Abstract
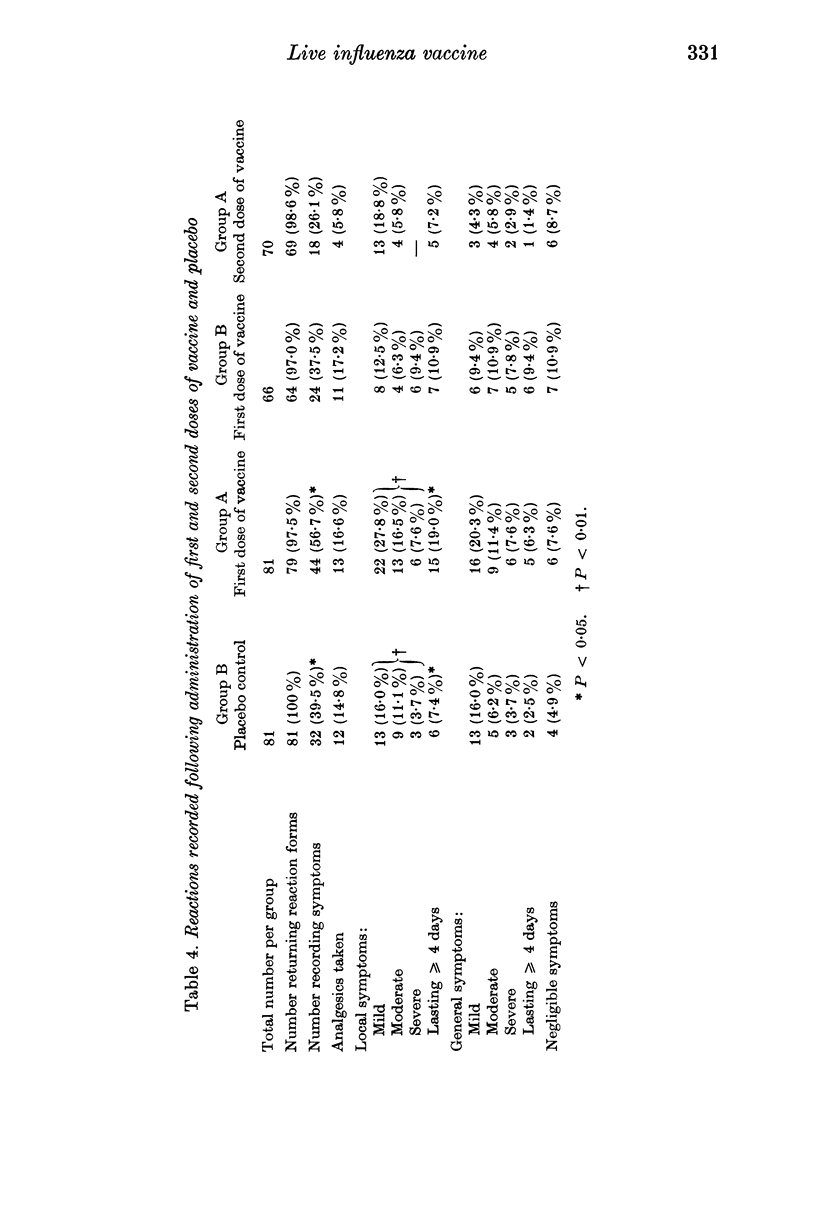
Haemagglutinating inhibiting antibody (HAI) responses were determined and clinical reactions recorded in 162 adult volunteers who received either 1 or 2 intranasal doses of 10(7-0) EID50 WRL 105 strain live influenza vaccine or placebo. After administration of a single dose of vaccine significant antibody responses were obtained in 69 (70%) of 98 volunteers with initial antibody titres of less than or equal to 1/20. Of the 70 volunteers who received a second dose of vaccine, 62 provided a further post-vaccination sample of serum, and only 3 (4-8%), who had not responded to the first dose of vaccine, produced a significant antibody response. Local, upper respiratory and constitutional symptoms were recorded more frequently after the administration of a first dose of vaccine than after placebo or a second dose of vaccine. The symptoms were of a minor nature except in one volunteer who, after the first dose of vaccine, developed influenzal symptoms followed by bronchitis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Freestone D. S., Bowker C. H., Letley E., Ferris R. D., White W. G., Barnes G. M. A clinical trial of WRL 105 strain live attenuated influenza vaccine comparing four methods of intranasal vaccination. J Hyg (Lond) 1976 Jun;76(3):459–466. doi: 10.1017/s002217240005539x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary I. B. Trials of intranasally administered rubella vaccine. J Hyg (Lond) 1971 Dec;69(4):547–552. doi: 10.1017/s0022172400021811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. A., Freestone D. S., Stealey V. M., Oliver P. R. Recombinant WRL 105 strain live attenuated influenza vaccine. Immunogenicity, reactivity, and transmissibility. Lancet. 1975 Aug 2;2(7927):196–199. doi: 10.1016/s0140-6736(75)90670-4. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Zealley H., Morrison A. M., Freestone D. S. Dose response studies with Wistar RA 27/3 strain live attenuated rubella vaccine. J Biol Stand. 1974 Apr;2(2):111–119. doi: 10.1016/0092-1157(74)90025-0. [DOI] [PubMed] [Google Scholar]


