Abstract
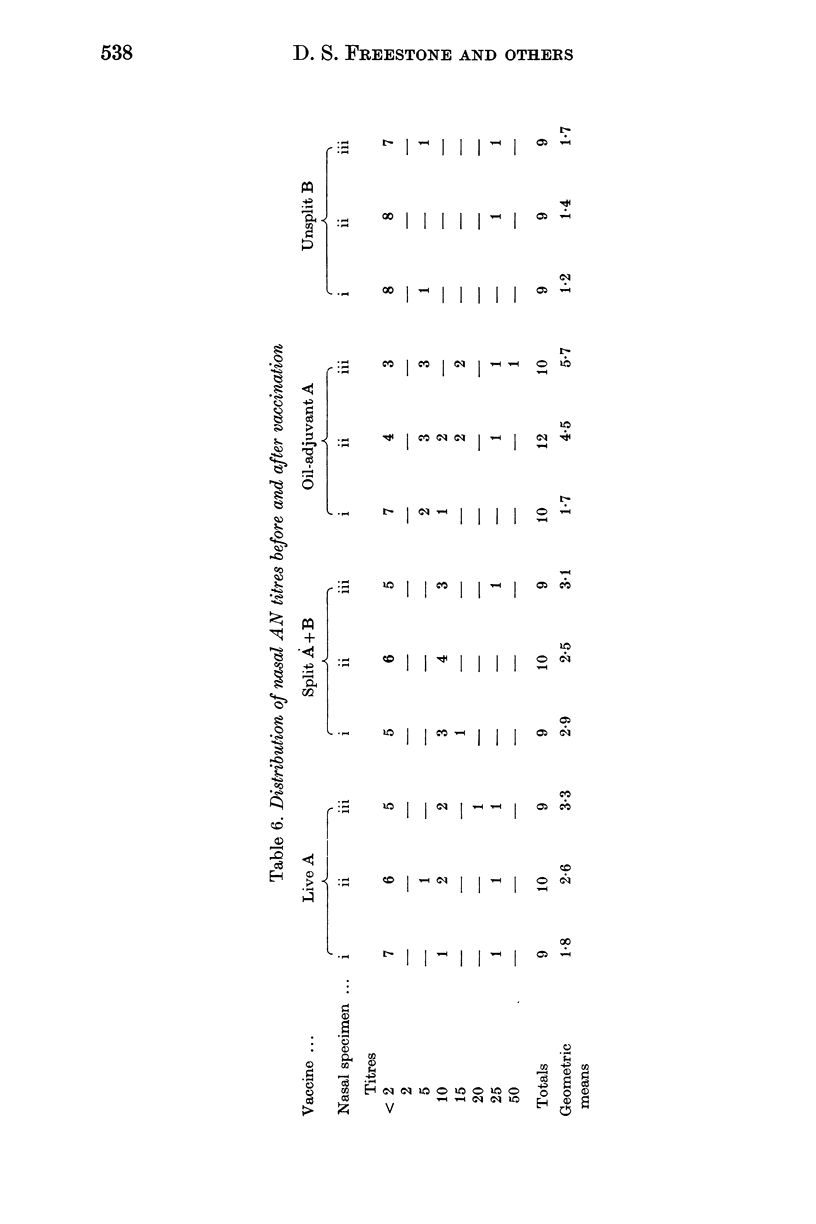
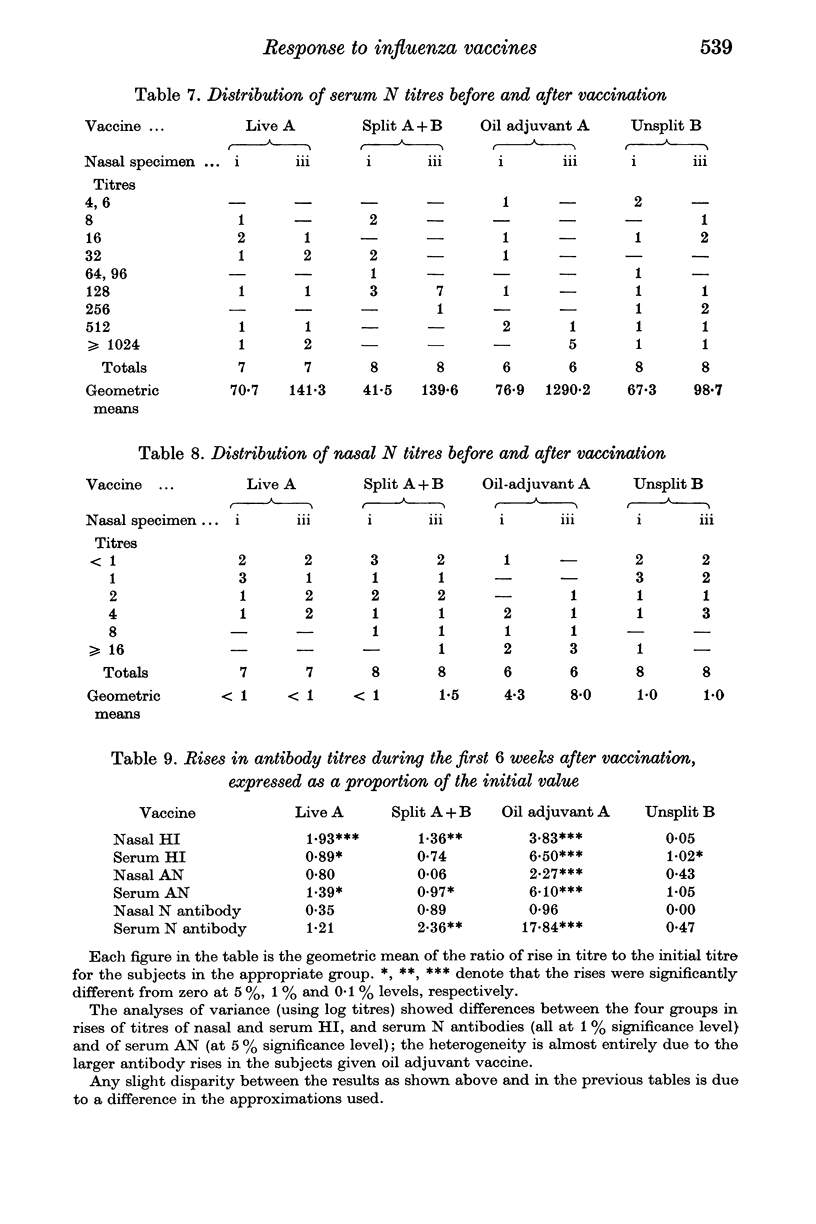
Forty-nine subjects were vaccinated with either live attenuated, detergent split, or oil adjuvant A2/Hong Kong influenza vaccines, or a saline influenza B vaccine as control. Respiratory symptoms occurred more frequently in subjects who received the live vaccine but in total there was little difference between the symptoms in the four groups. Antibody titres in nasal washings and serum were measured by haemagglutination inhibition, neuraminidase inhibition and virus neutralization tests. The oil adjuvant vaccine stimulated larger antibody responses than the other procedures. Six weeks after vaccination the volunteers were challenged with partially attenuated live A2/Hong Kong influenza virus administered intranasally. The live attenuated and oil adjuvant vaccines provided the best protection against challenge.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beare A. S., Bynoe M. L. Attenuation of human influenza A viruses. Br Med J. 1969 Oct 25;4(5677):198–201. doi: 10.1136/bmj.4.5677.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beare A. S., Hobson D., Reed S. E., Tyrrell D. A. A comparison of live and killed influenza-virus vaccines. Report to the Medical Research Council's Committee on Influenza and other Respiratory Virus Vaccines. Lancet. 1968 Aug 24;2(7565):418–422. doi: 10.1016/s0140-6736(68)90463-7. [DOI] [PubMed] [Google Scholar]
- Beare A. S., Hobson D., Reed S. E., Tyrrell D. A. Antibody responses to and efficacy of an inactivated spray vaccine. Bull World Health Organ. 1969;41(3):549–551. [PMC free article] [PubMed] [Google Scholar]
- Downie J. C. Neuraminidase- and hemagglutinin-inhibiting antibodies in serum and nasal secretions of volunteers immunized with attenuated and inactivated influenza B-Eng-13-65 virus vaccines. J Immunol. 1970 Sep;105(3):620–626. [PubMed] [Google Scholar]
- Schild G. C. Antibody against influenza A2 virus neuraminidase in human sera. J Hyg (Lond) 1969 Jun;67(2):353–365. doi: 10.1017/s0022172400041759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepushkin A. N., Schild G. C., Beare A. S., Chinn S., Tyrrell D. A. Neuraminidase and resistance to vaccination with live influenza A2 Hong Kong vaccines. J Hyg (Lond) 1971 Dec;69(4):571–578. doi: 10.1017/s0022172400021847. [DOI] [PMC free article] [PubMed] [Google Scholar]


