Abstract
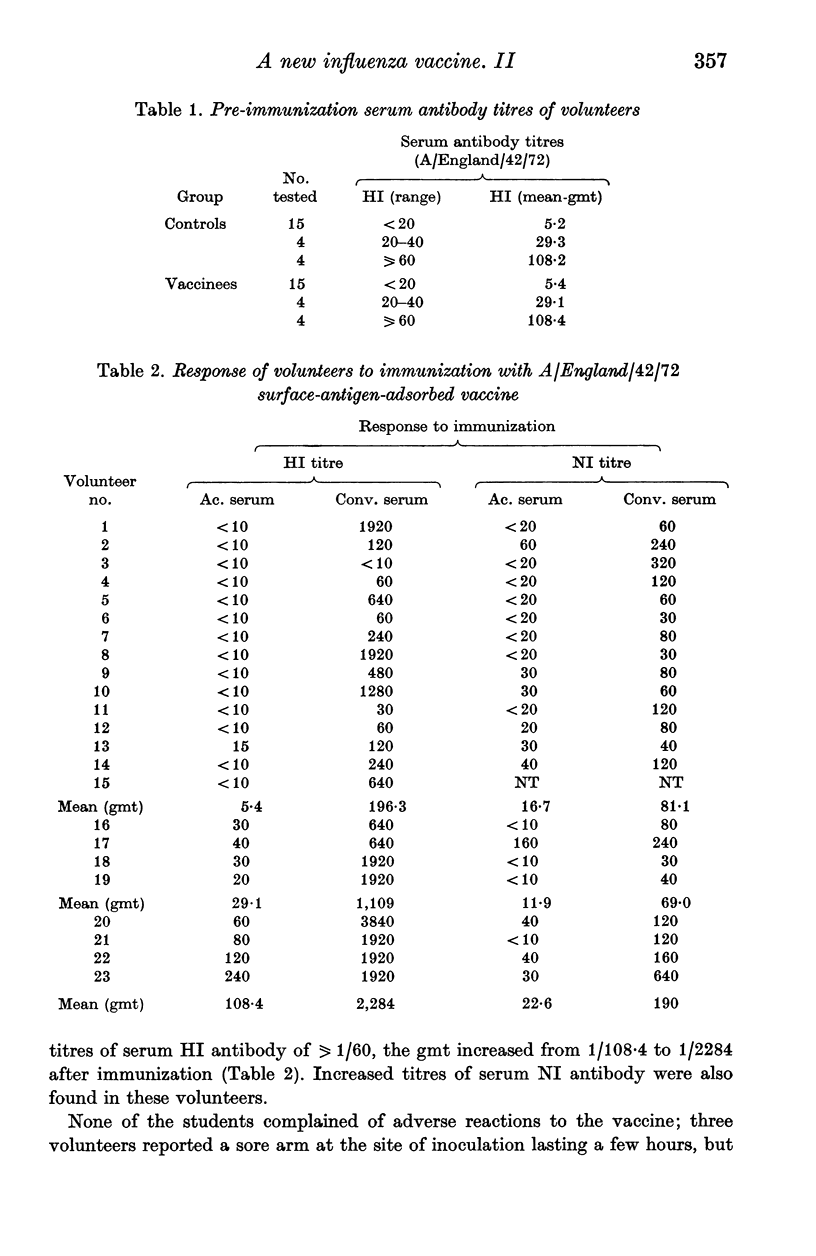
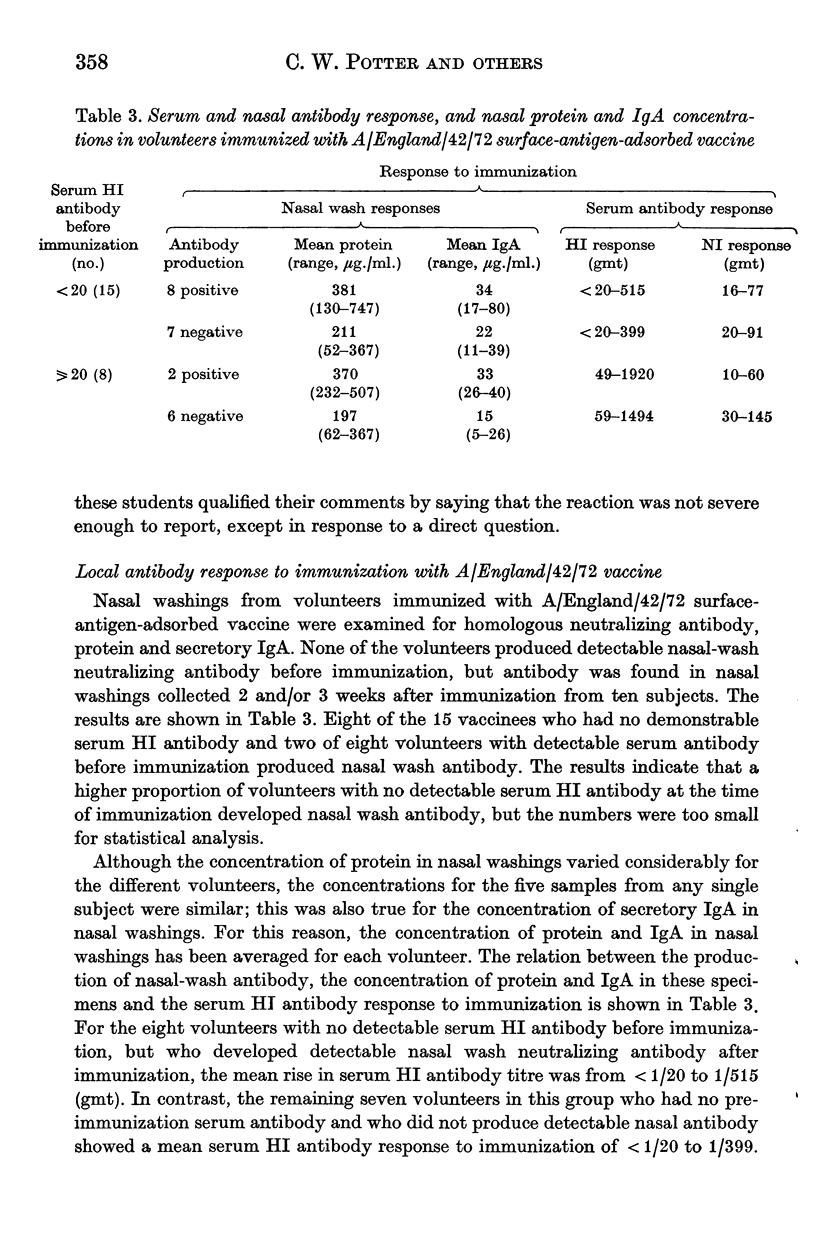
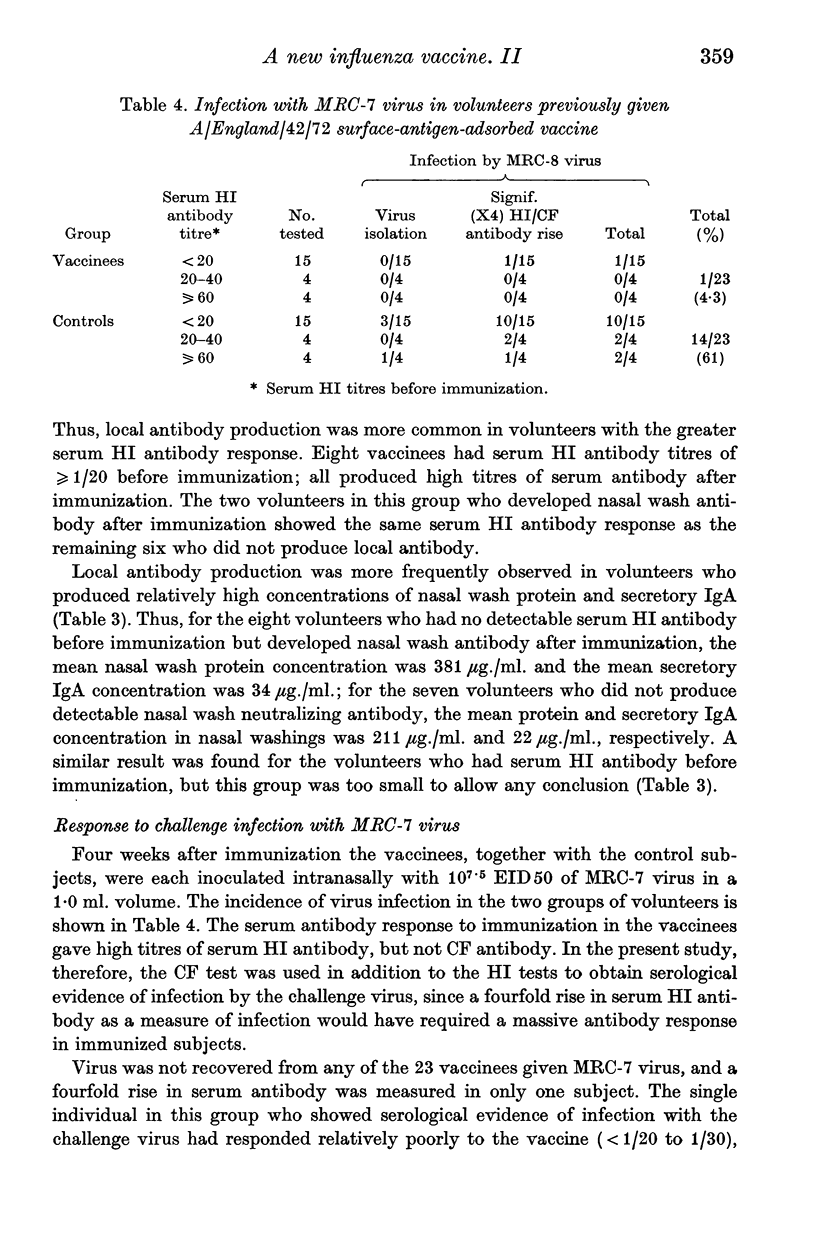
A group of 23 volunteers were each inoculated with 600 CCA of a new form of influenza virus A/England/42/72 vaccine; this vaccine consisted of purified haemagglutinin and neuraminidase antigens adsorbed to alhydrogel. No significant reactions to the vaccine were reported. Twenty-two volunteers produced increased titres of serum HI antibody, and all showed increased titres of NI antibody after immunization. Thus, for volunteers with no pre-immunization serum HI antibody, the geometric mean titre of serum antibody increased from 1/5 to 1/196 after immunization. Ten volunteers developed local neutralizing antibody after immunization; this antibody response was detected most frequently in volunteers who showed the greater serum antibody response to immunization, and in nasal washings with the higher concentrations of protein and IgA. Ten weeks after immunization, the vaccinees and a group of matched controls were inoculated intranasally with attenuated A/England/42/72 virus. Evidence of infection with the challenge virus was found in 14 of the control subjects and in one of the vaccinees. The results indicate that the surface-antigen-adsorbed vaccine induced high titres of serum antibody, and gave significant protection against challenge infection.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aymard-Henry M., Coleman M. T., Dowdle W. R., Laver W. G., Schild G. C., Webster R. G. Influenzavirus neuraminidase and neuraminidase-inhibition test procedures. Bull World Health Organ. 1973;48(2):199–202. [PMC free article] [PubMed] [Google Scholar]
- BRADSTREET C. M., TAYLOR C. E. Technique of complementfixation test applicable to the diagnosis of virus diseases. Mon Bull Minist Health Public Health Lab Serv. 1962 May;21:96–104. [PubMed] [Google Scholar]
- Brand C. M., Skehel J. J. Crystalline antigen from the influenza virus envelope. Nat New Biol. 1972 Aug 2;238(83):145–147. doi: 10.1038/newbio238145a0. [DOI] [PubMed] [Google Scholar]
- Brandon F. B., Barrett C. D., Jr, Hook A. E., Lease G. O. Human febrile response to influenza virus or its ether isolated hemagglutinins. Proc Soc Exp Biol Med. 1967 Jul;125(3):683–686. doi: 10.3181/00379727-125-32180. [DOI] [PubMed] [Google Scholar]
- DAVENPORT F. M., HENNESSY A. V., BRANDON F. M., WEBSTER R. G., BARRETT C. D., Jr, LEASE G. O. COMPARISONS OF SEROLOGIC AND FEBRILE RESPONSES IN HUMANS TO VACCINATION WITH INFLUENZA A VIRUSES OR THEIR HEMAGGLUTININS. J Lab Clin Med. 1964 Jan;63:5–13. [PubMed] [Google Scholar]
- DE ST GROTH S. F., WITHELL J., LAFFERTY K. J. An improved assay method for neutralizing antibodies against influenza viruses. J Hyg (Lond) 1958 Sep;56(3):415–426. doi: 10.1017/s0022172400037906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport F. M. Antigenic enhancement of ether-extracted influenza virus vaccines by AIPO4. Proc Soc Exp Biol Med. 1968 Feb;127(2):587–590. doi: 10.3181/00379727-127-32748. [DOI] [PubMed] [Google Scholar]
- Davenport F. M., Hennessy A. V., Askin F. B. Lack of adjuvant effect of A1PO4 on purified influenza virus hemagglutinins in man. J Immunol. 1968 May;100(5):1139–1140. [PubMed] [Google Scholar]
- Downie J. C., Stuart-Harris C. H. The production of neutralizing activity in serum and nasal secretion following immunization with influenza B virus. J Hyg (Lond) 1970 Jun;68(2):233–244. doi: 10.1017/s0022172400028709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY J. L., MCLAUGHLIN C. PREPARATION OF ANTISERA SPECIFIC FOR 6.6 S GAMMA-GLOBULINS, BETA 2A-GLOBULINS, GAMMA-1.-MACROGLOBULINS, AND FOR TYPE I AND II COMMON GAMMA-GLOBULIN DETERMINANTS. J Immunol. 1963 Oct;91:484–497. [PubMed] [Google Scholar]
- Fenters J. D., Yamashiroya H. M., Petzold R. F., Tolkacz V. K. Enhanced immunogenicity in mice of a purified, tween-ether-treated influenza vaccine. Appl Microbiol. 1970 Oct;20(4):544–550. doi: 10.1128/am.20.4.544-550.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy A. V., Davenport F. M. Relative antigenic potency in man of polyvalent influenza virus vaccines containing isolated hemagglutinins or intact viruses. J Immunol. 1966 Aug;97(2):235–238. [PubMed] [Google Scholar]
- Jennings R., Potter C. W., McLaren C., Brady M. A new, surface-antigen-adsorbed influenza virus vaccine. I. Studies on immunogenicity in hamsters. J Hyg (Lond) 1975 Dec;75(3):341–352. doi: 10.1017/s0022172400024402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McLaren C., Potter C. W., Jennings R. Immunity to influenza in ferrets. XII. Immunization of ferrets with TNBP-split influenza virus vaccine. Arch Gesamte Virusforsch. 1974;45(1-2):99–105. [PubMed] [Google Scholar]
- Neurath A. R., Rubin B. A., Sillaman J., Tint H. The effect of nonaqueous solvents on the quaternary structure of viruses: a procedure for the simultaneous concentration, purification and disruption of influenza viruses. Microbios. 1971 Sep;4(14):145–150. [PubMed] [Google Scholar]
- Ruben F. L., Jackson G. G. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. J Infect Dis. 1972 Jun;125(6):656–664. doi: 10.1093/infdis/125.6.656. [DOI] [PubMed] [Google Scholar]
- Ruben F. L., Potter C. W., Stuart-Harris C. H. Humoral and secretory antibody responses to immunization with low and high dosage split influenza virus vaccine. Arch Virol. 1975;47(2):157–166. doi: 10.1007/BF01320555. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Webster R. G., Laver W. G. Influenza virus subunit vaccines: immunogenicity and lack of toxicity for rabbits of ether- and detergent-disrupted virus. J Immunol. 1966 Apr;96(4):596–605. [PubMed] [Google Scholar]
- Závadová H., Vonka V., Adam E., Domorázková E., Davenport F. M. Clinical reactivity and immunogenicity of hemagglutinin influenza vaccine. I. Clinical reactions, hemagglutination-inhibiting and strain and type-specific complement-fixing antibody responses in subjects aged 3-6, 16-17 and 27-50 years. Arch Gesamte Virusforsch. 1972;36(1):43–52. [PubMed] [Google Scholar]


