Abstract
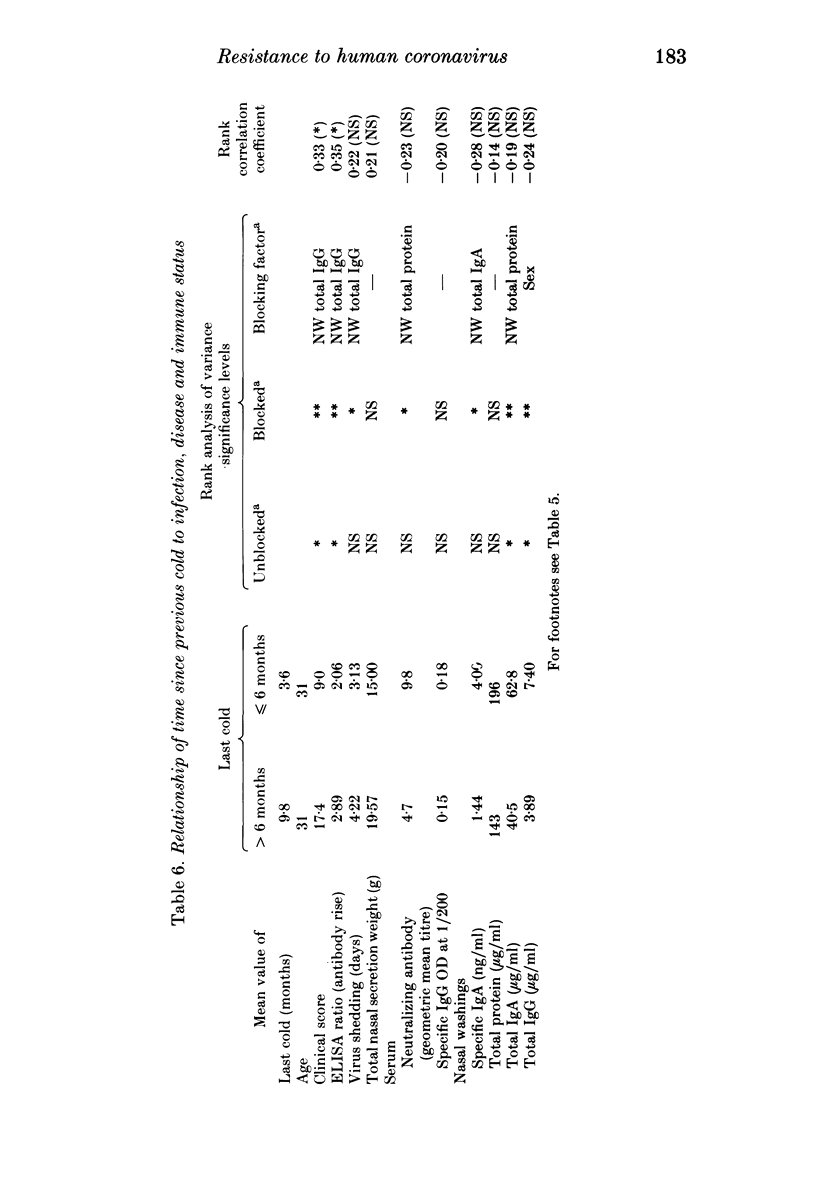
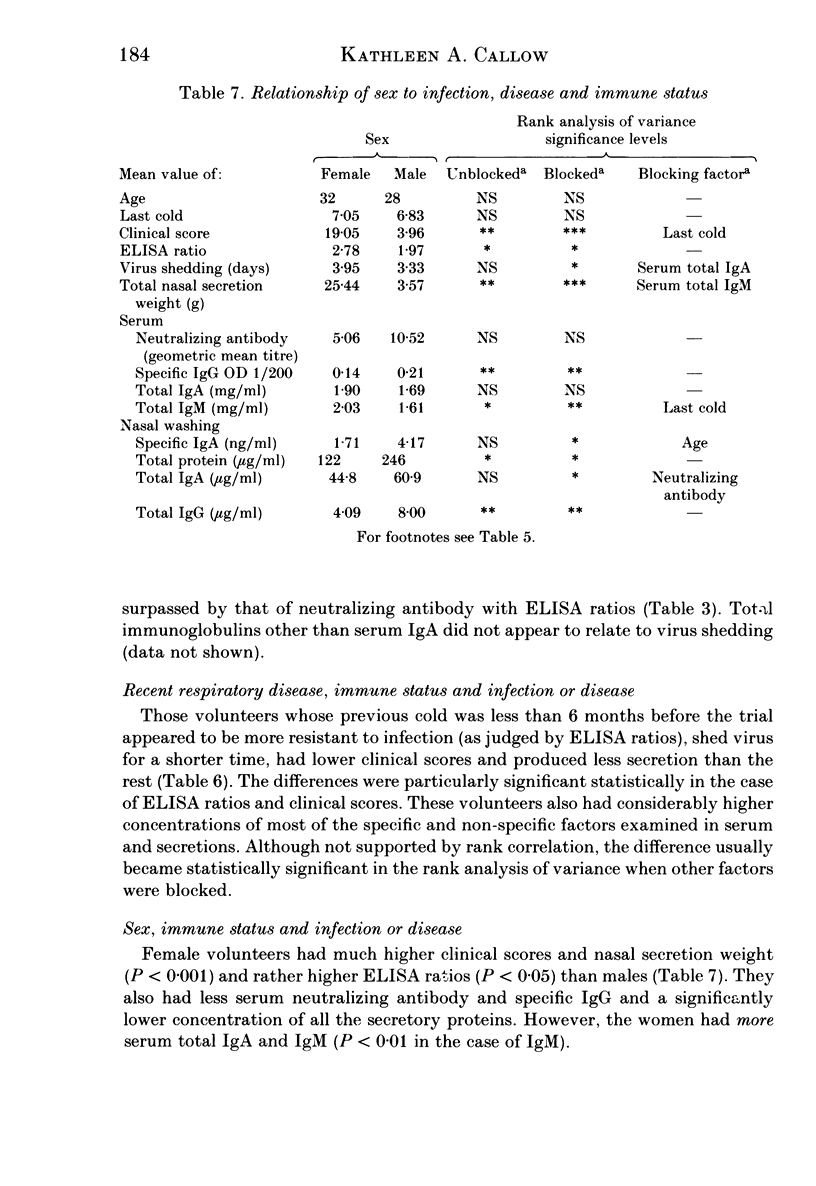
Thirty-three volunteers were inoculated intranasally with coronavirus 229 E, and their responses monitored by antibody rises, symptomatology and virus excretion. These were related to their pre-trial immune status as indicated by concentrations of specific antibodies and non-specific proteins in serum and nasal washings. Both circulating and local specific antibodies were associated with protection from infection and disease, but only specific IgA antibodies of either type appeared to shorten the period of virus shedding. Although total secretory IgA was significantly associated only with reduction of symptoms, total protein in nasal washings appeared to protect against infection also, indicating that other locally produced proteins, not identified, may be associated with resistance. Two of the many factors which may affect the concentration of circulating and local protective proteins and thus influence the outcome of virus inoculation, namely, sex of the volunteer and the interval since the previous cold, were examined. Male volunteers or volunteers who had had evidence of a recent respiratory infection were less likely to be infected, but if they were infected, they had lower clinical scores and stopped shedding virus earlier than the rest. These groups possessed higher concentrations of specific antibodies and non-specific proteins in their pre-challenge sera and/or nasal washings. The significance of these findings is discussed.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARTENSTEIN M. S., BELLANTI J. A., BUESCHER E. L. IDENTIFICATION OF THE ANTIVIRAL SUBSTANCES IN NASAL SECRETIONS. Proc Soc Exp Biol Med. 1964 Nov;117:558–564. doi: 10.3181/00379727-117-29637. [DOI] [PubMed] [Google Scholar]
- Arnold R. R., Prince S. J., Mestecky J., Lynch D., Lynch M., McGhee J. R. Secretory immunity and immunodeficiency. Adv Exp Med Biol. 1978;107:401–410. doi: 10.1007/978-1-4684-3369-2_45. [DOI] [PubMed] [Google Scholar]
- Buscho R. F., Perkins J. C., Knopf H. L., Kapikian A. Z., Chanock R. M. Further characterization of the local respiratory tract antibody response induced by intranasal instillation of inactivated rhinovirus 13 vaccine. J Immunol. 1972 Jan;108(1):169–177. [PubMed] [Google Scholar]
- Butler W. T., Waldmann T. A., Rossen R. D., Douglas R. G., Jr, Couch R. B. Changes in IgA and IgG concentrations in nasal secretions prior to the appearance of antibody during viral respiratory infection in man. J Immunol. 1970 Sep;105(3):584–591. [PubMed] [Google Scholar]
- CATE T. R., COUCH R. B., JOHNSON K. M. STUDIES WITH RHINOVIRUSES IN VOLUNTEERS: PRODUCTION OF ILLNESS, EFFECT OF NATURALLLY ACQUIRED ANTIBODY, AND DEMONSTRATION OF A PROTECTIVE EFFECT NOT ASSOCIATED WITH SERUM ANTIBODY. J Clin Invest. 1964 Jan;43:56–67. doi: 10.1172/JCI104894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow K. A. Measurement of antibodies to influenza virus neuraminidase by an enzyme-linked immunosorbent assay. Infect Immun. 1983 Aug;41(2):650–656. doi: 10.1128/iai.41.2.650-656.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate T. R., Rossen R. D., Douglas R. G., Jr, Butler W. T., Couch R. B. The role of nasal secretion and serum antibody in the rhinovirus common cold. Am J Epidemiol. 1966 Sep;84(2):352–363. doi: 10.1093/oxfordjournals.aje.a120648. [DOI] [PubMed] [Google Scholar]
- Clements M. L., O'Donnell S., Levine M. M., Chanock R. M., Murphy B. R. Dose response of A/Alaska/6/77 (H3N2) cold-adapted reassortant vaccine virus in adult volunteers: role of local antibody in resistance to infection with vaccine virus. Infect Immun. 1983 Jun;40(3):1044–1051. doi: 10.1128/iai.40.3.1044-1051.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crifö S., Vella S., Filiaci F., Resta S., Rocchi G. Secretory immune response after nasal vaccination with live attenuated influenza viruses. Rhinology. 1980 Jun;18(2):87–92. [PubMed] [Google Scholar]
- Edmondson W. P., Purcell R. H., Gundelfinger B. F., Love J. W., Ludwig W., Chanock R. M. Immunization by selective infection with type 4 adenovirus grown in human diploid tissue culture. II. specific protective effect against epidemic disease. JAMA. 1966 Feb 7;195(6):453–459. [PubMed] [Google Scholar]
- Fleet W. F., Couch R. B., Cate T. R., Knight V. Homologous and heterologous resistance to rhinovirus common cold. Am J Epidemiol. 1965 Sep;82(2):185–196. doi: 10.1093/oxfordjournals.aje.a120543. [DOI] [PubMed] [Google Scholar]
- Freestone D. S., Hamilton-Smith S., Schild G. C., Buckland R., Chinn S., Tyrrell D. A. Antibody responses and resistance to challenge in volunteers vaccinated with live attenuated, detergent split and oil adjuvant A2-Hong Kong-68 (H 3 N 2 ) influenza vaccines. A report to the Medical Research Council Committee on Influenza and other Respiratory Virus Vaccines. J Hyg (Lond) 1972 Sep;70(3):531–543. doi: 10.1017/s0022172400063117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamre D., Beem M. Virologic studies of acute respiratory disease in young adults. V. Coronavirus 229E infections during six years of surveillance. Am J Epidemiol. 1972 Aug;96(2):94–106. doi: 10.1093/oxfordjournals.aje.a121445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendley J. O., Gwaltney J. M., Jr, Jordan W. S., Jr Rhinovirus infections in an industrial population. IV. Infections within families of employees during two fall peaks of respiratory illness. Am J Epidemiol. 1969 Feb;89(2):184–196. doi: 10.1093/oxfordjournals.aje.a120928. [DOI] [PubMed] [Google Scholar]
- Higgins P. G., Phillpotts R. J., Scott G. M., Wallace J., Bernhardt L. L., Tyrrell D. A. Intranasal interferon as protection against experimental respiratory coronavirus infection in volunteers. Antimicrob Agents Chemother. 1983 Nov;24(5):713–715. doi: 10.1128/aac.24.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M. J., Reed S. E., Stott E. J., Tyrrell D. A. Studies of experimental rhinovirus type 2 infections in polar isolation and in England. J Hyg (Lond) 1976 Jun;76(3):379–393. doi: 10.1017/s0022172400055303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemmott J. B., 3rd, Borysenko J. Z., Borysenko M., McClelland D. C., Chapman R., Meyer D., Benson H. Academic stress, power motivation, and decrease in secretion rate of salivary secretory immunoglobulin A. Lancet. 1983 Jun 25;1(8339):1400–1402. doi: 10.1016/s0140-6736(83)92354-1. [DOI] [PubMed] [Google Scholar]
- Kraaijeveld C. A., Madge M. H., Macnaughton M. R. Enzyme-linked immunosorbent assay for coronaviruses HCV 229E and MHV 3. J Gen Virol. 1980 Jul;49(1):83–89. doi: 10.1099/0022-1317-49-1-83. [DOI] [PubMed] [Google Scholar]
- LIDWELL O. M., SOMMERVILLE T. Observations on the incidence and distribution of the common cold in a rural community during 1948 and 1949. J Hyg (Lond) 1951 Dec;49(4):365–381. doi: 10.1017/s0022172400066699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIDWELL O. M., WILLIAMS R. E. The epidemiology of the common cold. I. J Hyg (Lond) 1961 Sep;59:309–319. doi: 10.1017/s0022172400038973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIDWELL O. M., WILLIAMS R. E. The epidemiology of the common cold. II. Cross-infection and immunity. J Hyg (Lond) 1961 Sep;59:321–334. doi: 10.1017/s0022172400038985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matthews T. H., Nair C. D., Lawrence M. K., Tyrrell D. A. Antiviral activity in milk of possible clinical importance. Lancet. 1976 Dec 25;2(8000):1387–1389. doi: 10.1016/s0140-6736(76)91922-x. [DOI] [PubMed] [Google Scholar]
- McCormick D. P., Wenzel R. P., Davies J. A., Beam W. E. Nasal secretion protein responses in patients with wild-type adenovirus disease. Infect Immun. 1972 Sep;6(3):282–288. doi: 10.1128/iai.6.3.282-288.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J., 5th, Van Kirk J. E., Wright P. F., Chanock R. M. Experimental respiratory syncytial virus infection of adults. Possible mechanisms of resistance to infection and illness. J Immunol. 1971 Jul;107(1):123–130. [PubMed] [Google Scholar]
- Monto A. S. Medical reviews. Coronaviruses. Yale J Biol Med. 1974 Dec;47(4):234–251. [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Chalhub E. G., Nusinoff S. R., Kasel J., Chanock R. M. Temperature-sensitive mutants of influenza virus. 3. Further characterization of the ts-1(E) influenza A recombinant (H3N2) virus in man. J Infect Dis. 1973 Oct;128(4):479–487. doi: 10.1093/infdis/128.4.479. [DOI] [PubMed] [Google Scholar]
- Ogra P. L., Coppola P. R., MacGillivray M. H., Dzierba J. L. Mechanism of mucosal immunity to viral infections in gammaA immunoglobulin-deficiency syndromes. Proc Soc Exp Biol Med. 1974 Mar;145(3):811–816. doi: 10.3181/00379727-145-37900. [DOI] [PubMed] [Google Scholar]
- Perkins J. C., Tucker D. N., Knopf H. L., Wenzel R. P., Kapikian A. Z., Chanock R. M. Comparison of protective effect of neutralizing antibody in serum and nasal secretions in experimental rhinovirus type 13 illness. Am J Epidemiol. 1969 Dec;90(6):519–526. doi: 10.1093/oxfordjournals.aje.a121098. [DOI] [PubMed] [Google Scholar]
- Phillpotts R. J. Clones of MRC-C cells may be superior to the parent line for the culture of 229E-like strains of human respiratory coronavirus. J Virol Methods. 1983 May;6(5):267–269. doi: 10.1016/0166-0934(83)90041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. E., Hall T. S. Hemagglutination-inhibition test in rhinovirus infections of volunteers. Infect Immun. 1973 Jul;8(1):1–3. doi: 10.1128/iai.8.1.1-3.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. E. The behaviour of recent isolates of human respiratory coronavirus in vitro and in volunteers: evidence of heterogeneity among 229E-related strains. J Med Virol. 1984;13(2):179–192. doi: 10.1002/jmv.1890130208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes K., Scott A., Markham R. L., Monk-Jones M. E. Immunological sex differences. A study of patients with rheumatoid arthritis, their relatives, and controls. Ann Rheum Dis. 1969 Mar;28(2):104–120. doi: 10.1136/ard.28.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen R. D., Butler W. T., Waldman R. H., Alford R. H., Hornick R. B., Togo Y., Kasel J. A. The proteins in nasal secretion. II. A longitudinal study of IgA and neutralizing antibody levels in nasal washings from men infected with influenza virus. JAMA. 1970 Feb 16;211(7):1157–1161. doi: 10.1001/jama.211.7.1157. [DOI] [PubMed] [Google Scholar]
- Rossen R. D., Schade A. L., Butler W. T., Kasel J. A. The proteins in nasal secretion: a longitudinal study of the gammaA-globulin, gammaG-globulin, albumin, siderophilin, and total protein concentrations in nasal washings from adult male volunteers. J Clin Invest. 1966 May;45(5):768–776. doi: 10.1172/JCI105391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savilahti E. IgA deficiency in children. Immunoglobulin-containing cells in the intestinal mucosa, immunoglobulins in secretions and serum IgA levels. Clin Exp Immunol. 1973 Mar;13(3):395–406. [PMC free article] [PubMed] [Google Scholar]
- Smith C. B., Purcell R. H., Bellanti J. A., Chanock R. M. Protective effect of antibody to parainfluenza type 1 virus. N Engl J Med. 1966 Nov 24;275(21):1145–1152. doi: 10.1056/NEJM196611242752101. [DOI] [PubMed] [Google Scholar]
- South M. A., Copper M. D., Wollheim F. A., Good R. A. The IgA system. II. The clinical significance of IgA deficiency: studies in patients with agammaglobulinemia and ataxia-telangiectasia. Am J Med. 1968 Feb;44(2):168–178. doi: 10.1016/0002-9343(68)90148-4. [DOI] [PubMed] [Google Scholar]
- TOMASI T. B., Jr, TAN E. M., SOLOMON A., PRENDERGAST R. A. CHARACTERISTICS OF AN IMMUNE SYSTEM COMMON TO CERTAIN EXTERNAL SECRETIONS. J Exp Med. 1965 Jan 1;121:101–124. doi: 10.1084/jem.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi T. B., Jr, DeCoteau E. Mucosal antibodies in respiratory and gastrointestinal disease. Adv Intern Med. 1970 Jun;116(535):401–425. [PubMed] [Google Scholar]
- Totman R., Kiff J., Reed S. E., Craig J. W. Predicting experimental colds in volunteers from different measures of recent life stress. J Psychosom Res. 1980;24(3-4):155–163. doi: 10.1016/0022-3999(80)90037-9. [DOI] [PubMed] [Google Scholar]
- Totman R., Reed S. E., Craig J. W. Congnitive dissonance, stress and virus-induced common colds. J Psychosom Res. 1977;21(1):55–63. doi: 10.1016/0022-3999(77)90026-5. [DOI] [PubMed] [Google Scholar]
- Tremonti L. P., Lin J. S., Jackson G. G. Neutralizing activity in nasal secretions and serum in resistance of volunteers to parainfluenza virus type 2. J Immunol. 1968 Sep;101(3):572–577. [PubMed] [Google Scholar]
- Waldman R. H., Henney C. S. Cell-mediated immunity and antibody responses in the respiratory tract after local and systemic immunization. J Exp Med. 1971 Aug 1;134(2):482–494. doi: 10.1084/jem.134.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yodfat Y., Silvian I. A prospective study of acute respiratory tract infections among children in a kibbutz: the role of secretory IgA and serum immunoglobulins. J Infect Dis. 1977 Jul;136(1):26–30. doi: 10.1093/infdis/136.1.26. [DOI] [PubMed] [Google Scholar]



