., CUTTING R. RUBELLA EPIDEMIC ON ST. PAUL ISLAND IN THE PRIBILOFS, 1963. II. CLINICAL AND LABORATORY FINDINGS FOR THE INTENSIVE STUDY POPULATION. JAMA. 1965 Feb 22;191:624–626. doi: 10.1001/jama.1965.03080080014003. [DOI] [PubMed] [Google Scholar]
- Shelton J. D., Jacobson J. E., Orenstein W. A., Schulz K. F., Donnell H. D., Jr Measles vaccine efficacy: influence of age at vaccination vs. duration of time since vaccination. Pediatrics. 1978 Dec;62(6):961–964. [PubMed] [Google Scholar]
- Sutherland I., Fayers P. M. Effect of measles vaccination on incidence of measles in the community. Br Med J. 1971 Mar 27;1(5751):698–702. doi: 10.1136/bmj.1.5751.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E. B., Worcester J. Contact and Measles. Proc Natl Acad Sci U S A. 1941 Jan 15;27(1):7–13. doi: 10.1073/pnas.27.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorke J. A., London W. P. Recurrent outbreaks of measles, chickenpox and mumps. II. Systematic differences in contact rates and stochastic effects. Am J Epidemiol. 1973 Dec;98(6):469–482. doi: 10.1093/oxfordjournals.aje.a121576. [DOI] [PubMed] [Google Scholar]
- Yorke J. A., Nathanson N., Pianigiani G., Martin J. Seasonality and the requirements for perpetuation and eradication of viruses in populations. Am J Epidemiol. 1979 Feb;109(2):103–123. doi: 10.1093/oxfordjournals.aje.a112666. [DOI] [PubMed] [Google Scholar]


