Abstract
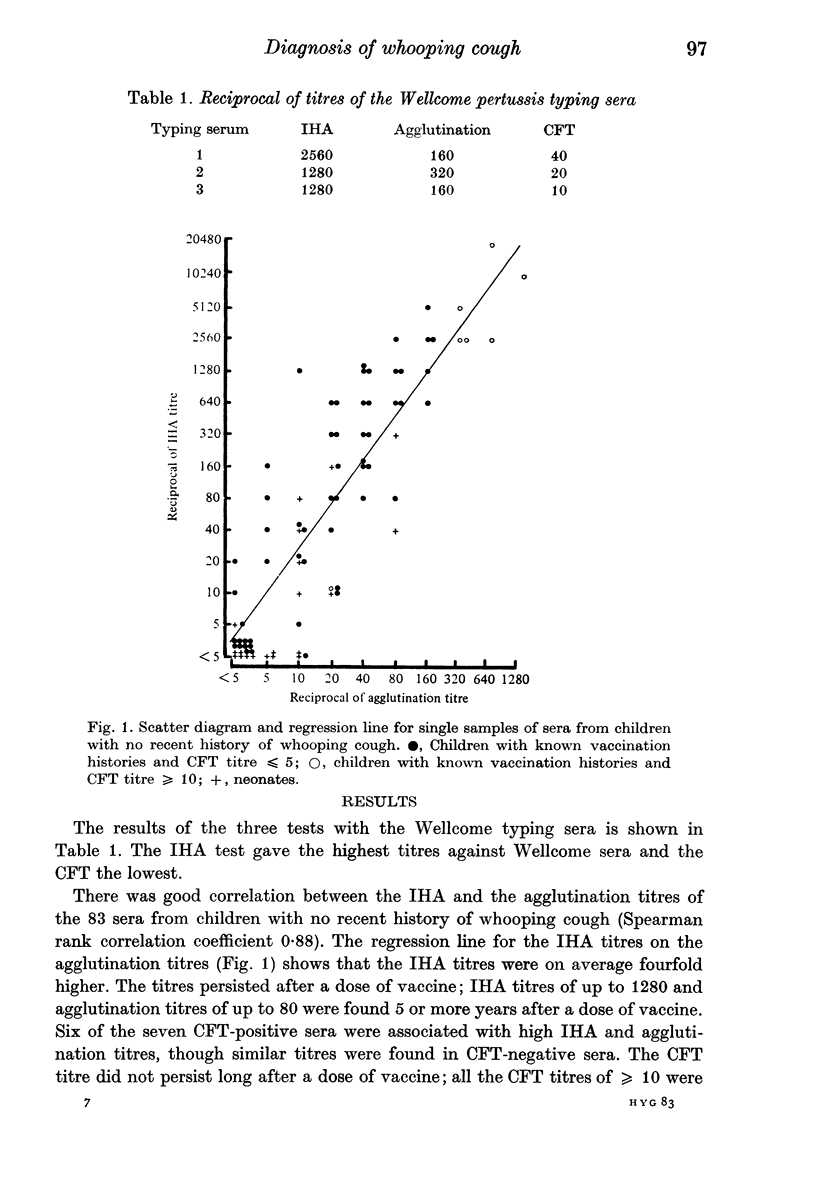
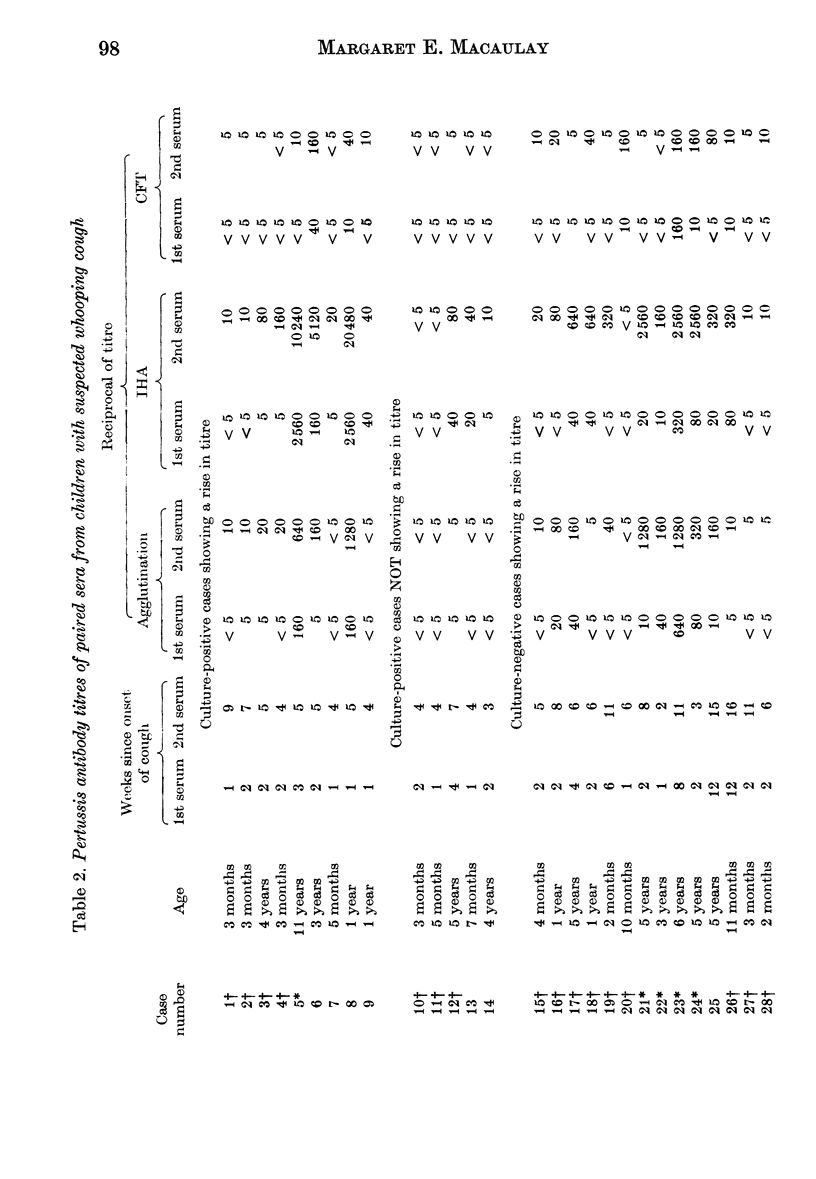
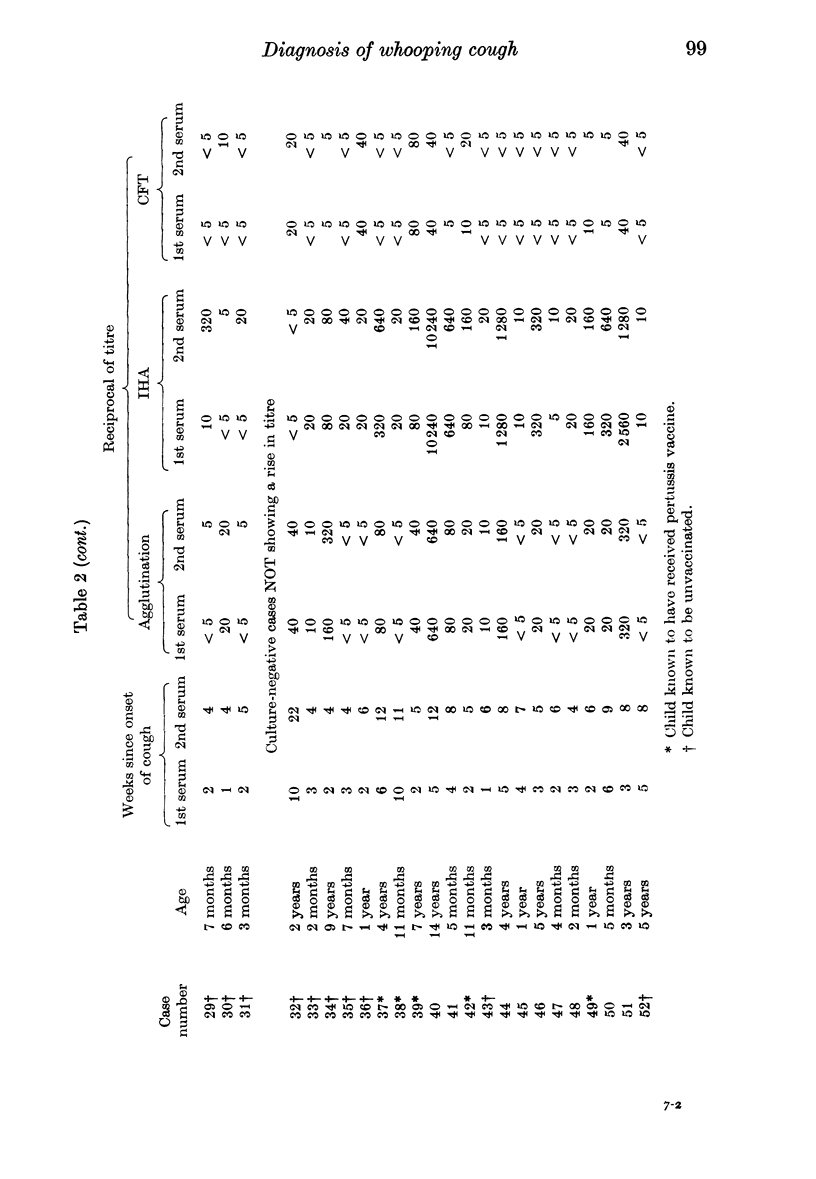
Indirect haemagglutination (IHA), agglutination and complement fixation tests (CFT) for Bordetella pertussis antibodies were compared on paired sera from 52 suspected cases of whooping cough and single sera from 83 children with no recent history of whooping cough. All three tests detected serotype antibodies 1, 2 and 3, but the IHA test was the most sensitive; in seven cases it was the only test to show a rise in titre. It is recommended, particularly with vaccinated children, that the serological diagnosis of whooping cough should be based upon a rise in titre. There should be a gap of at least 2-4 weeks between serum samples, depending on the age and vaccination state of the child. The CFT appears to detect a different antibody from that detected by the other two tests, and in three cases it was the only test to show a rise in titre.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN E. K. Serological studies on H. pertussis H. parapertussis and H. bronchisepticus. Acta Pathol Microbiol Scand. 1953;33(2):202–224. doi: 10.1111/j.1699-0463.1953.tb01512.x. [DOI] [PubMed] [Google Scholar]
- Bradstreet C. M., Tannahill A. J., Edwards J. M., Benson P. F. Detection of Bordetella pertussis antibodies in human sera by complement-fixation and immunofluorescence. J Hyg (Lond) 1972 Mar;70(1):75–83. doi: 10.1017/s0022172400022117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt L. B., Spasojevíc V. The role of surface antigens in the protective potency of Bordetella pertussis suspensions as measured by the intracerebral challenge technique in mice. J Med Microbiol. 1968 Aug;1(1):119–126. doi: 10.1099/00222615-1-1-119. [DOI] [PubMed] [Google Scholar]
- Linnemann C. C., Jr, Bass J. W., Smith M. H. The carrier state in pertussis. Am J Epidemiol. 1968 Nov;88(3):422–427. doi: 10.1093/oxfordjournals.aje.a120903. [DOI] [PubMed] [Google Scholar]
- Preston N. W., Stanbridge T. N. Efficacy of pertussis vaccines: a brighter horizon. Br Med J. 1972 Aug 19;3(5824):448–451. doi: 10.1136/bmj.3.5824.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston N. W. Technical problems in the laboratory diagnosis and prevention of whooping-cough. Lab Pract. 1970 May;19(5):482–486. [PubMed] [Google Scholar]
- SCHUBERT J. H., ELEFF M. G., HERMANN G. J. Hemagglutination test for pertussis antibody with a soluble extract of Bordetella pertussis. Am J Public Health Nations Health. 1961 Mar;51:441–445. doi: 10.2105/ajph.51.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira P. J., Eldridge A. E. Treponemal haemagglutination test. Br J Vener Dis. 1973 Jun;49(3):242–248. doi: 10.1136/sti.49.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soare I., Barber C., Dumitresco M., Scurtu A. Sur l'hémagglutination passive (indirecte) dans le diagnostic de la coqueluche; corrélation avec la réaction d'agglutination. Arch Roum Pathol Exp Microbiol. 1964 Mar;23(1):35–44. [PubMed] [Google Scholar]
- Thomas G. Respiratory and humoral immune response to aerosol and intramuscular pertussis vaccine. J Hyg (Lond) 1975 Apr;74(2):233–237. doi: 10.1017/s0022172400024293. [DOI] [PMC free article] [PubMed] [Google Scholar]


