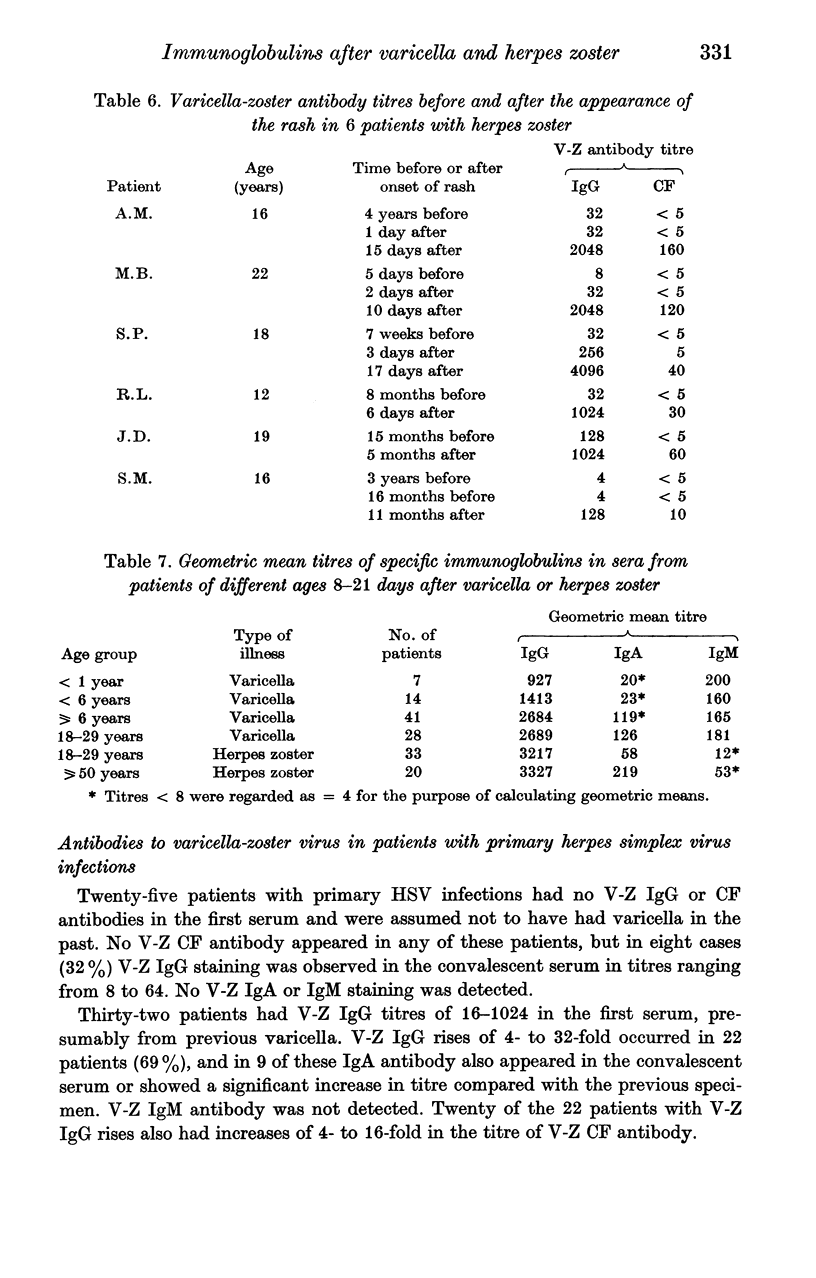
Abstract
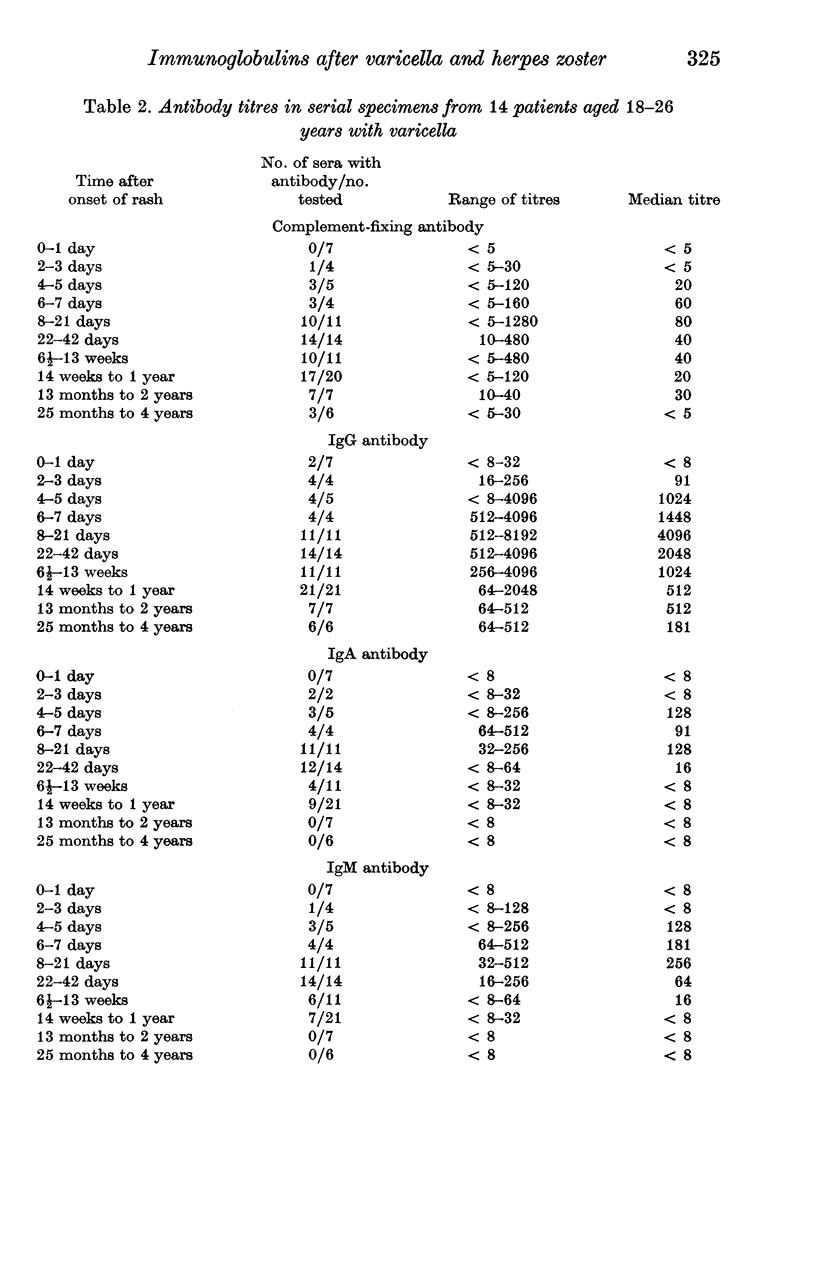
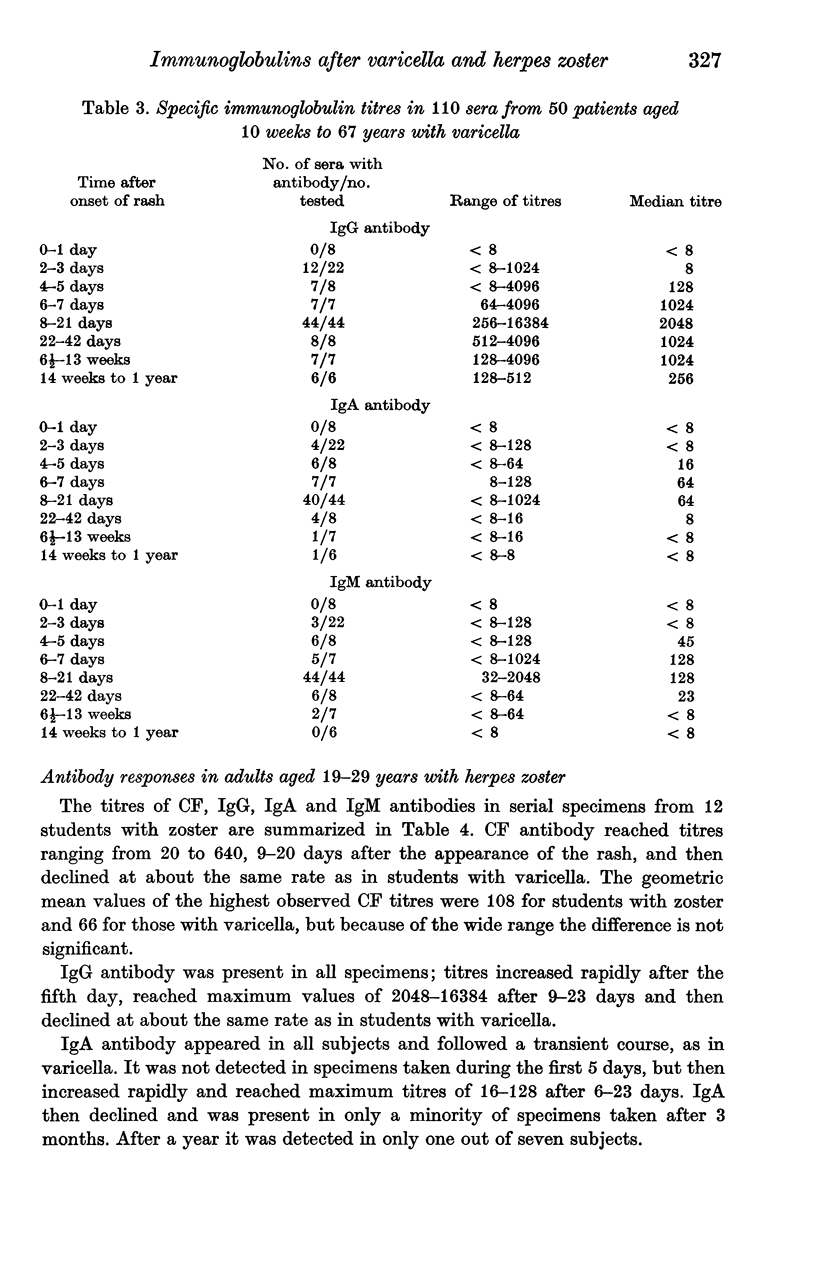
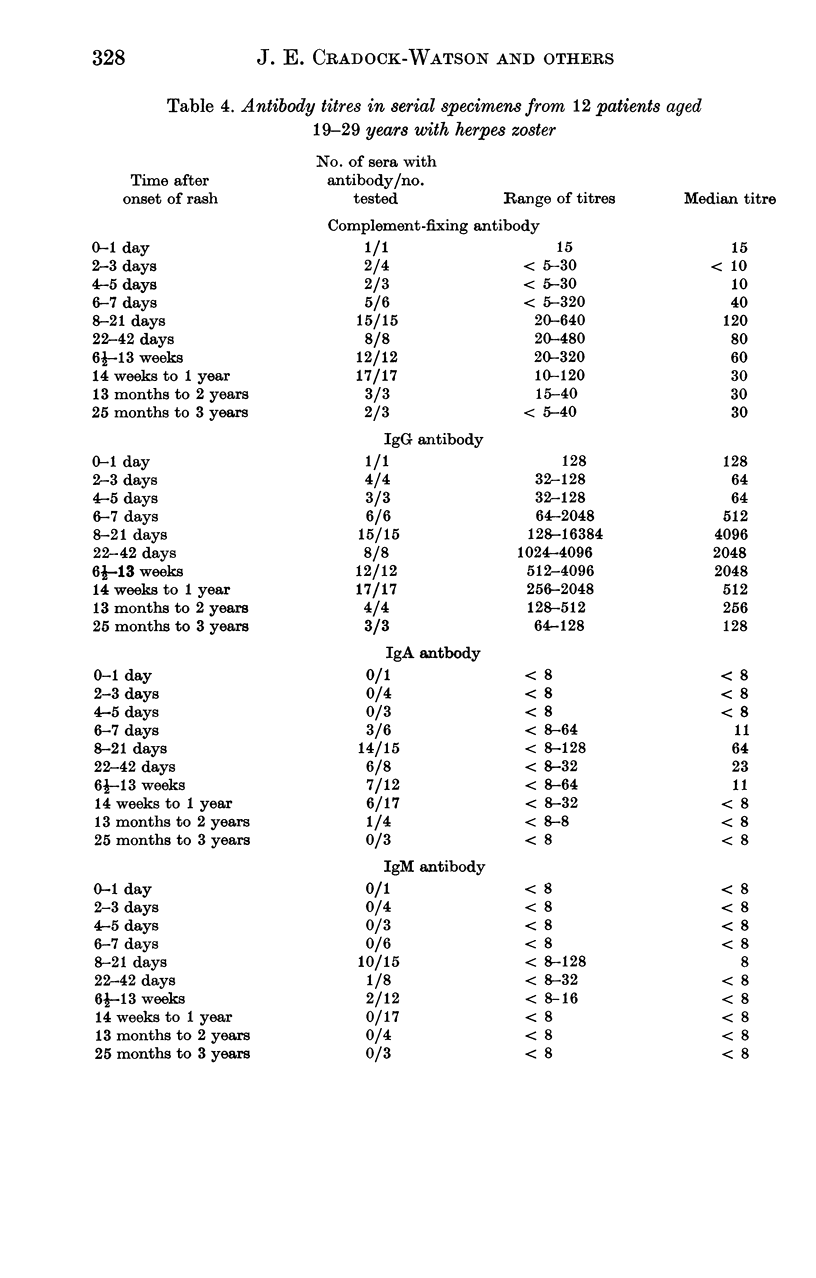
The indirect immunofluorescence technique has been used to titrate the specific immunoglobulins in 200 sera from 64 patients with varicella, and 195 sera from 67 patients with herpes zoster. IgG and IgM antibodies were detected in all patients with varicella, and IgA in 59 (92%). All three classes of antibody appeared 2--5 days after the onset of the rash, increased virtually simultaneously and reached maximum titres during the second and third weeks. IgG then declined slowly, but never became undetectable and was still present in five subjects who were retested after 2--4 years; it was present in 88 out of 100 healthy young adults and probably persists indefinitely after varicella. IgA and IgM antibodies declined more rapidly and were not detected in specimens taken more than a year after the illness. IgA, however, may possibly persist in some cases since low titres were found in 8 out of 88 young adults who possessed IgG antibody and had presumably had varicella in the past. IgA responses were significantly weaker in children under the age of 6 years than in older children and adults. Six out of 67 patients with zoster were tested at various times before the onset of the rash: IgG antibody was detected in all. IgG was present in all sera taken after the onset of the rash, increased rapidly after 2--5 days, reached maximum titres during the second and third weeks and then declined slowly. IgA antibody was detected in 66 patients (99%) and IgM in 52 (78%); both types of antibody followed transient courses, as in varicella. Maximum titres of IgG and complement-fixing antibodies were greater after zoster than after varicella, but the differences were not significant. IgA and IgM titres in young adults with zoster were significantly lower than in older patients, and also lower than in young adults with varicella. Increases in varicella-zoster antibody in patients with herpes simplex virus infections consisted mainly of IgG, sometimes IgA, but never IgM.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Nakib W., Best J. M., Banatvala J. E. Rubella-specific serum and nasopharygeal immunoglobulin responses following naturally acquired and vaccine-induced infection. Prolonged persistence of virus-specific IgM. Lancet. 1975 Jan 25;1(7900):182–185. doi: 10.1016/s0140-6736(75)91356-2. [DOI] [PubMed] [Google Scholar]
- Allansmith M., McClellan B. H., Butterworth M., Maloney J. R. The development of immunoglobulin levels in man. J Pediatr. 1968 Feb;72(2):276–290. doi: 10.1016/s0022-3476(68)80324-5. [DOI] [PubMed] [Google Scholar]
- BRADSTREET C. M., TAYLOR C. E. Technique of complementfixation test applicable to the diagnosis of virus diseases. Mon Bull Minist Health Public Health Lab Serv. 1962 May;21:96–104. [PubMed] [Google Scholar]
- BRUNELL P. A., CASEY H. L. CRUDE TISSUE CULTURE ANTIGEN FOR DETERMINATION OF VARICELLA-ZOSTER COMPLEMENT FIXING ANTIBODY. Public Health Rep. 1964 Sep;79:839–842. [PMC free article] [PubMed] [Google Scholar]
- Best J. M., Banatvala J. E., Watson D. Serum IgM and IgG responses in postnatally acquired rubella. Lancet. 1969 Jul 12;2(7611):65–68. doi: 10.1016/s0140-6736(69)92386-1. [DOI] [PubMed] [Google Scholar]
- Boué A., Nicolas A., Montagnon B. Reinfection with rubella in pregnant women. Lancet. 1971 Jun 19;1(7712):1251–1253. doi: 10.1016/s0140-6736(71)91775-2. [DOI] [PubMed] [Google Scholar]
- Brunell P. A., Gershon A. A., Uduman S. A., Steinberg S. Varicella-Zoster Immunoglobulins during Varicella, Latency, and Zoster. J Infect Dis. 1975 Jul;132(1):49–54. doi: 10.1093/infdis/132.1.49. [DOI] [PubMed] [Google Scholar]
- Buck A. C., Cooke E. M. The fate of ingested Pseudomonas aeruginosa in normal persons. J Med Microbiol. 1969 Nov 4;2(4):521–525. doi: 10.1099/00222615-2-4-521. [DOI] [PubMed] [Google Scholar]
- Buckley R. H., Dees S. C., O'Fallon W. M. Serum immunoglobulins. I. Levels in normal children and in uncomplicated childhood allergy. Pediatrics. 1968 Mar;41(3):600–611. [PubMed] [Google Scholar]
- Caunt A. E., Shaw D. G. Neutralization tests with varicella-zoster virus. J Hyg (Lond) 1969 Jun;67(2):343–352. doi: 10.1017/s0022172400041747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke E. M., Brayson J. C., Edmondson A. S., Hall D. An investigation into the incidence and sources of klebsiella infections in hospital patients. J Hyg (Lond) 1979 Jun;82(3):473–480. doi: 10.1017/s0022172400053997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke E. M., Hettiaratchy I. G., Buck A. C. Fate of ingested Escherichia coli in normal persons. J Med Microbiol. 1972 Aug;5(3):361–369. doi: 10.1099/00222615-5-3-361. [DOI] [PubMed] [Google Scholar]
- Cradock-Watson J. E., Bourne M. S., Vandervelde E. M. IgG, IgA and IgM responses in acute rubella determined by the immunofluorescent technique. J Hyg (Lond) 1972 Sep;70(3):473–485. doi: 10.1017/s0022172400063063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cradock-Watson J. E., Ridehalgh M. K. Specific immunoglobulins in infants with the congenital rubella syndrome. J Hyg (Lond) 1976 Feb;76(1):109–123. doi: 10.1017/s0022172400055005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson A. S., Cooke E. M. The development and assessment of a bacteriocin typing method for Klebsiella. J Hyg (Lond) 1979 Apr;82(2):207–223. doi: 10.1017/s0022172400025626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser K. B., Shirodaria P. V., Stanford C. F. Fluorescent staining and human IgM. Br Med J. 1971 Sep 18;3(5776):707–707. doi: 10.1136/bmj.3.5776.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Plotkin S. A. Indirect hemagglutination test for varicella-zoster infection. Infect Immun. 1972 Jun;5(6):835–839. doi: 10.1128/iai.5.6.835-839.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Achilli G., Chambers R. W. Determination of neutralizing antibody and IgG antibody to varicella-zoster virus and of IgG antibody to membrane antigens by the immunoperoxidase technique. J Infect Dis. 1977 Jun;135(6):975–979. doi: 10.1093/infdis/135.6.975. [DOI] [PubMed] [Google Scholar]
- Gershon A. A., Krugman S. Seroepidemiologic survey of varicella: Value of specific fluorescent antibody test. Pediatrics. 1975 Dec;56(6):1005–1008. [PubMed] [Google Scholar]
- Gershon A. A., Steinberg S., Brunell P. A. Zoster immune globulin. A further assessment. N Engl J Med. 1974 Jan 31;290(5):243–245. doi: 10.1056/NEJM197401312900503. [DOI] [PubMed] [Google Scholar]
- Gold E., Godek G. Complement fixation studies with a varicella-zoster antigen. J Immunol. 1965 Oct;95(4):692–695. [PubMed] [Google Scholar]
- Horstmann D. M., Liebhaber H., Le Bouvier G. L., Rosenberg D. A., Halstead S. B. Rubella: reinfection of vaccinated and naturally immune persons exposed in an epidemic. N Engl J Med. 1970 Oct 8;283(15):771–778. doi: 10.1056/NEJM197010082831501. [DOI] [PubMed] [Google Scholar]
- Jacobs J. P., Jones C. M., Baille J. P. Characteristics of a human diploid cell designated MRC-5. Nature. 1970 Jul 11;227(5254):168–170. doi: 10.1038/227168a0. [DOI] [PubMed] [Google Scholar]
- KRUGMAN S., GILES J. P., FRIEDMAN H., STONE S. STUDIES ON IMMUNITY TO MEASLES. J Pediatr. 1965 Mar;66:471–488. doi: 10.1016/s0022-3476(65)80112-3. [DOI] [PubMed] [Google Scholar]
- Leonard L. L., Schmidt N. J., Lennette E. H. Demonstration of viral antibody activity in two immunoglobulin G subclasses in patients with varicella-zoster virus infection. J Immunol. 1970 Jan;104(1):23–27. [PubMed] [Google Scholar]
- MCGREGOR R. M. Herpes zoster, chicken-pox, and cancer in general practice. Br Med J. 1957 Jan 12;1(5010):84–87. doi: 10.1136/bmj.1.5010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald H., Tobin J. O., Cradock-Watson J. E., Lomax J. Antibody titres in women six to eight years after the administration of RA2713 and Cendehill rubella vaccines. J Hyg (Lond) 1978 Jun;80(3):337–347. doi: 10.1017/s0022172400024785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. H., Brunell P. A. Zoster, reinfection or activation of latent virus? Observations on the antibody response. Am J Med. 1970 Oct;49(4):480–483. doi: 10.1016/s0002-9343(70)80042-0. [DOI] [PubMed] [Google Scholar]
- Ogra P. L., Kerr-Grant D., Umana G., Dzierba J., Weintraub D. Antibody response in serum and nasopharynx after naturally acquired and vaccine-induced infection with rubella virus. N Engl J Med. 1971 Dec 9;285(24):1333–1339. doi: 10.1056/NEJM197112092852401. [DOI] [PubMed] [Google Scholar]
- Ohta-Hatano R., Hinuma Y. Mercaptoethanol-sensitive neutralizing-antibody in natural infection with coxsackievirus B5. Proc Soc Exp Biol Med. 1966 Jul;122(3):725–729. doi: 10.3181/00379727-122-31237. [DOI] [PubMed] [Google Scholar]
- Pattison J. R., Dane D. S. The routine serological investigation of cases and contacts of rubella. J Hyg (Lond) 1975 Aug;75(1):91–98. doi: 10.1017/s0022172400047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike R. M. Antibody heterogeneity and serological reactions. Bacteriol Rev. 1967 Jun;31(2):157–174. doi: 10.1128/br.31.2.157-174.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C. A., McDaid R. Specific IgM antibody in serum of patients with herpes zoster infections. Br Med J. 1972 Dec 2;4(5839):522–523. doi: 10.1136/bmj.4.5839.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVEHAG S. E., MANDEL B. THE FORMATION AND PROPERTIES OF POLIOVIRUS-NEUTRALIZING ANTIBODY. II. 19S AND 7S ANTIBODY FORMATION: DIFFERENCES IN ANTIGEN DOSE REQUIREMENT FOR SUSTAINED SYNTHESIS, ANAMNESIS, AND SENSITIVITY TO X-IRRADIATION. J Exp Med. 1964 Jan 1;119:21–39. doi: 10.1084/jem.119.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H., Magoffin R. L. Immunological relationship between herpes simplex and varicella-zoster viruses demonstrated by complement-fixation, neutralization and fluorescent antibody tests. J Gen Virol. 1969 Apr;4(3):321–328. doi: 10.1099/0022-1317-4-3-321. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H. Neutralizing antibody responses to varicella-zoster virus. Infect Immun. 1975 Sep;12(3):606–613. doi: 10.1128/iai.12.3.606-613.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H., Woodie J. D., Ho H. H. Immunofluorescent staining in the laboratory diagnosis of varicella-zoster virus infections. J Lab Clin Med. 1965 Sep;66(3):403–412. [PubMed] [Google Scholar]
- Selden R., Lee S., Wang W. L., Bennett J. V., Eickhoff T. C. Nosocomial klebsiella infections: intestinal colonization as a reservoir. Ann Intern Med. 1971 May;74(5):657–664. doi: 10.7326/0003-4819-74-5-657. [DOI] [PubMed] [Google Scholar]
- Shinebaum R., Cooke E. M., Brayson J. C. Acquisition of Klebsiella aerogenes by neonates. J Med Microbiol. 1979 May;12(2):201–205. doi: 10.1099/00222615-12-2-201. [DOI] [PubMed] [Google Scholar]
- TAYLOR-ROBINSON D., DOWNIE A. W. Chickenpox and herpes zoster. 1. Complement fixation studies. Br J Exp Pathol. 1959 Aug;40:398–409. [PMC free article] [PubMed] [Google Scholar]
- TAYLOR-ROBINSON D., RONDLE C. J. Chickenpox and herpes zoster. II. Ouchterlony precipitation studies. Br J Exp Pathol. 1959 Dec;40:517–520. [PMC free article] [PubMed] [Google Scholar]
- WELLER T. H., COONS A. H. Fluorescent antibody studies with agents of varicella and herpes zoster propagated in vitro. Proc Soc Exp Biol Med. 1954 Aug-Sep;86(4):789–794. doi: 10.3181/00379727-86-21235. [DOI] [PubMed] [Google Scholar]
- WELLER T. H., WITTON H. M. The etiologic agents of varicella and herpes zoster; serologic studies with the viruses as propagated in vitro. J Exp Med. 1958 Dec 1;108(6):869–890. doi: 10.1084/jem.108.6.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams V., Gershon A., Brunell P. A. Serologic response to varicella-zoster membrane antigens measured by direct immunofluorescence. J Infect Dis. 1974 Dec;130(6):669–672. doi: 10.1093/infdis/130.6.669. [DOI] [PubMed] [Google Scholar]