Abstract
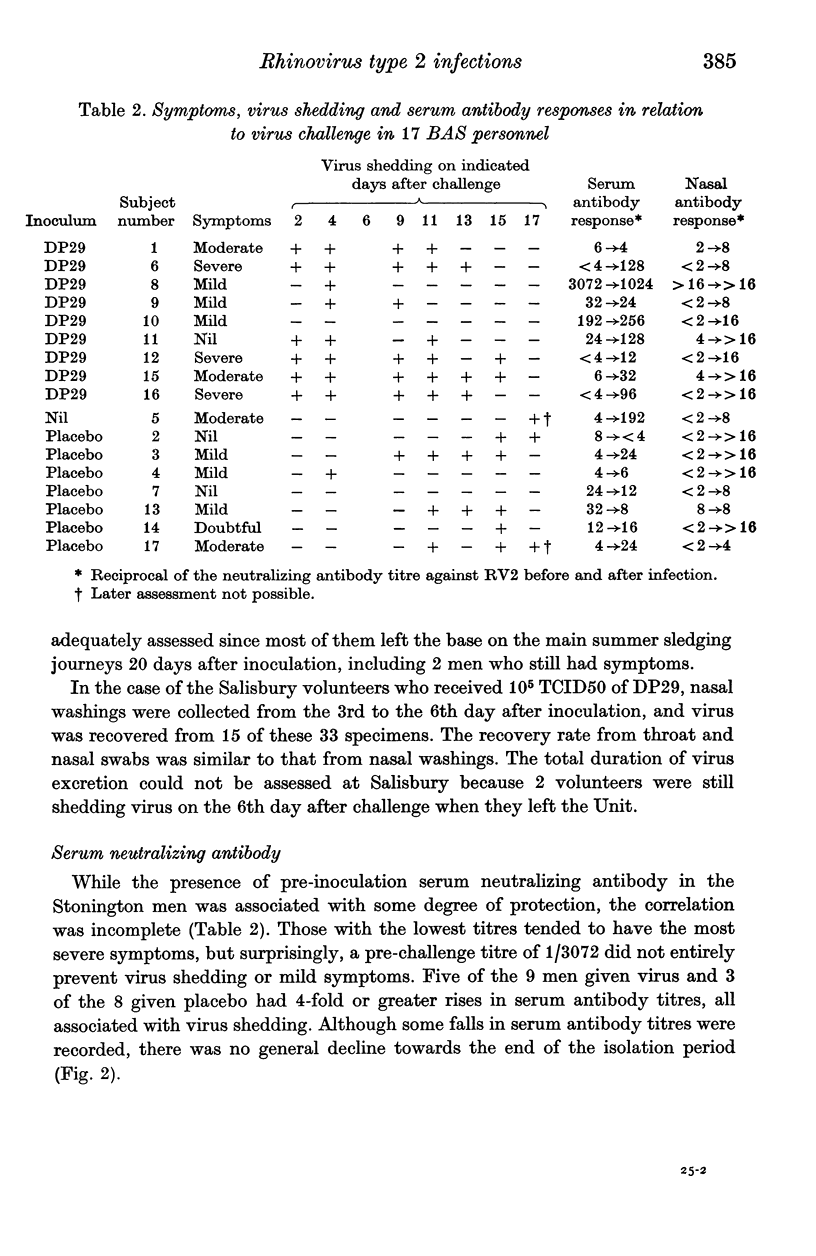
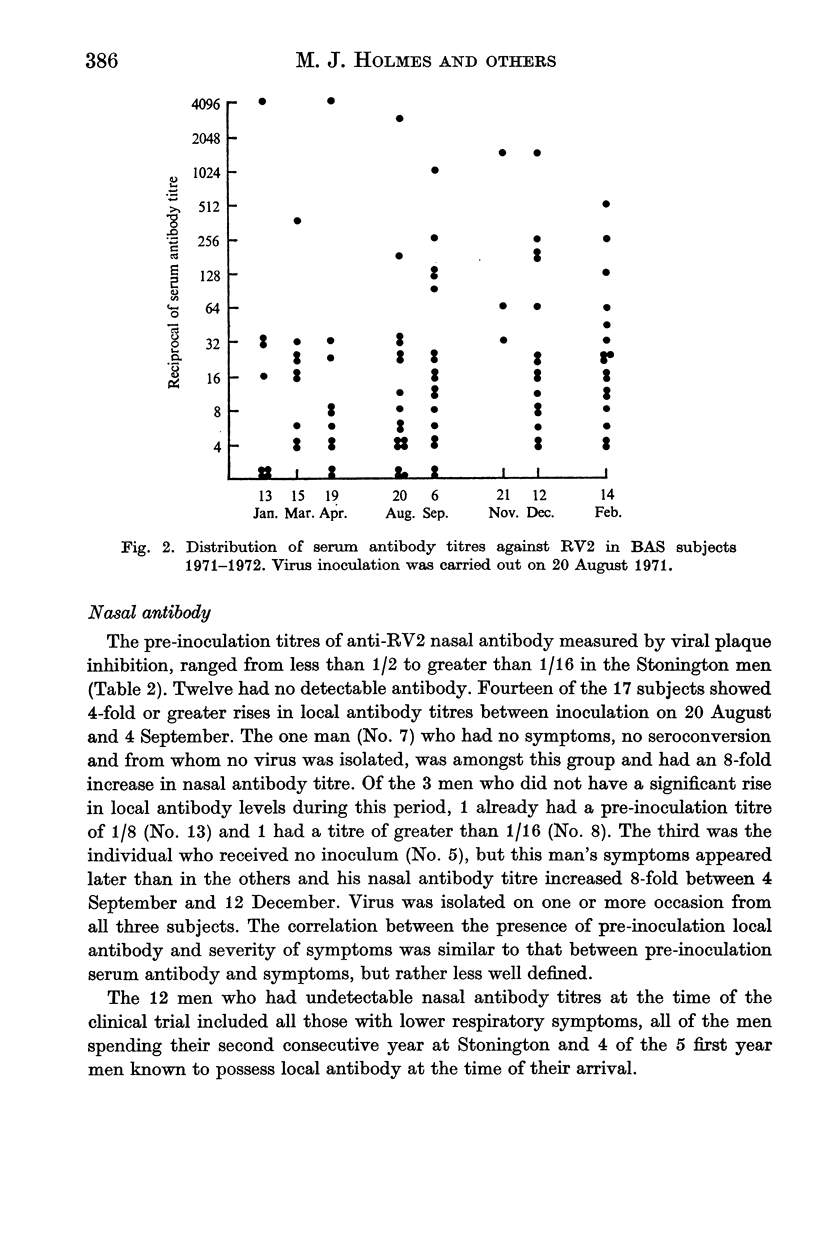
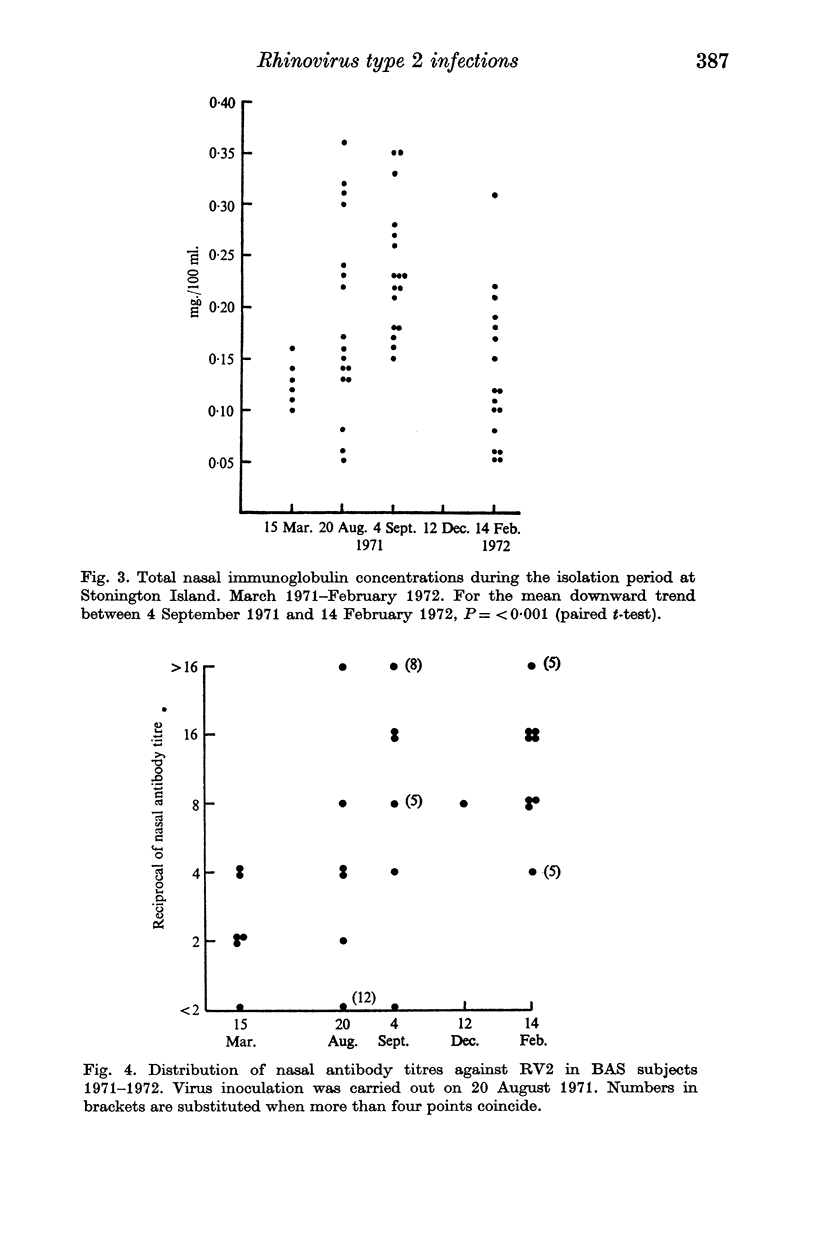
After five months of total isolation a wintering party of seventeen British Antarctic Survey (BAS) personnel was inoculated under double blind concitions with placebo, or rhinovirus type 2 which had been propagated in tissue culture. The clinical and virological responses of these subjects were compared with those of volunteers in England who received a similar dose of the same strain. The virus used was apparently partly attenuated for man; at the dosage used its effects in England were similar to a smaller dose of an unattenuated strain, but in the Antarctic it caused relatively severe infections. Both the symptoms and the laboratory evidence of virus infection appeared to be more pronounced in the BAS subjects than in the volunteers in England who received the same challenge. In the former group the infection readily spread to those who were originally given placebo. In the BAS subjects serum antibody titres were well maintained during the isolation period but a significant fall in nasal immunoglobulin concentration was recorded during the 5 months of isolation after the virus challenge. Possible mechanisms for the increased sensitivity to rhinovirus of subjects who have been totally isolated in a small closed community are discussed.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradburne A. F., Bynoe M. L., Tyrrell D. A. Effects of a "new" human respiratory virus in volunteers. Br Med J. 1967 Sep 23;3(5568):767–769. doi: 10.1136/bmj.3.5568.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscho R. F., Perkins J. C., Knopf H. L., Kapikian A. Z., Chanock R. M. Further characterization of the local respiratory tract antibody response induced by intranasal instillation of inactivated rhinovirus 13 vaccine. J Immunol. 1972 Jan;108(1):169–177. [PubMed] [Google Scholar]
- Butler W. T., Waldmann T. A., Rossen R. D., Douglas R. G., Jr, Couch R. B. Changes in IgA and IgG concentrations in nasal secretions prior to the appearance of antibody during viral respiratory infection in man. J Immunol. 1970 Sep;105(3):584–591. [PubMed] [Google Scholar]
- Cameron A. S., Moore B. W. The epidemiology of respiratory infection in an isolated Antarctic community. J Hyg (Lond) 1968 Sep;66(3):427–437. doi: 10.1017/s0022172400041292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate T. R., Douglas R. G., Jr, Couch R. B. Interferon and resistance to upper respiratory virus illness. Proc Soc Exp Biol Med. 1969 Jun;131(2):631–636. doi: 10.3181/00379727-131-33941. [DOI] [PubMed] [Google Scholar]
- Chaniot S. C., Holmes M. J., Stott E. J., Tyrrell D. A. An investigation of media for the long term storage of three respiratory viruses. Arch Gesamte Virusforsch. 1974;44(4):396–400. doi: 10.1007/BF01251022. [DOI] [PubMed] [Google Scholar]
- Couch R. B., Douglas R. G., Jr, Lindgren K. M., Gerone P. J., Knight V. Airborne transmission of respiratory infection with coxsackievirus A type 21. Am J Epidemiol. 1970 Jan;91(1):78–86. doi: 10.1093/oxfordjournals.aje.a121115. [DOI] [PubMed] [Google Scholar]
- Douglas R. G., Jr, Couch R. B. Attenuation of rhinovirus type 15 for humans. Nature. 1969 Jul 12;223(5202):213–214. doi: 10.1038/223213b0. [DOI] [PubMed] [Google Scholar]
- Holmes M. J., Allen T. R., Bradburne A. F., Stott E. J. Studies of respiratory viruses in personnel at an Antarctic base. J Hyg (Lond) 1971 Jun;69(2):187–199. doi: 10.1017/s0022172400021422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist U., Ceska M. Effect of non-ionic polymers on the formation of immuno-precipitates in single radial immunodiffusion techniques. Immunology. 1972 Sep;23(3):413–422. [PMC free article] [PubMed] [Google Scholar]
- Perkins J. C., Tucker D. N., Knopf H. L., Wenzel R. P., Kapikian A. Z., Chanock R. M. Comparison of protective effect of neutralizing antibody in serum and nasal secretions in experimental rhinovirus type 13 illness. Am J Epidemiol. 1969 Dec;90(6):519–526. doi: 10.1093/oxfordjournals.aje.a121098. [DOI] [PubMed] [Google Scholar]
- Stott E. J., Eadie M. B., Grist N. R. Rhinovirus infections of children in hospital; isolation of three possibly new rhinovirus serotypes. Am J Epidemiol. 1969 Jul;90(1):45–52. doi: 10.1093/oxfordjournals.aje.a121048. [DOI] [PubMed] [Google Scholar]
- Stott E. J., Tyrrell D. A. Some improved techniques for the study of rhinoviruses using HeLa cells. Arch Gesamte Virusforsch. 1968;23(3):236–244. doi: 10.1007/BF01241896. [DOI] [PubMed] [Google Scholar]
- Strizova V., Brown P. K., Head B., Reed S. E. The advantages of HeLa cells for isolation of rhinoviruses. J Med Microbiol. 1974 Nov;7(4):433–438. doi: 10.1099/00222615-7-4-433. [DOI] [PubMed] [Google Scholar]
- TYRRELL D. A. THE USE OF VOLUNTEERS. Am Rev Respir Dis. 1963 Sep;88:SUPPL–134. doi: 10.1164/arrd.1963.88.3P2.128. [DOI] [PubMed] [Google Scholar]
- Urquhart G. E., Stott E. J. Rhinoviraemia. Br Med J. 1970 Oct 3;4(5726):28–30. doi: 10.1136/bmj.4.5726.28. [DOI] [PMC free article] [PubMed] [Google Scholar]



