Abstract
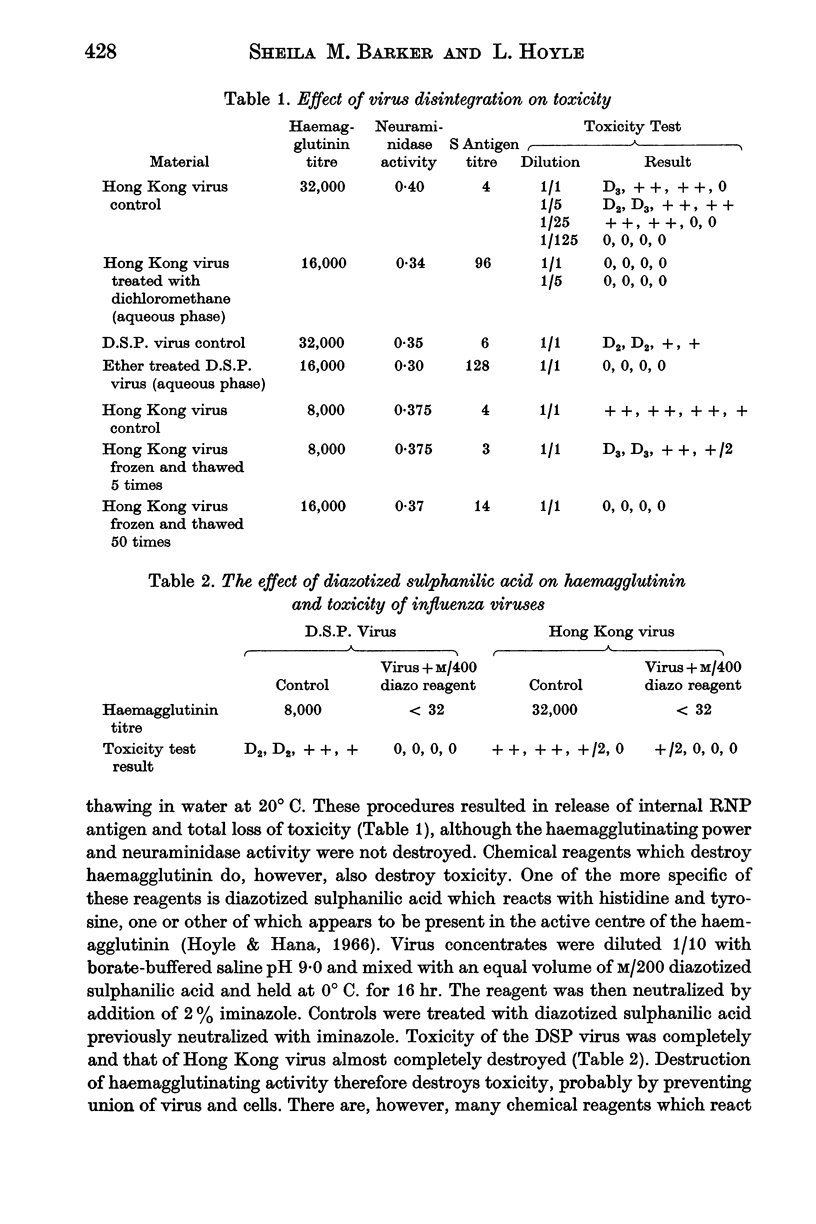
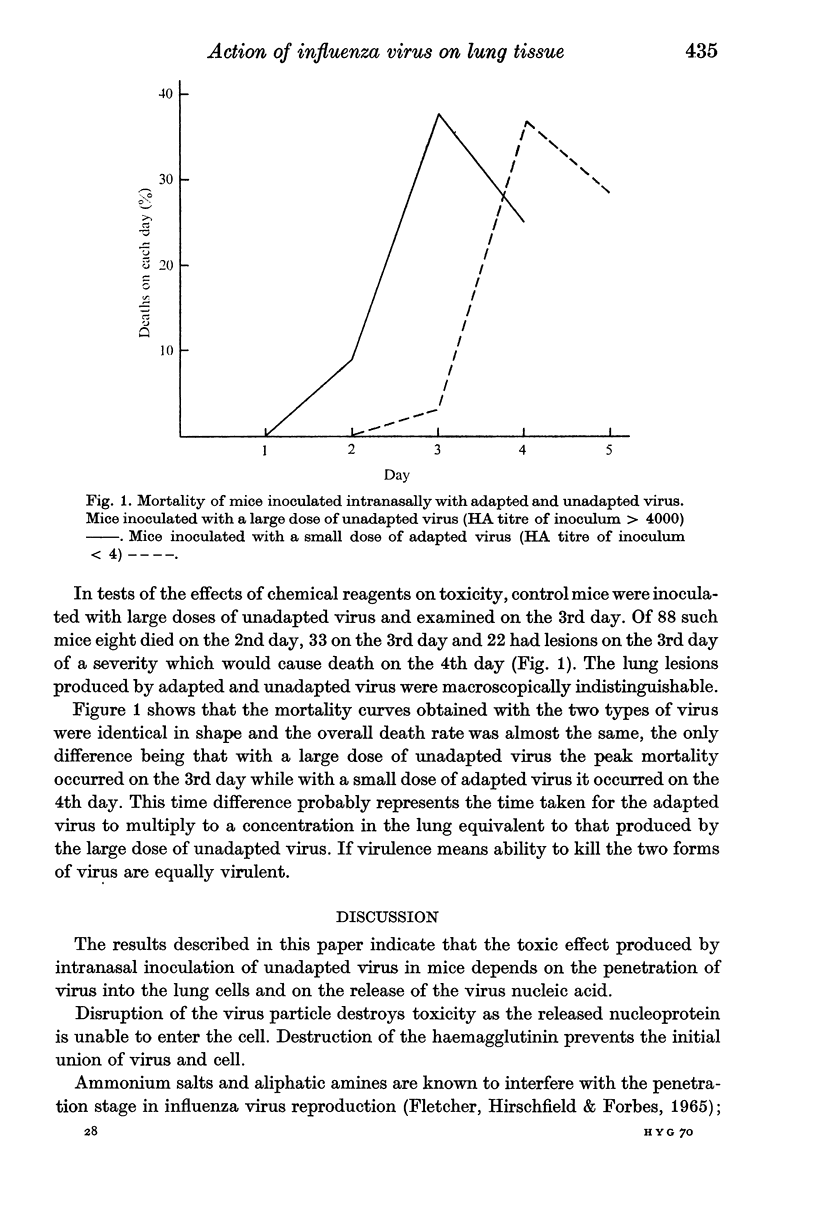
The inoculation of large doses of unadapted influenza virus intranasally into mice results in the production of severe lung lesions. This toxic effect is a result of the entry of virus particles into the lung cells followed by uncoating of the virus ribonucleic acid.
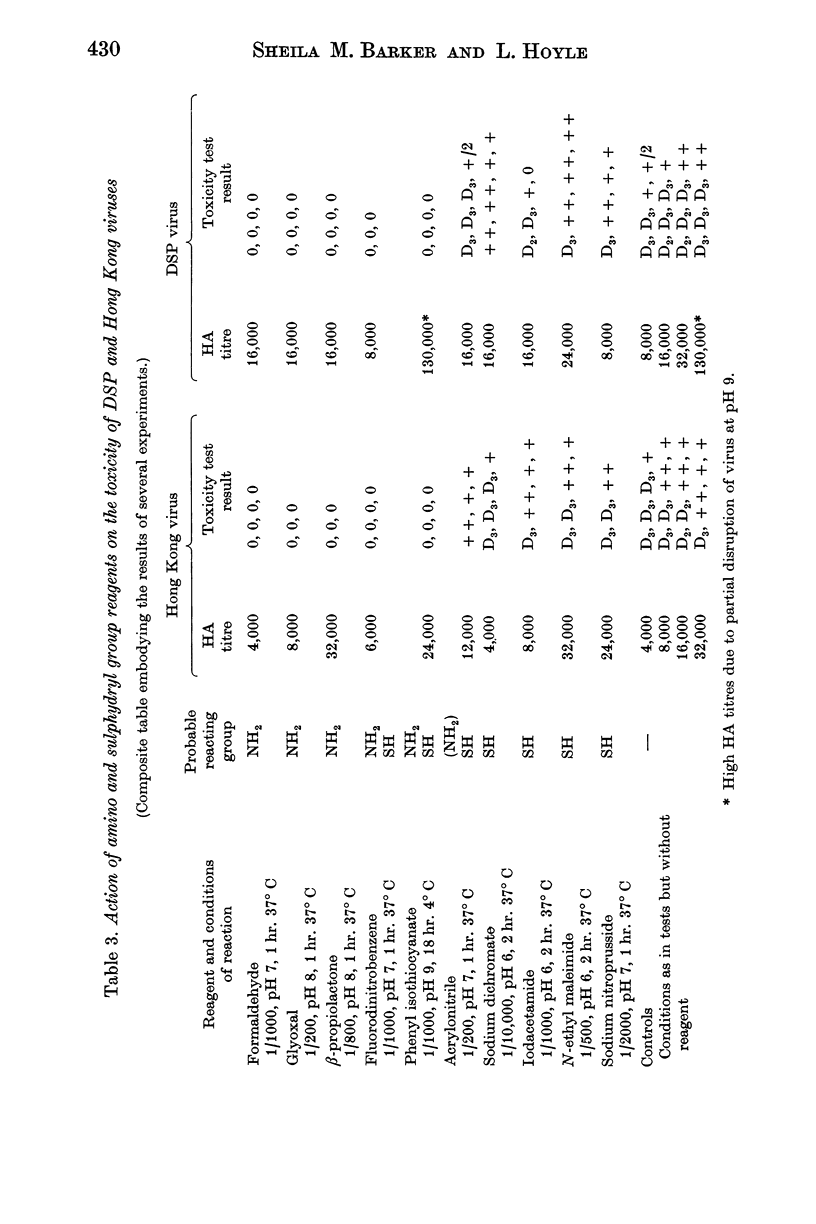
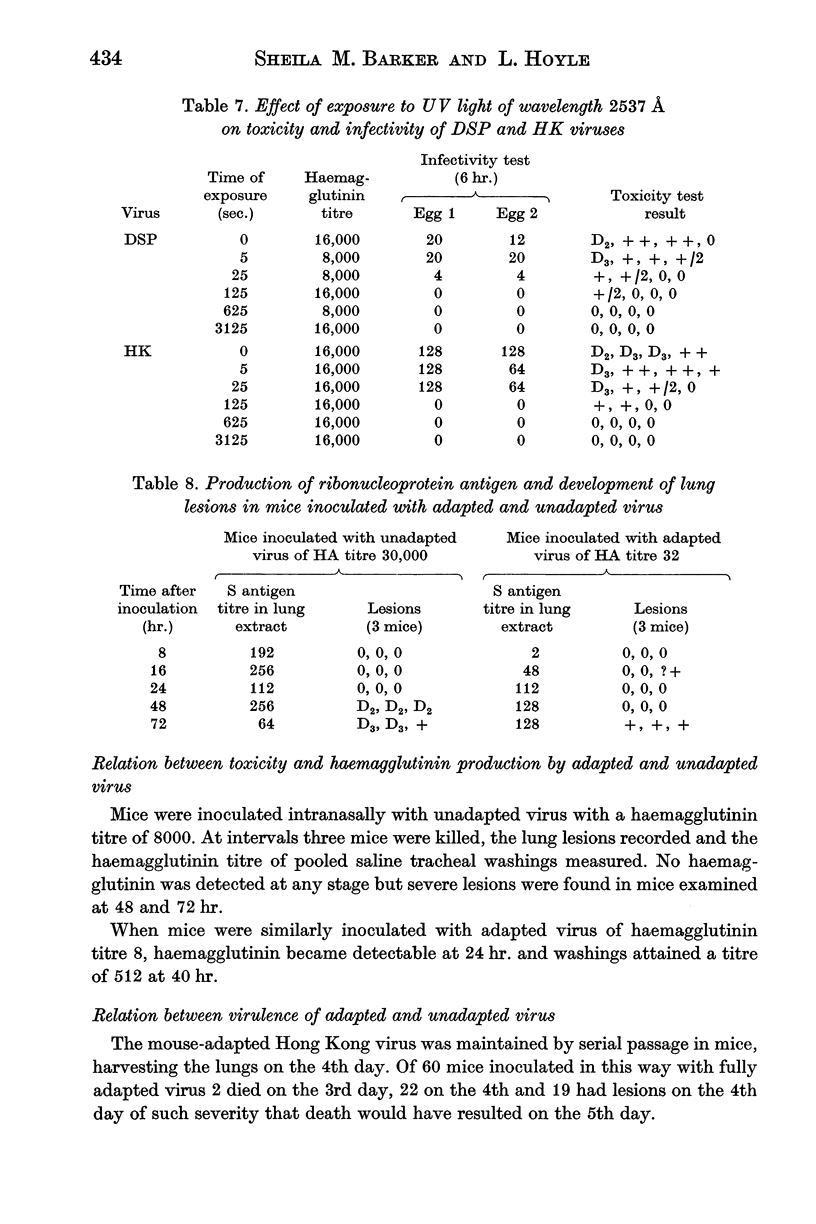
The toxic property of the virus is destroyed by procedures which destroy or modify the nucleic acid such as exposure to monochromatic UV light of wavelength 2537 Å, or treatment with hydroxylamine or Bayer A139. Reagents acting on amino groups are particularly effective as they react with the nucleic acid and probably also interfere with penetration of virus into the cell.
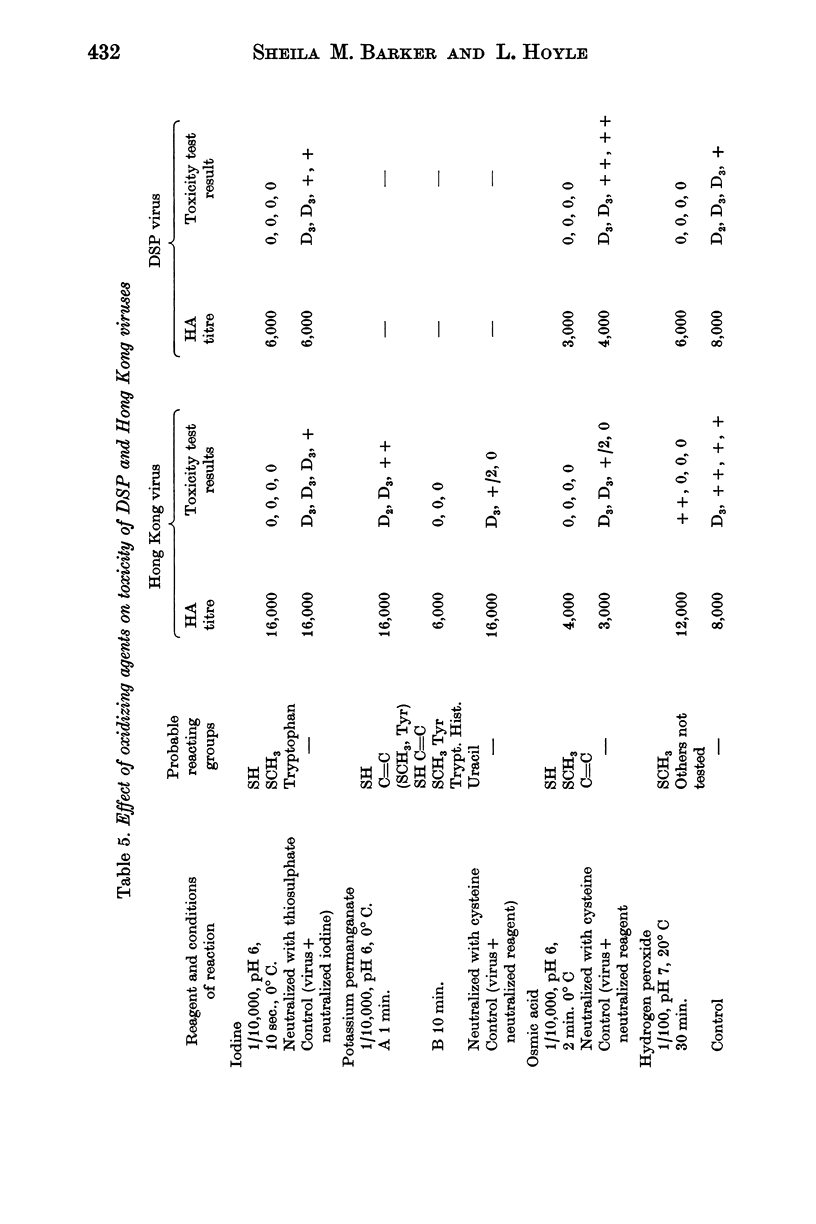
Toxicity is also destroyed by mercurials which probably prevent uncoating of the nucleic acid by union with disulphide bonds, and by oxidizing agents such as iodine, permanganate, osmic acid and hydrogen peroxide under condition which suggest possible action on some constituent of the virus containing methionine.
The toxic effect produced by the inoculation of large doses of unadapted virus intranasally in mice is associated with the occurrence of an incomplete growth cycle in which there is full production of RNP antigen but no production of haemagglutinin or infective virus.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERMANN W. W., MAASSAB H. F. Growth characteristics of influenza virus; biochemical differentiation of stages of development. II. J Exp Med. 1955 Oct 1;102(4):393–402. doi: 10.1084/jem.102.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLISON A. C. Observations on the inactivation of viruses by sulfhydryl reagents. Virology. 1962 May;17:176–183. doi: 10.1016/0042-6822(62)90095-8. [DOI] [PubMed] [Google Scholar]
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINTER N. B., BEALE A. J. The 6-hour soluble antigen production test for comparing the infectivity of influenza virus preparations. J Hyg (Lond) 1956 Mar;54(1):58–67. doi: 10.1017/s0022172400044302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher R. D., Hirschfield J. E., Forbes M. A common mode of antiviral action for ammonium ions and various amines. Nature. 1965 Aug 7;207(997):664–665. doi: 10.1038/207664a0. [DOI] [PubMed] [Google Scholar]
- GINSBERG H. S. Formation of non-infectious influenza virus in mouse lungs: its dependence upon extensive pulmonary consolidation initiated by the viral inoculum. J Exp Med. 1954 Dec 1;100(6):581–603. doi: 10.1084/jem.100.6.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle L., Hana L. The chemical reactions of influenza virus proteins. J Pathol Bacteriol. 1966 Oct;92(2):447–460. doi: 10.1002/path.1700920224. [DOI] [PubMed] [Google Scholar]
- SCHOLTISSEK C., ROTT R. SYNTHESIS OF VIRAL RIBONUCLEIC ACID BY A CHEMICALLY INACTIVATED INFLUENZA VIRUS. Nature. 1963 Jul 13;199:200–201. doi: 10.1038/199200a0. [DOI] [PubMed] [Google Scholar]
- Sugg J. Y. AN INFLUENZA VIRUS PNEUMONIA OF MICE THAT IS NONTRANSFERABLE BY SERIAL PASSAGE. J Bacteriol. 1949 Apr;57(4):399–403. doi: 10.1128/jb.57.4.399-403.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]


