Abstract
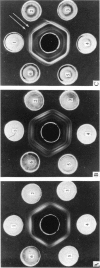
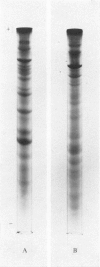
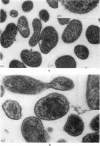
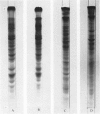
Ten mycoplasmas were isolated from 130 nasopharyngeal swabs from thoroughbred horses with acute respiratory disease and three from 198 apparently normal horses. Two mycoplasmas were isolated from 21 tracheal swabs taken at necropsy. These mycoplasmas, together with six isolated from the equine respiratory tract by other workers, were subjected to biochemical and serological tests. Other properties examined in certain representative strains were appearance under the electron microscope, ability to adsorb or agglutinate the erythrocytes of various animal species and the electrophoretic pattern of the cell proteins. On the basis of these test, mycoplasmas from the equine respiratory tract were divided into seven species. Three species belonged to the genus Acholeplasma, members of which do not require sterol for growth, and were identified as A. laidlawii, A. oculi (formerly A. oculusi) originally isolated from the eyes of goats, and a recently named species A. equifoetale, previously isolated from aborted equine fetuses. Of the four sterol-dependent Mycoplasma species, one was indentified as M. pulmonis, a common rodent pathogen. Another cross-reacted serologically with M. felis and should probably be classified as that species. The other two species probably represent new species peculiar to the horse. One of these, represented by the strains N3 and N11, ferments glucose and is serologically distinct from 19 recognized species of glucose-utilizing mycoplasmas and from two species which do not metabolize either glucose or arginine. The other species, represented by four strains, hydrolyses arginine and, because it is serologically distinct from all the named arginine-hydrolysing Mycoplasma species, the name M. equirhinis sp.nov. is proposed for it. Of the seven species, only M. pulmonis and the glucose-utilizing species represented by N3 and N11 were found exclusively in horses with acute respiratory disease. A. oculi was isolated from an apparently normal horse. The other four species were found in normal horses as well as those with respiratory disease, although three out of the four strains of M. equirhinis were from sick horses.
Full text
PDF




























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allam N. M., Powell D. G., Andrews B. E., Lemcke R. M. The isolation of mycoplasma species from horses. Vet Rec. 1973 Oct 6;93(14):402–402. doi: 10.1136/vr.93.14.402. [DOI] [PubMed] [Google Scholar]
- Boatman E. S., Kenny G. E. Three-dimensional morphology, ultrastructure, and replication of Mycoplasma felis. J Bacteriol. 1970 Jan;101(1):262–267. doi: 10.1128/jb.101.1.262-277.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLYDE W. A., Jr MYCOPLASMA SPECIES IDENTIFICATION BASED UPON GROWTH INHIBITION BY SPECIFIC ANTISERA. J Immunol. 1964 Jun;92:958–965. [PubMed] [Google Scholar]
- Dellinger J. D., Jasper D. E. Polyacrylamide-gel electrophoresis of cell proteins of mycoplasma isolated from cattle and horses. Am J Vet Res. 1972 Apr;33(4):769–775. [PubMed] [Google Scholar]
- Edward D. G. Determination of sterol requirement for Mycoplasmatales. J Gen Microbiol. 1971 Dec;69(2):205–210. doi: 10.1099/00221287-69-2-205. [DOI] [PubMed] [Google Scholar]
- Goel M. C. New method for the isolation of membranes from Mycoplasma gallisepticum. J Bacteriol. 1973 Nov;116(2):994–1000. doi: 10.1128/jb.116.2.994-1000.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingdale M. R., Lemcke R. M. Antigenic differences within the species Mycoplasma hominis. J Hyg (Lond) 1970 Sep;68(3):469–477. doi: 10.1017/s0022172400042376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingdale M. R., Lemcke R. M. Membrane antigens of Mycoplasma hominis. J Hyg (Lond) 1972 Mar;70(1):85–98. doi: 10.1017/s0022172400022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff H., Deegen E., Zeller R., Floer W. Nachweis von Mykoplasmen in Luftsack und Pharynx von Pferden mit akuten Erkrankungen der Respirationsorgane. Dtsch Tierarztl Wochenschr. 1972 Oct 1;79(19):465–468. [PubMed] [Google Scholar]
- Kirchhoff H. Neue Spezies der Fam. Acholeplasmataceae un der Fam. Mykoplasmataceae bei Pferden. Zentralbl Veterinarmed B. 1974 Mar;21(3):207–210. [PubMed] [Google Scholar]
- LEMCKE R. M. A SEROLOGICAL COMPARISON OF VARIOUS SPECIES OF MYCOPLASMA BY AN AGAR GEL DOUBLE-DIFFUSION TECHNIQUE. J Gen Microbiol. 1965 Jan;38:91–100. doi: 10.1099/00221287-38-1-91. [DOI] [PubMed] [Google Scholar]
- Langford E. V. Isolation of Mycoplasma bovigenitalium from an aborted equine foetus. Vet Rec. 1974 Jun 8;94(23):528–528. doi: 10.1136/vr.94.23.528. [DOI] [PubMed] [Google Scholar]
- Leach R. H. Comparative studies of mycoplasma of bovine origin. Ann N Y Acad Sci. 1967 Jul 28;143(1):305–316. doi: 10.1111/j.1749-6632.1967.tb27670.x. [DOI] [PubMed] [Google Scholar]
- Leach R. H. Further studies on classification of bovine strains of mycoplasmatales, with proposals for new species, Acholeplasma modicum and Mycoplasma alkalescens. J Gen Microbiol. 1973 Mar;75(1):135–153. doi: 10.1099/00221287-75-1-135. [DOI] [PubMed] [Google Scholar]
- Lemcke R. M. Osmolar concentration and fixation of mycoplasmas. J Bacteriol. 1972 Jun;110(3):1154–1162. doi: 10.1128/jb.110.3.1154-1162.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Haemadsorption and haemagglutination by mycoplasmas. J Gen Microbiol. 1968 Mar;50(3):465–478. doi: 10.1099/00221287-50-3-465. [DOI] [PubMed] [Google Scholar]
- Ogata M., Watabe J., Koshimizu K. Classification of Acholeplasmas isolated from horses. Nihon Juigaku Zasshi. 1974 Feb;36(1):43–51. doi: 10.1292/jvms1939.36.43. [DOI] [PubMed] [Google Scholar]
- Purcell R. H., Taylor-Robinson D., Wong D. C., Chanock R. M. A color test for the measurement of antibody to the non-acid-forming human Mycoplasma species. Am J Epidemiol. 1966 Jul;84(1):51–66. doi: 10.1093/oxfordjournals.aje.a120627. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. Mycoplasma taxonomy studiedy electrophoresis of cell proteins. J Bacteriol. 1968 Sep;96(3):687–694. doi: 10.1128/jb.96.3.687-694.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWITZER W. P. Studies on infectious atrophic rhinitis. IV. Characterization of a pleuropneumonia-like organism isolated from the nasal cavities of swine. Am J Vet Res. 1955 Oct;16(61 Pt 1):540–544. [PubMed] [Google Scholar]
- Stipkovits L., Romváry J., Nagy Z., Bodon L., Varga L. Studies on the pathogenicity of Acholeplasma axanthum in swine. J Hyg (Lond) 1974 Apr;72(2):289–296. doi: 10.1017/s0022172400023500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan R. J., Miles J. A. Incidence and significance of mycoplasmas in sick cats. Res Vet Sci. 1974 Jan;16(1):27–34. [PubMed] [Google Scholar]
- Windsor G. D. Letter: The isolation of mycoplasma from horses. Vet Rec. 1973 Dec 1;93(22):593–594. doi: 10.1136/vr.93.22.593. [DOI] [PubMed] [Google Scholar]
- al-Aubaidi J. M., Dardiri A. H., Muscoplatt C. C., McCauley E. H. Identification and characterization of Acholeplasma oculusi spec. nov. from the eyes of goats with keratoconjunctivitis. Cornell Vet. 1973 Jan;63(1):117–129. [PubMed] [Google Scholar]