Abstract
As a result of an energetic accretion, the Earth is a volatile-poor and strongly differentiated planet. The volatile elements can be accounted for by a late veneer (≈1% of total mass of the Earth). The incompatible elements are strongly concentrated into the exosphere (atmosphere, oceans, sediments, and crust) and upper mantle. Recent geochemical models invoke a large primordial undegassed reservoir with chondritic abundances of uranium and helium, which is clearly at odds with mass and energy balance calculations. The basic assumption behind these models is that excess “primordial” 3He is responsible for 3He/4He ratios higher than the average for midocean ridge basalts. The evidence however favors depletion of 3He and excessive depletion of 4He and, therefore, favors a refractory, residual (low U, Th) source Petrological processes such as melt-crystal and melt-gas separation fractionate helium from U and Th and, with time, generate inhomogeneities in the 3He/4He ratio. A self-consistent model for noble gases involves a gas-poor planet with trapping of CO2 and noble gases in the shallow mantle. Such trapped gases are released by later tectonic and magmatic processes. Most of the mantle was depleted and degassed during the accretion process. High 3He/4He gases are viewed as products of ancient gas exsolution stored in low U environments, rather than products of primordial reservoirs.
The Earth accreted energetically in a high temperature part of the solar nebula (1, 2). It therefore is depleted in the volatile elements, and the large-ion elements are strongly concentrated toward the surface (3). The Earth is cooling down and this accounts for approximately one-half of the heat flow (4). These physical constraints are violated by recent geochemical models that imply a volatile-rich planet (5) and a balance between radioactive heat productivity and present heat flow (6).
The average midocean ridge basalt (MORB), the most voluminous terrestrial magma, has 3He/4He ratios (R) of approximately eight times atmospheric (8 Ra). Large volumes of the mantle are processed to continuously generate the oceanic crust, and blending inevitably takes place. Ocean island basalts (OIB), mantle xenoliths, and small volume melts from the mantle sometimes have R of up to 30 Ra although the average and median values from a given region are generally close to the MORB average. Because high values are discarded in compiling the MORB average (7, 8) and because mid-ocean ridge processes are so effective in blending material from large volumes, it is not clear what the distribution of R is in the source region of MORB and what the extreme values in the distribution are. The common practice of comparing extremes (OIB) with means (MORB) is not valid. Some of the so-called high 3He/4He ratio hotspots exhibit a population that have means and medians well within the MORB range, and from this point of view, they could represent samples drawn from the same population. The 3He content of MORB is generally much higher than in OIB. Because the non-MORB samples are so small, and so heterogeneous in helium content and in the isotopic ratios, and because so few atoms are represented in these samples, the variation in R may be due to very local heterogeneities in the distribution of U and He.
Standard Model.
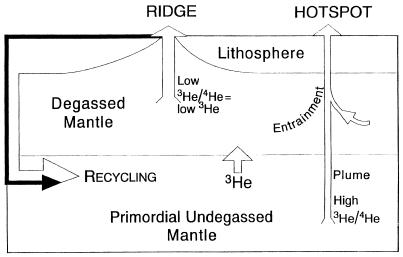
A widely accepted box model (5, 6, 9) for the distribution of helium in the Earth’s mantle is shown in Fig. 1. The upper mantle was degassed during crustal formation and is poor in He and U. The lower mantle, in this model, has escaped degassing and has primordial abundances of the elements. Hotspots tap this deep primordial reservoir via narrow plumes and provide high 3He/4He basalts.
Figure 1.
The “Standard Model.” Hotspots are fed by narrow plumes from primordial undegassed lower mantle. The recycled component, treated as a package, goes deep into the mantle before it returns as a plume. The upper mantle is completely degassed but is injected with 3He leaking from the lower mantle (5, 6, 9). High 3He/4He ratios are interpreted as excess 3He. Temporal variations in magmas at hotspots reflect a compositionally zoned plume and motion of the plate across the plume. In this model, magmatism is initiated by impingement of a hot plume on the base of the plate. In contrast, Pb-isotopes have been used to argue that U, and other lithophile elements, was transported to the outer layers in early Earth history (3, 40, 41).
There are various problems with this model:
1. High 3He/4He ratio basalts have very little 3He, often two orders of magnitude less than MORB;
2. the He/Ar, He/Ne, etc., ratios in high R basalts do not indicate that they have lost more He than MORB;
3. the inferred He content of the primordial reservoir requires a gas-rich planet;
4. many high R environments have average R values similar to MORB;
5. basalts from the conjectured primordial mantle do not have other primordial characteristics, such as primitive Pb-isotopic compositions;
6. the 3He gas flux from hotspot volcanoes is orders of magnitude less than from mid-ocean ridges and island arcs (10), contrary to predictions of the Standard Model;
7. the present 4He flux from the mantle is an order of magnitude less than predicted from primordial U and Th abundances and the observed heat flow; and
8. the main carrier of He, CO2, is depleted in the outer parts of the Earth by an order of magnitude compared with other volatiles.
These problems are known as “paradoxes” in the noble gas literature.
Alternative Model.
High R also may represent low 4He or low time integrated U, Th abundances. In this case, the high R reservoir is not primitive; it can be a shallow refractory reservoir such as residual peridotite, harzburgite, or magma chamber cumulates. There are various mechanisms for separating U, Th from He and, therefore, for generating an inhomogeneous 3He/4He distribution. Large-ion lithophile elements such as U and Th are excluded from mantle minerals and are concentrated in melts. Helium is a gas, is neutral, and has a small radius. It therefore has quite a different melt-solid partition coefficient than U and Th, and the U/He ratio in residual crystals is likely to be lower than in silicate melts. Even if He is expelled from the crystal during melting, some is reincorporated, as fluid-filled inclusions or vugs, during shallow crystallization of the melt. This process need not be efficient because high 3He/4He melts and gases account for a very small fraction of the total 3He brought to the Earth’s surface. Melt-gas separation also separates U, Th from He and melt can escape from the mantle easier than can a free-gas phase that does not wet grain boundaries.
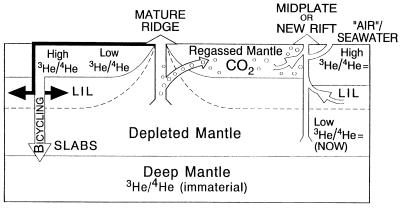
Fig. 2 illustrates a mechanism whereby a magma degasses at shallow depth and stores some of the gases in refractory (U, Th-poor) mantle. In this case, the gas is separated from MORB at or near a ridge and released at midplate environments at a much later time. This model explains the low 3He content and low He/Ne and He/Ar ratios of high R basalts because the noble gases in these basalts are contaminants derived from ancient MORB-degassing events. CO2 is the main carrier phase.
Figure 2.
The Plate Model. High R basalts contain a component from a high 3He/U reservoir rather than a high 3He-undegassed reservoir. Magma degasses CO2 and He at shallow depth that are partially trapped in fluid inclusions or vugs in refractory (U, Th-poor) shallow mantle, (high 3He/U). The 3He/4He ratio in the gas is the same as, and the Ar/He and Ne/He ratios are higher than, in the melt. With time, the 3He/4He ratio in the newly formed crust and the original MORB reservoir (low 3He/U) decline, but it stays high in the fluid inclusions. Midplate or new rift magmatism interacts with the shallow mantle, picking up large-ion lithophile elements, from recycling, and old trapped melts, and seawater contamination (implied by heavy noble gas abundances) and CO2 with “frozen-in” high 3He/4He ratios. Recycling returns large-ion lithophiles to the shallow mantle (perisphere) by dehydration reactions and returns depleted slab material to the deep mantle (bicycling). High 3He/4He ratios are due to low U and low 4He abundances, compared with high U and Th crust and mantle source regions. Midplate magmatism involves near-surface interaction and/or recycled material. High 3He/4He ratios may have been MORB values when the shallow refractory mantle, or recycled lithosphere, formed. 3He/4He ratios of crust, lithosphere, and mantle source diverge at the time of magma emplacement, outgassing, and formation of fluid-filled inclusions. Eventually, the lithosphere in the vicinity of the conduit will be degassed but the volcano load may initiate a new crack, and the degassing process of a new section of lithosphere can start anew. Temporal variations are due to magmatism induced changes in the plate (depletion, degassing). Shallow material is incorporated into midplate volcanoes by wall-rock reactions, melting, magma-mixing, degassing, and near-surface contamination. In a propagating magma-filled crack, the magma runs out of pressure before it makes it to the very narrow crack tip, and there is a gap between the tip and the magma front. The tip cavity will therefore be filled with volatiles exsolving from the magma or pore fluid from the host rock. Gases can therefore exsolve from magma at greater depth than usually assumed, and wall rock gases can enter new cracks.
The parameters controlling 3He/4He variations in the mantle are 238U/3He (NU) and the Th/U ratio. The production of 4He is low in low 238U/3He (LONU) environments, such as peridotites residual after melt (i.e., U, Th) extraction. With time, such environments have high 3He/4He ratios compared with basalts, fertile mantle or recycled material, or the MORB reservoir.
Tests of the Model.
Noble gas ratios in midplate volcanics suggest relative depletion in those elements most soluble in melts and contamination with seawater infiltrated crust (11, 12). CO2 and noble gases exsolved from magmas are partially trapped in fluid-filled inclusions and vugs in the shallow mantle and carry with them the 3He/4He ratio of MORB, at the time of degassing. If these gases are stored in refractory, U-poor, mantle, the R values of the inclusions and the parent magma reservoir will diverge. R in old inclusions can be much greater than in modern basalts from the same reservoir, and these can contaminate midplate magmas. Fig. 2 illustrates the process.
Trapping of CO2-He in lower crustal or upper mantle cumulates provides a potentially high 3He/4He component for future magmas to inherit, especially at the onset of magmatism. An estimate of the dimensions of the source, if it is in the current lithosphere, is provided by the deep seismicity under Kilauea and Loihi (13). 3He abundances in deep xenoliths (14, 15) yield an estimate of 2 × 109 cm3 of 3He in the seismically active portion of the lithosphere under Hawaii by using the seismic zone as an estimate of degassing volume (2 × 104 km3). This represents 2,000 years of 3He available at the current eruption rates. 3He/4He ratios in Hawaiian basalts approach MORB values on time scales of thousands of years (16). The temporal decrease of R in Hawaiian volcanoes, generally attributed to drifting off of a deep mantle plume, may instead be due to exhaustion of CO2-rich pockets in the shallow mantle. A new conduit will restart a high R pathway. The source of helium in this model is very local. Such a model explains why there are no global correlations of helium with other isotopes but sometimes very good local correlations and why there are large local variations in R.
As volcanoes grow and erode, new cracks open up and new lithosphere is exposed to ascending magmas. I attribute the temporal change of noble gas chemistry at Hawaiian volcanoes to lithospheric or shallow mantle characteristics (new cracks and new conduits) rather than to motion of the plate over a static plume.
The 3He/4He ratio in Réunion Island basalts has remained high for approximately 360,000 years, ≈10 times longer than in Hawaiian volcanoes (17). Réunion is much less active than Hawaii, and the eruption rates are an order of magnitude lower. Any helium stored in the shallow mantle should therefore survive much longer.
Midplate volcanoes initiate with cracks, caused by plate-tectonic stresses and volcanic loading. Magma, in fact, can fracture its way to the surface in lithosphere exposed to tensile stresses. Generally, lithosphere is under compression and magma pressure is not high enough to fracture it. The initial magmas interact with CO2-rich fluid-filled inclusions with 3He/4He ratios frozen in at the time of stabilization of the lithosphere (Fig. 2). Virgin lithosphere, or recycled slab, with fluid-filled inclusions is a potential low-υ (LONU) reservoir (low 238U, high CO2, “high” He). As magmas ascend, they interact with shallow mantle, altered oceanic crust and seawater infiltrated rocks, and sediments. As magma conduits get established, the nature and extent of contamination change. In particular, the high 3He/4He fluid inclusions get used up and low 3He/4He crustal xenoliths (in a HINU environment) may lower the helium ratio. The crust may have lost most of its helium so lithospheric contamination may be more important than crustal contamination. When a conduit gets plugged, or a new crack opens, a new transient stage of high 3He/4He magmas can start. This evolutionary history explains why hotspot magmas are diverse and why atmospheric components are intimately related to “primordial” components (11). It also explains the range of R found on oceanic islands and the temporal evolution toward MORB values (16). Ocean island basalts should have lower helium abundances than MORB or than gas-rich mantle xenoliths, as observed.
DISCUSSION
There are several possible refractory LONU reservoirs: (1) ancient lithosphere, (2) deep crustal cumulates, and (3) the lithosphere beneath the island.
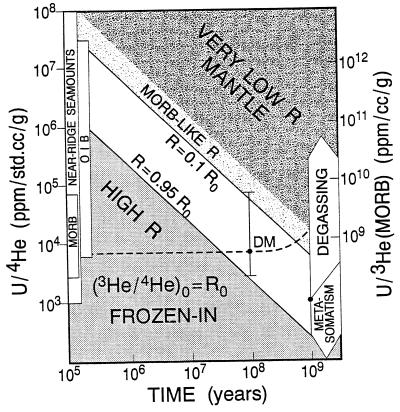
The latter is the most interesting case, but the question arises, can the 3He/4He ratio in the reservoir from which the oceanic crust and lithosphere formed have decreased by a factor of three since the fluid inclusions were emplaced? This depends on υ of the MORB mantle (MM). Fig. 3 shows the tradeoffs between U/He ratios and time. MORB-like R values can be generated from Loihi-like values in 108–109 years if υ of MM is similar to MORB. High R reservoirs can be preserved if υ is lower than in MM by 1–2 orders of magnitude. Refractory mantle with CO2-rich inclusions, can have very low υ. Although we do not have a good estimate of υ of MM, or how it changes with time, high values of υ lead to difficulties when extrapolated too far into the past. There is some evidence for rapid increases of U in the upper mantle (18, 19). Because the value and history of the 238U/204Pb ratio (μ) in the upper mantle are uncertain, it is premature to rule out a high or increasing 238U/3He ratio (υ). It also is not certain that the age of the mantle under Hawaii is the same age as the crust. Swells such as the Hawaiian swell may be partially held up by buoyant refractory residue and may be unsubductible. Young crust can form on old lithosphere. There is, in fact, evidence that the Hawaiian Swell is not a thermal swell but may be a thick section of depleted peridotite (20). It may be older than the overlying crust. Other oceanic swells or plateaus are suspected of having an ancient, or continental, component.
Figure 3.
Trade-off curves between U/4He (ppm g/cc) and time, which give high and low 3He/4He reservoirs [modified from Zindler and Hart (42)]. The ordinate is the present day U/4He ratio. Ranges for MORB, OIB, and near-ridge seamounts are shown to the left. The dashed horizontal line is based on a composition of the MORB-reservoir (DM) using U = 0.06 ppm and [He] = 8 × 10−6 std cc/g (23, 34). These values correspond to 20% melting of DM to provide values in the range of MORB observations. The absolute values do not matter for the growth of 4He/3He, only the 238U/3He or 238U/4He ratios and time are involved. For this value of U/4He, the 3He/4He ratio declines from an assumed initial value of 100 Ra to MORB-like values in ≈6 × 108 years. The range of observed U/4He in MORB, if typical of the DM source, give a range of aging times from ≈108 to >109 years, to evolve from a high R source to MORB-like values. Reservoirs with U/4He ratios <104 (ppm g/cc) can maintain nearly their initial values for times in excess of 50 million years. If high and low R basalts come from reservoirs that differ only in U/4He, then the difference must be 1 or 2 orders of magnitude to explain the observed spread in 3He/4He ratios. The triangular region to the lower left shows combinations of U/4He and time for which initial R values are essentially frozen in. The region to the upper right indicates conditions under which low R reservoirs can form. The large arrows to the right show the effects on U/4He of degassing and metasomatism with a gas-rich, low-U, fluid. Parameters used are 232Th/238U = 3.0 and Ro = 100 Ra (42).
An alternative low υ reservoir for high 3He/4He inclusions is ancient lithosphere that has been recycled and stored for longer periods of time. This would be similar to the recycling of ancient oceanic crust, which is the conventional explanation for some high 238U/204Pb (HIMU) plume components (19, 21, 22, 23).
The range in μ for MORB is approximately a factor of 6 (23). Because He is a gaseous species and recycling and degassing both increase the U/He ratio, a time integrated two-order of magnitude variation in υ (5, 6) seems plausible (Fig. 3), even in the upper mantle.
Change of 3He/4He with Time.
If high R locales, such as Loihi, represent old MM values of R, we can estimate the 238U/3He and 3He/22Ne ratios of MM.
I adopt R for Loihi as the value for MM at the time of formation of the oceanic crust at Hawaii (≈100 Ma) and solve for υ of MM, which yields current MORB values for 3He/4He. Because this is a minimum age for the LONU reservoir, it yields a maximum NU for MM. The result, assuming Th/U = 3.9, is 2.2 × 105 for 238U/3He (atomic ratio), or 2.6 × 109 ppm⋅g/cc. This is in the range of MORB (24). If NU (MM) is much lower than this, then the degassing event must be much older than the age of the crust under Hawaii.
If noble gases presently at Loihi were exsolved from the MORB reservoir at some time in the past, and subsequently stored in a refractory (LONU) environment, one can calculate the 21Ne/22Ne and 3He/22Ne ratios of MM, from the difference in 3He/4He between MORB and Loihi and the 4He/21Ne production ratio.
The growth of 21Ne, calculated from the growth of 4He via the production ratio, yields 3.4–4.6 for the atomic ratio 3He/22Ne. The lower values are close to the solar ratio, consistent with the solar ratios for 20Ne/22Ne found in MORB. Ratios involving the radiogenic isotope 4He and the nucleogenic isotope 21Ne depend on υ. The production ratio 4He/21Ne is approximately 1–3.5 (× 107). If the Loihi 3He/4He ratio represents the MORB value at some time in the past, the 22Ne/21Ne ratio in MORB can be calculated from this ratio in Loihi basalts and the difference in R values. For a MM 22Ne/3He ratio of 0.24, a typical MORB value (11), a decrease of R from 30 Ra to 8 Ra (MORB) is accompanied by an increase in 21Ne/22Ne of 0.03, which agrees with the increase from Loihi basalts to MORB. This calculation has been used in the past to support the undegassed primitive mantle model. Furthermore, if gases inherited by Loihi basalts and xenoliths, and other OIB, were in equilibrium with MORB, they should have lower 3He/22Ne ratios than MORB (as well as high 3He/4He ratio) (Figs. 2 and 4).
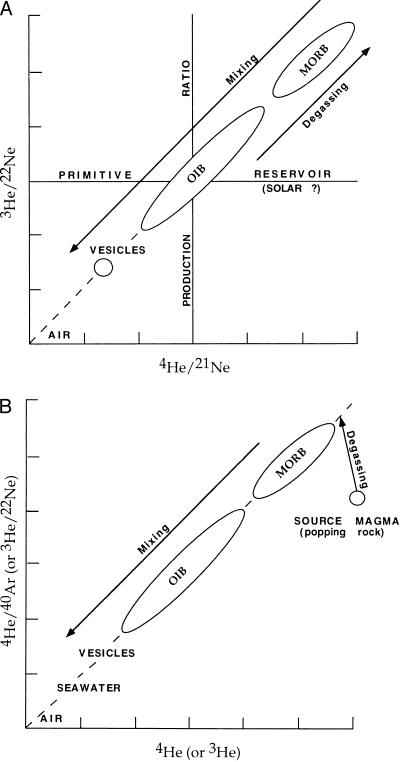
Figure 4.
(A) Degassing drives residual magmas toward high values of He/Ne. Reference values are production ratios for 4He/21Ne and solar values for 3He/22Ne. Gas evolved from decompressing magmas, and atmosphere/seawater values, are low. OIB fall in the field between MORB and air and may represent degassed magmas contaminated by atmospheric, xenolithic, and shallow mantle gases. (B) Degassing reduces the helium content of magmas and increases ratios such as 4He/40Ar and 3He/22Ne. Contamination with air- and fluid-filled inclusions (vesicles or vugs) decreases the noble gas ratios. Thus, both OIB and MORB can have higher 4He/40Ar and 3He/22Ne ratios than production ratios or primordial (solar) ratios.
Loihi basalts have much lower 3He/22Ne ratios than MORB (11, 25, 26), consistent with vesicles (and air) being the source of Loihi gases. Estimates of 3He/22Ne ratios of MORB (9.4) and Loihi (6.9) are both above the solar ratio (27). These high ratios can be explained by degassing of the MORB precursor, at depth, raising the He/Ne ratio in the residual melt and then contaminating this melt with shallow mantle vesicles and seawater-infiltrated crust, both serving to decrease He/Ne (Fig. 4A). In the steady-state primordial mantle models with noble gases leaking into the upper mantle, the 3He/22Ne ratios in EM and DUM must be identical. Porcelli and Wasserburg (5) predict a ratio of 4.5 for both reservoirs, much lower than observed.
Degassing of magma increases the He/Ne and He/Ar ratios of the residual magma because of the high solubility of He in the melt. MORB have higher ratios than OIB (Fig. 4). Mixing of degassed MORB with air or seawater gases or with gases expelled from previous magmatic episodes gives trends of 3He/22Ne vs. 4He/22Ne (Fig. 4A) and 4He/40Ar and 3He/22Ne vs. 4He or 3He (Fig. 4B), similar to those observed.
A decrease in 3He/4He over 108 years from ≈30 Ra to 8 Ra requires a υ of ≈2 × 105. If MM contains 10 ppb of 238U, the implied 3He content is 108 atoms/g, an order of magnitude lower than MORB that concentrates U and He, relative to MM, by the melting process. For an undegassed Earth, υ has been estimated to be ≈103 (5, 11, 26). Degassing increases this ratio. The He abundances and U/He ratios implied by the shallow mantle contamination model are therefore reasonable. One implication is that the MORB reservoir (MM) is depleted in noble gases, compared with chondrites, by ≈two orders of magnitude relative to uranium. Other volatile elements are depleted in MM by factors of >10 (3), and more extreme depletion of He is expected. The bulk of the mantle was degassed during accretion and should have even lower volatile contents. Part of the mantle may be volatile-rich because of recycling. However, it is the top of the slab that contains these volatiles, and they enter the mantle wedge at shallow depths; noble gases may not be efficiently recycled. Massive deep (>200 km) recycling of upper slab volatiles is unlikely (28). The shallow mantle is the main depository of the incompatible and volatile recycled components, even if the demetasomatised slab dives deeply into the mantle. The conclusion that the Earth is extensively depleted in very volatile elements relative to chondritic meteorites is not new but is different from the implications of current gas-rich models (5, 6, 9, 26). If the Earth differentiates during accretion (3), or if a late veneer is involved in the volatile budget of the Earth (29), the upper mantle should be more volatile-rich than the bulk of the mantle. The present model resolves one other minor paradox. R of HIMU basalts are slightly lower than MORB but 206Pb/204Pb ratios are substantially higher. Mixing calculations with low 3He contents in MORB do not satisfy the data (44). If the MORB source has higher He/Pb ratios than the endmember HIMU source, the basalt data for HIMU islands can be satisfied.
Ridges and subduction zones migrate about the surface of the Earth. These processes introduce U (22) into the mantle and remove He, giving a progressive increase in υ (and μ) of the upper mantle. The upper mantle is processed about once every 1 or 2 Ga (30). The present high value for υ cannot be extrapolated far into the past and excessively large 3He/4He ratios in the distant past are not expected. Multiple stage evolutionary models are required to explain Pb and other isotopes. Nevertheless, some MORB have 238U/206Pb ratios close to the geochron and therefore are consistent with a closed system evolution for 4.5 Ga. The most gas-rich MORB have He-U ages of ≈1–2 Ga (31), a minimum age assuming undegassed magma. The relatively high 129Xe content of MORB also is consistent with an ancient reservoir. If the difference between MORB and Loihi helium (and neon) isotopic ratios evolved over ≈109 years, instead of the age of the crust under Hawaii, then the LONU source of Loihi simply may be the mantle complement to the crustal HIMU component.
The 4He-Heat Flow Paradox.
Only ≈10% of the 4He flux predicted from heat flow is observed to degas from the Earth (32). In contrast, ≈70% of the 40Ar produced by 40K decay is in the atmosphere, indicating efficient degassing of Ar in early Earth history (3, 33). Part of the discrepancy may be due to the relatively high solubility of He in magma and the retention of CO2 and helium in the shallow mantle. The gas content of average MORB vs. popping rock implies that most MORB have lost 90% or more of their gas, at depth, before extrusion and eruption (34). The He-heat flow paradox indicates that He is not easily or promptly degassed. Helium in undegassed magmas, or trapped in the upper mantle, may be recycled and retained in the upper mantle for a considerable period. With usual estimates of U abundances in MM, it takes the age of the Earth to build up the observed levels of 4He and 21Ne in a closed system (30, 31). With 200–300 ppb of U, the observed 4He levels (both concentrations referring to the basalt, not the source) can be built up during periods of time comparable with the isolation time [(1 to 2) × 109 years] of a given portion of the upper mantle (30).
There is no support for the hypotheses that hotspots dominate the 3He-degassing budget of the Earth and that high R magmas are more extensively degassed than MORB. The relatively high 4He/21Ne, 3He/22Ne, and 4He/CO2 ratios in MORB suggest that MORB is helium-rich compared with high R basalts and, therefore, that the high 4He/3He ratio in MORB is due to excess 4He, not a deficit in the “primordial” isotope 3He. The 238U/206Pb ratio in some MORB imply closed system evolution for nearly the age of the Earth. If MORB are 80% degassed just before or during eruption, then the helium age of the MORB reservoir (inferred from U/4He) also is comparable to the age of the Earth, and MM can be viewed as an ancient, relatively undegassed reservoir, the opposite of the usual interpretation.
As magmas ascend to the surface, or crystallize, CO2 is released, some of which is trapped in refractory crystals and in magma chambers. The trapping temperature is ≈600°C (35), roughly the temperature at which the lithosphere forms (36). These CO2–He-rich fluid inclusions represent time capsules, which retain the high 3He/4He ratios of a previous generation of ocean ridge magmatism. These time capsules are isolated from mantle convection by the buoyancy and strength of the lithosphere and perisphere. The upper mantle may contain large reservoirs of free fluid CO2 in fluid-filled cavities. Up to 3 vol percent fluid (CO2, He, Ar … ) has been found in cavities up to 1.5 cm in length in mantle xenoliths from Australia (42). Decompressing or crystallizing magma releases volatiles to the wall rock, and these volatiles may be picked up by magmas passing through this vesicular upper mantle (42). A CO2-rich shallow mantle may be at least a partial explanation for the missing CO2 in the atmosphere, oceans, sediments, and crust.
The heavy noble gases in Loihi basalts are the result of contamination with atmosphere derived noble gases dissolved in seawater (25). Other characteristics of hotspot basalts and xenoliths with high R, include carbonate and other contamination indicators. Iodine is a tracer of pelagic sediments, and I is also high in Loihi basalts (37). Interplanetary dust particles (IDP), abundant in pelagic sediments, have high 3He contents and 3He/4He ratios. It has been a paradox that midplate basalts and xenoliths with the most “primitive” character (high R) also show extensive contamination with material of atmospheric, seawater and sedimentary heritage.
The standard models of noble gas geochemistry involve two large hypothetical reservoirs, the upper (degassed) and lower (undegassed) mantles (5, 6, 9). The present model also involves two reservoirs, the MORB and the shallow (regassed) mantles, but the total fertile and gas-rich mantle is a small fraction of the Earth. Helium and CO2 are trapped in the shallow mantle and are released by midplate tectonic and magmatic processes, rather than by deep-seated convective processes. Melt-vapor fractionation has been invoked before (33, 38) but usually as related to early processes and atmospheric formation, not the final stages of midplate magmatism.
The present paper interprets the spread in R as being due to differing ages and υ of the reservoirs. It is also possible that high R sources have a different origin than MORB gases; for example, a late veneer (30).
Most of the characteristics of “hotspot” magmas such as isotopic chemistry (Sr, Nd, Pb, O, and C), oxidation state, and noble gas abundance patterns are consistent with near-surface interactions. The recognition that ocean-island basalts are deficient in 4He means that there is no evidence for an undegassed reservoir. All of the characteristic geochemical signatures in ocean island basalts can be attributed to near surface processes.
The MORB reservoir is often treated as a depleted, degassed reservoir, complementary to the continental crust. The present model views these two reservoirs as complementary to the rest of the mantle and together these three reservoirs add up to “primordial mantle,” a mantle that has ceased to exist a long time ago, probably during accretion (3). The abundances of volatile and very incompatible elements (I, Cl, Ba, Cs … ) in the exosphere imply that most of the Earth has been processed, depleted, and degassed (3, 43).
The tectonic model developed in this paper resolves many of the paradoxes associated with the “primordial, undegassed” reservoir model (45). The idea that the Hawaiian Swell, and other hotspot swells, may be thick refractory peridotite has recently received additional support from seismology (47). These swells may predate the crust.
Acknowledgments
I thank David Graham, John Eiler, Des Patterson, Youxue Zhang, Pete Burnard, Masahiko Honda, and Don Porcelli for constructive comments. Conversations with Ken Farley, Marc Javoy, David Graham, and Barry Hanan have been helpful. Supported by National Science Foundation Grant EAR 97-26252 Contribution no. 8530, Division of Geological and Planetary Sciences, California Institute of Technology.
ABBREVIATIONS
- MORB
midocean ridge basalt
- OIB
ocean island basalts
- MM
MORB mantle
- HIMU
high values of 238U/204Pb
- LONU
low values of 238U/3He
Footnotes
To whom reprint requests should be addressed. e-mail: dla@gps.caltech.edu.
References
- 1.Boss A P. In: Origin of the Earth. Newson H E, Johns J H, editors. New York: Oxford Univ. Press; 1990. p. 375. [Google Scholar]
- 2.Schubert G, Turcotte D L, Solomon S C, Sleep N. In: Origin and Evolution of Planetary and Satellite Atmospheres. Atreya S K, Pollack J B, Matthews M S, editors. Tucson, AZ: Univ. Arizona Press; 1989. p. 881. [Google Scholar]
- 3.Anderson D L. Theory of the Earth. Oxford: Blackwell Scientific; 1989. p. 366. [Google Scholar]
- 4.Schubert G, Stevenson D, Cassen P. J Geophys Res. 1980;85:2531–2538. [Google Scholar]
- 5.Porcelli D, Wasserburg G J. Geochim Cosmochim Acta. 1995;59:4921–4937. [Google Scholar]
- 6.Kellogg L H, Wasserburg G J. Earth Planet Sci Lett. 1990;99:276–289. [Google Scholar]
- 7.Hilton D R, Hammerschmidt K, Loock G, Friedrichsen H. Geochim Cosmochim Acta. 1993;57:2819–2841. [Google Scholar]
- 8.Kurz M D, Jenkins W J, Hart S R, Schilling J-D. Earth Planet Sci Lett. 1982;58:1–14. [Google Scholar]
- 9.O’Nions R K, Tolstikhin I N. Earth Planet Sci Lett. 1994;124:131–138. [Google Scholar]
- 10.Sano Y, Williams S. Geophys Res Lett. 1996;23:2749–2752. [Google Scholar]
- 11.Farley K A, Poreda R J. Earth Planet Sci Lett. 1993;114:325–339. [Google Scholar]
- 12.Matsuda J, Marty B. Geophys Res Lett. 1995;22:1937–1940. [Google Scholar]
- 13.Klein F, Koyanagi R. In: The Geology of North America. Winterer E L, Hussong D, Decker R, editors. N. Boulder, CO: Geol. Soc. Am.; 1989. p. 238. [Google Scholar]
- 14.Vance D, Stone J O H, O’Nions R K. Earth Planet Sci Lett. 1989;96:147–169. [Google Scholar]
- 15.Poreda R J, Farley K A. Earth Planet Sci Lett. 1992;113:129–144. [Google Scholar]
- 16.Kurz M, Garcia M, Frey F, O’Brien P. Geochim Cosmochim Acta. 1987;51:2905–2914. [Google Scholar]
- 17.Graham D, Lupton J, Albarede F, Condomines M. Nature (London) 1990;347:545–548. [Google Scholar]
- 18.Class C, Goldstein S L, Galer S J G, Weis D. Nature (London) 1993;362:715–721. [Google Scholar]
- 19.Halliday A N, Davies G R, Lee D C, Tommasini S, Paslick C R, Fitton J G, James D E. Nature (London) 1992;359:623–627. [Google Scholar]
- 20.Woods M, Okal E, Cara M. Geophys Res Lett. 1991;18:105–108. [Google Scholar]
- 21.Chauvel C, Hofmann A W, Vidal P. Earth Planet Sci Lett. 1992;110:99–119. [Google Scholar]
- 22.McCulloch M T. Earth Planet Sci Lett. 1993;115:89–100. [Google Scholar]
- 23.White W M. Earth Planet Sci Lett. 1993;115:211–226. [Google Scholar]
- 24.Zindler A, Hart S. Earth Planet Sci Lett. 1986;79:1–8. [Google Scholar]
- 25.Honda M, McDougall I, Patterson D. Lithos. 1993;30:257–265. [Google Scholar]
- 26.Harper C L, Jacobsen S B. Science. 1996;273:1814–1818. [Google Scholar]
- 27.Honda M, McDougall I. Seventh Annual V. M. Goldschmidt Conference. Houston: Lunar Planetary Inst.; 1997. , no. 921, pp. 98–99. [Google Scholar]
- 28.Anderson D L. Int Geol Rev. 1996;38:1–21. [Google Scholar]
- 29.Wänke H. Philos Trans R Soc London A. 1981;303:287–302. [Google Scholar]
- 30.Anderson D L. Science. 1993;261:170–176. doi: 10.1126/science.261.5118.170. [DOI] [PubMed] [Google Scholar]
- 31.Jambon A. In: Volatiles in Magmas. Carrol M R, Holloway J R, editors. Washington, DC: Mineral. Soc. Am.; 1994. , Chap. 12. [Google Scholar]
- 32.O’Nions R K, Oxburgh E R. Nature (London) 1983;306:429–431. [Google Scholar]
- 33.Zhang Y, Zindler A. J Geophys Res. 1989;94:13719–13737. [Google Scholar]
- 34.Sarda P, Graham D. Earth Planet Sci Lett. 1990;97:268–289. [Google Scholar]
- 35.Dunai T J, Touret J L R. Earth Planet Sci Lett. 1993;119:271–281. [Google Scholar]
- 36.Anderson D L. Rev Geophys. 1995;33:125–149. [Google Scholar]
- 37.Dérvelle B, Dreibus G, Jambon A. Earth Planet Sci Lett. 1992;108:217–227. [Google Scholar]
- 38.Hart R. Chem Geol. 1985;52:45–73. [Google Scholar]
- 39.Patterson C, Tatsumoto M. Geochim Cosmochim Acta. 1964;28:1–22. [Google Scholar]
- 40.Birch F. Geol Soc Am Bull. 1965;76:133–154. [Google Scholar]
- 41.Zindler A, Hart S. Earth Planet Sci Lett. 1986;79:1–8. [Google Scholar]
- 42.Anderson T, O’Reilly S Y, Griffin W L. Contrib Mineral Petrol. 1984;88:72–85. [Google Scholar]
- 43.Lodders K, Fegley B. Icarus. 1997;126:373–394. [Google Scholar]
- 44.Hanyu T, Kaneoka I. Geophys Res Lett. 1998;25:687–690. [Google Scholar]
- 45.Anderson D. Proc Natl Acad Sci USA. 1998;95:4822–4827. doi: 10.1073/pnas.95.9.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubin A M. Annu Rev Earth Planet Sci. 1995;23:287–336. doi: 10.1146/annurev.ea.23.050195.001243. [DOI] [PubMed] [Google Scholar]
- 47.Katzman R. Ph.D. thesis. Cambridge, MA: Mass. Inst. of Technol.; 1998. [Google Scholar]