Abstract
Cardiac sarcolemmal ATP-sensitive K+ (KATP) channels, composed of Kir6.2 and SUR2A subunits, couple the metabolic status of cells with the membrane excitability. Based on previous functional studies, we have hypothesized that creatine kinase (CK) may be a part of the sarcolemmal KATP channel protein complex. The inside-out and whole cell patch clamp electrophysiology applied on guinea pig cardiomyocytes showed that substrates of CK regulate KATP channels activity. Following immunoprecipitation of guinea-pig cardiac membrane fraction with the anti-SUR2 antibody, Coomassie blue staining revealed, besides Kir6.2 and SUR2A, a polypeptide at ∼48 kDa. Western blotting analysis confirmed the nature of putative Kir6.2 and SUR2A, whereas matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis identified p48 kDa as a muscle form of CK. In addition, the CK activity was found in the anti-SUR2A immunoprecipitate and the cross reactivity between an anti-CK antibody and the anti-SUR2A immunoprecipitate was observed as well as vice verse. Further results obtained at the level of recombinant channel subunits demonstrated that CK is directly physically associated with the SUR2A, but not the Kir6.2, subunit. All together, these results suggest that the CK is associated with SUR2A subunit in vivo, which is an integral part of the sarcolemmal KATP channel protein complex.
Keywords: heart, KATP channels, SUR2A, Kir6.2
In the heart, creatine kinase (CK) is a major phosphotransfer system essential in supporting cardiac energy balance (1). To ensure communications between sites that generate, use, and sense ATP, cardiac cells rely on phosphotransfer networks that facilitate the transfer and distribution of energy-rich phosphoryls between cellular compartments in a kinetically and thermodynamically efficient manner mediated by CK (2).
ATP-sensitive K+ (KATP) channels belong to a group of intracellular ATP sensors and they couple the metabolic status of cell with membrane excitability (3). Numerous studies have demonstrated that potassium channel openers, drugs that promote opening of KATP channels, decrease infarct size, mimic ischemic preconditioning, and improve functional and energetic recovery of cardiac muscle following ischemic and hypoxic insults (4, 5). More recently, evidence has suggested that activation of both sarcolemmal and mitochondrial KATP channels may promote cellular survival (4), which would agree with the idea that these channels may communicate through phosphotransfer reactions from an intracellular compartment to the cell membrane (1). The structure of mitochondrial KATP channels is still unknown, but the proteins constituting the sarcolemmal KATP channel complex have been cloned recently (6-8). Sarcolemmal KATP channels are heteromultimers composed of two structurally distinct proteins (Kir6.2 and SUR2A) (7). The Kir6.2 subunit was shown to form the inwardly rectifying K+ channel core, primarily responsible for K+ permeance, whereas SUR2A has been implicated in ligand-dependent regulation of channel gating (7). The activity of KATP channels is controlled by the complex interaction of potentially linked intracellular signaling pathways (1, 9). From reports of channel regulation to date, the intracellular ATP/ADP (adenosine diphosphate) ratio would seem to be the most important factor in the regulation of sarcolemmal KATP channels, where ATP and ADP act as endogenous blockers and openers of the channels (9, 10). Recently, it has been suggested that there is a close functional relationship between adenylate kinase (AK) and CK phosphotransfer defining the directionality of nucleotide exchange within the sarcolemmal KATP channel vicinity. Specifically, it has been proposed that activation of KATP channels by AK (via AMP+ATP↔2ADP) may be counteracted by CK (via ADP+phosphocreatine↔ATP+creatine) as a system for ATP production and scavenger of the products of AK catalysis, thereby keeping KATP channels closed (11). Admittedly, for such action of two major phosphotransfer systems it is probably necessary that both AK and CK are physically associated with sarcolemmal KATP channels, where they can synchronously act as anchoring sites for the cellular phosphotransfer network. Indeed, Carrasco et al. (11) recently showed that AK physically associates with cardiac KATP channel protein complex, but whether this may be true for CK remains unknown. If CK is also associated with the KATP channel, it would strongly support the novel concept that AK and CK, as parts of the KATP channel complex, act together to efficiently couple cardiac energetics with membrane excitability (11).
In the present study, we have provided evidence that CK is associated with the SUR2A subunit of the cardiac KATP channel complex in vivo, where it serves to regulate channel behavior. These results support the concept of KATP channels regulation proposed by Carrasco et al. (11) and provide a new avenue for investigating relationships between cardiac metabolism and cardiac membrane excitability.
MATERIALS AND METHODS
Cardiac cells isolation
Ventricular cardiomyocytes were dissociated from guinea-pig hearts, as described (11). In brief, hearts were perfused retrogradely (at 37°C) with medium 199 for 2-3 min, followed by Ca2+-EGTA-buffered low-Ca2+ medium (pCa=7) for 80 s, and finally low-Ca2+ medium containing pronase E (8 mg/100 ml), proteinase K (1.7 mg/100 ml), bovine albumin (0.1 g/100 ml, fraction V), and 200 μM CaCl2. Ventricles were cut into small fragments (6-10 mm3) in the low-Ca2+ medium enriched with 200 μM CaCl2. Single cells were isolated by stirring the tissue (at 37°C) in a solution containing pronase E and proteinase K supplemented with collagenase (5 mg/10 ml). After 10 min, the first aliquot was removed, filtered through a nylon sieve, centrifuged for 60 s (300-400 rpm), and washed twice. Remaining tissue fragments were re-exposed to collagenase, and isolation continued for 2-3 such cycles. We stored isolated cardiomyocytes in low-Ca2+ medium with 200 μM CaCl2. Only rod-shaped cardiomyocytes with clear striations and smooth surfaces were used for electrophysiological recordings.
Patch clamp electrophysiology
To monitor on-line behavior of single channel molecules, we applied the giagaohm seal patch-clamp technique in the inside-out configuration (13). Cells were superfused with (in mM): potassium gluconate 140, MgCl2 1, ethylenediaminetetraacetic acid (EGTA 5), and N-(2-hydroxyethyl) piperazine-2′-(2-ethanesulphonic acid) (HEPES)-KOH 5 (pH 7.4). Fire-polished pipettes, coated with Sylgard (resistance 5-7 MΩ), were filled with the following (in mM): KCl 140, CaCl2 1, MgCl2 1, and HEPES-KOH 5 (pH 7.3). We took recordings at room temperature (22°C) by using a patch-clamp amplifier (Axopatch-200B, Axon Instruments, Inc., Foster City, CA). Single-channel activity was monitored on-line and stored on a PC. Data were reproduced, low-pass filtered at 1 KHz (-3 dB), sampled at 100 μs rate, and analyzed further by using the “pClamp8” software (Axon Instruments, Inc.). Channel activity, assayed by digitizing segments of current recordings and forming histograms of base line and open level data points, were expressed as NPo (N, number of channels in the patch; Po, probability of each channel to be open). For whole-cell electrophysiology (14), cells were superfused with Tyrode solution (in mM: 136.5 NaCl; 5.4 KCl; 1.8 CaCl2; 0.53 MgCl2; 5.5 glucose; and 5.5 HEPES-NaOH, pH 7.4). Pipettes (resistance 3-5 MΩ) were filled with the following (in mM): potassium gluconate 140, MgCl2 1, HEPES-KOH 5, pH 7.3, plus either adenosine triphosphate [ATP] (1 mM) or ATP (1 mM) plus creatine (3 mM) or ADP (1 mM) plus phosphocreatine (3 mM). During each experiment, the membrane potential was normally held at -40 mV, and the currents evoked by a series of 400 ms depolarizing and hyperpolarizing current steps (-100 mV to +80 mV in 20 mV steps) recorded directly to hard disk by using an Axopatch-200B amplifier, Digidata-1321 interface, and pClamp8 software (Axon Instruments, Inc.). The capacitance compensation was adjusted to null the additional whole-cell capacitative current. The slow capacitance component measured by this procedure was used as an approximation of the cell surface area and allowed normalization of current amplitude (i.e., current density). Currents were low-pass-filtered at 10 kHz digitized at 2 kHz.
A549 cells and gene transfection
A549 cells (ATCC), cells that are natively devoid of KATP channels, were cultured in a tissue flask (at 5% CO2) containing Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum and 2 mM glutamine. At 40%-60% confluence, cells were transfected by using 8-24 μl lipofectamine (Gibco, Great Island, NY), with 2-6 μg of total plasmid DNA (full length Kir6.2 and/or SUR2A and/or muscle creatine kinase cDNA subcloned into the mammalian expression vector pcDNA3.1+) (success of rate of transfection was ∼50% of cells, 15). Kir6.2 was a gift from S. Seino (Chiba University, Chiba, Japan), and SUR2A was kindly provided by Y. Kurachi (Osaka University, Osaka, Japan), while creatine kinase was cloned from the mouse heart RNA. We synthesized the first strand of cDNA from 1 μg tissue RNA by using 200 units of MML-V reverse transcriptase (Promega, Madison, WI) and an anchor-oligo(dT) (5′-GTCATGGCATGGGATCCTG(T;)15; total volume, 20 μl), according to the manufacturer instructions. We performed the polymerase chain reaction with muscle creatine kinase (Acc NM 007710)-specific primers (sense, 5′-CAAAGGCCGCCACCATGC-3′; antisense, 5′-CCCTGCGCCTACTTCTGC-3′). The obtained product (full-length cDNA), which contains the translatable part of the CK sequence was isolated from a 1% agarose gel by using Qiagen gel extraction kit, sequenced, ligated into the pcDNA3.1+ mammalian expression vector and used for transfection.
Immunoprecipitation, blue staining, and Western blotting analysis
Sheep antipeptide antibodies were raised against synthetic peptides comprising residues 33-47 in the Kir6.2 protein (ARFVSKKGNCNVAHK) and residues 321-334 in the SUR2A protein (CIVQRVNETQNGTNN), conjugated to a carrier protein, keyhole limpet hemocyanin, and used for immunoprecipitation and Western blotting (16, 17). To obtain the membrane cardiac fraction, guinea-pig ventricular tissue was homogenized in buffer I (TRIS 10 mM; NaH2PO4 20 mM; EDTA 1 mM; phenylmethylsulfonyl fluoride (PMSF) 0.1 mM; pepstatin 10 μg/ml; and leupeptin 10 μg/ml, pH 7.8) and incubated for 20 min (at 4°C). We restored the osmolarity with KCl, NaCl, and sucrose, and the obtained mixture was centrifugated at 500 g. The supernatant was diluted in buffer II (imidazole 30 mM; KCl 120 mM; NaCl 30 mM; NaH2PO4 20 mM; sucrose 250 mM; pepstatin 10 μg/ml; leupeptin 10 μg/ml, pH 6.8) and centrifugated at 7,000 g, pellet removed and supernatant centrifugated at 30,000 g. The obtained pellet contains membrane fraction. A549 Cells or membrane fraction were snap-frozen and ground to a powder under liquid nitrogen. The powder was resuspended in 10 ml of buffer (20 mM HEPES; 150 mM NaCl; and Triton-X 100 (1%), pH 7.5) and homogenized. Protein concentration was determined by using the Bradford method. A sheep antipeptide antibody was raised against the synthetic peptide comprising residues 321-334 in the SUR2A protein (CIVQRVNETQNGTNN), conjugated to a carrier protein, keyhole limpet hemocyanin and was used for immunoprecipitation. The epitope-specific anti-SUR2A antibody (40 μg) was prebound to protein-G sepharose beads and used to immunoprecipitate from 60 μg total of total protein extract. We ran the pellets of this precipitation on two sodium dodecylsulfate polyacrylamide gels, one for Coomassie blue stain and one for Western blot analysis. Blue staining was performed for 1 h and was destained for another 2 h. We performed Western blot probing by using a 1/200-1/1,000 dilution of anti-SUR2A, anti-Kir6.2, and anti-CK antibodies. For detection, we used Protein-G HRP and ECL reagents. In a separate series of experiments, immunoprecipitation was performed with the anti-CK antibody (Abcam, Cambridge, U.K.) by using the same protocol as described above.
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) analysis
Proteins from Coomassie blue-stained gels can be digested enzymatically with the resulting peptide analyzed and sequenced by mass spectrometry. This methodology is used often to identify an unknown protein, because proteins yield the same peptide maps when extracted from Coomassie- and silver-stained gels, as judged by electrospray and MALDI mass spectrometry (18). Thus, the band of interest was excised from the gel and the protein trapped within the gel was reduced and alkylated and then digested with trypsin (12.5 ng/μl) overnight (18). The peptides were extracted; first, by the addition of 25 mM ammonium bicarbonate, followed after a 10-min incubation by an equal volume of acetonitrile, and then by two extractions with 5% formic acid and acetonitrile. The pooled extracts were concentrated to near dryness in vacuo and were redissolved in 10% formic acid. We desalted and concentrated half of the sample by using a micro C18 column (0.2 μl ZapTip, Millipore, Gloucestershire, U.K.) according to the manufacturer instructions. The peptides were eluted directly from the tip onto the target in 1.5 μl alpha-cyano-4-hydroxycinnamic acid (10 mg/ml) in 75% acetonitrile/25% formic acid (10%). Spectra were obtained on a Micromass TofSpec 2E instrument (Micromass, Manchster, U.K.), equipped with a 337 nm laser and operated in reflectron mode. We combined 300 shots and calibrated the data against a mixture of known peptides. Peptide masses were searched against the Swiss-Prot/TREMBL databases by using the ExPASy PeptIdent program and against the NCBI database by using the MS-Fit search program (USCF Mass Spectrometry Facility, San Francisco, CA). The searches were limited to rodent proteins and included an appropriate mass range limit.
Creatine kinase assay
We determined creatine kinase activity in a coupled enzyme reaction by using pyruvate kinase (PK) and lactate dehydrogenase (LDH). Reagent solution contained: 8.5 mM ATP, 1.22 mM NADH, 2 mM PEP, 15 u/ml LDH, 7 u/ml PK, MgSO4 28 mM, and gluthatione (reduced) 26 mM; pH was adjusted to 7.4. Buffered creatine solution contained 0.4 mM glycine containing 53.2 mM creatine and 62 mM potassium carbonate; pH was adjusted to 8.9. CK activity was measured using a spectrophotometer (Unicam UV2 Spec, Cambridge, U.K.) set at wavelength 340 nm on either commercial CK (5 U) or pellets (20 μl) dissolved in phosphate buffered saline (total volume was 100 ⎧l) and put in mixture of reagent solution (0.7 ml) and buffered creatine (2.2 ml).
Statistical analysis
Data are presented as mean ± se, with n representing the number of patched cells or examined hearts. Mean values obtained were compared by the paired or unpaired Student’s t-test where appropriate. P < 0.05 was considered statistically significant.
RESULTS
CK substrates and cardiac KATP channels activity
On excision of a membrane patch from guinea-pig ventricular cardiomyocyte in an ATP-free environment, we observed vigorous openings of KATP channels (Fig. 1A, B). Addition of phosphocreatine (3 mM) in the presence of the endogenous channel opener ADP (1 mM) on the intracellular face of the excised membrane patch closed the channels (Npo was 3.1±0.5 under control condition and 1.1±0.4 in the presence of phosphocreatine plus ADP, n=4, I<0.01, Fig 1A), whereas creatine (3 mM) in the presence of the endogenous channel blocker, ATP (1 mM), opened the channels (Npo was 0.1±0.3 in the presence of ATP and 1.8±0.4 in the presence of creatine plus ATP, n=4, P<0.01, Fig 1B). To examine whether CK influences the channel activity to the extent of altering whole cell membrane excitability, we measured membrane currents from a guinea-pig ventricular myocyte in a whole-cell configuration. Dialysis of the pipette solution into the cell under whole cell patch clamp configuration led to complete replacement of intracellular solution with the pipette solution in a few minutes, thus removing cytosolic CK from the cell and leaving inside the cell only those CK fractions that are bound tightly to the cellular interior and the sarcolemma. When ATP (1 mM) alone was present in the pipette solution, the steady-state voltage-current (I-V) relationship (Fig. 1C, C1) was in an “N” shape due to the strong inward rectification of IK1 channels and absence of active KATP channels. However, when creatine (3 mM) plus ATP (1 mM) was added into the pipette solution, outward K+ current increased significantly at potentials more positive than -70 mV (at 80 mV current density was 14.6 pA/pF with ATP plus creatine compared with 4.8 pA/pF with ATP alone, P<0.01, n=4-5, Fig 1C-C2), and the inward rectification of the I-V relationship became much weaker, which is a typical finding when measuring K+ currents flow through KATP channels (see ref. 9). In contrast, when phosphocreatine (3 mM) plus ADP (1 mM) was present in the pipette solution, whole-cell membrane currents were similar to those obtained when the pipette solution contained ATP alone (at 80 mV current density was 3.3 pA/pF with ADP plus phosphorylcreatine; compared with 4.8 pA/pF with ATP alone, P>0.05, n=4, Fig 1C-C2).
Figure 1. Creatine kinase regulate KATP channels activity.
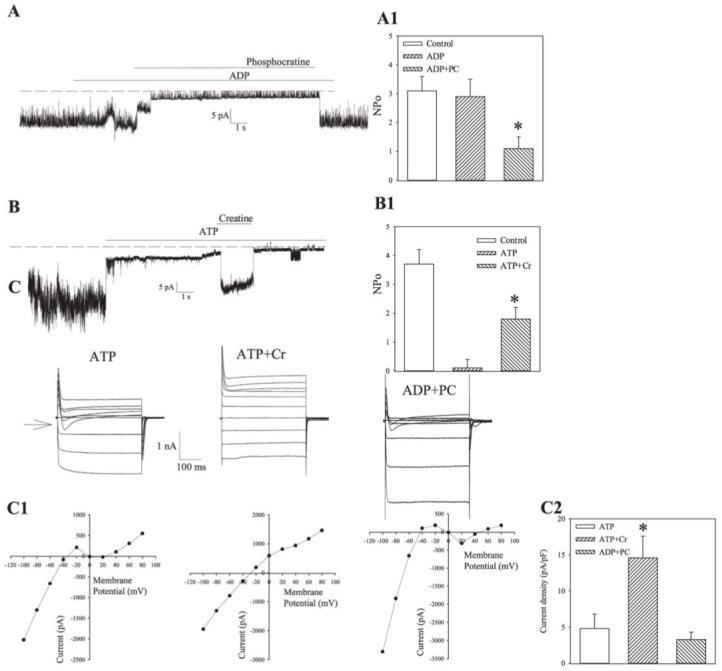
A, B) Recording of KATP channel activity in membrane patch treated with ADP (1 mM), ADP (1 mM) plus phosphocreatine (3 mM), and again with ADP (1 mM) (A) or treated with ATP (1 mM), ATP (1 mM) plus creatine (3 mM) and again with ATP (1 mM) (B). Holding potential: -60 mV. Doted lines correspond to the zero current levels, A1 and B1. Channel activity expressed as NPo under conditions in (A) and (B). Vertical bars represent mean ± se (n=4 for each). *P<0.01. C) Membrane currents evoked by identical families of 400 ms voltage pulses in cells filled with pipette solution containing either ATP (1 mM) or ATP (1 mM) plus creatine (3 mM) or ADP (1 mM) plus phosphocreatine (3 mM). Arrow points to the zero current level. C1. Current-voltage relationships corresponding to recording on (C), C2. Current densities at 80 mV for conditions in (C). Vertical bars represent mean ± se (n = 4-5). *P<0.01.
Coimmunoprecipitation of cardiac membrane fraction
To immunoprecipitate proteins associated with the cardiac sarcolemmal KATP channels, we have used anti-SUR2A antibody raised against a SUR2A epitope CIVQRVNETQNGTNN (residues 321-334; see also ref. 17). Specificity of the antibody was confirmed on total protein extract from A549 cells, cells natively devoid of KATP channels, cotransfected with cDNAs encoding Kir6.2 and SUR2A subunits (Fig. 2A). Western blotting analysis with the anti-SUR2A antibody of the immunoprecipitate uncovered a single signal at ∼150 kDa (expected size for the SUR2A subunit, 16), and only in cotransfected cells (Fig. 2A). Coomassie blue staining of the anti-SUR2A immunoprecipitate of cardiac membrane fraction revealed polypeptides with sizes corresponding to Kir6.2 (∼38 kDa), SUR2A (∼150 kDa), and CK (∼48 kDa) (2, 16) (Fig. 2B). The appearance of these polypeptide bands was blocked by incubation with the corresponding antigenic peptide, and these proteins did not precipitate if the membrane fraction was probed with a non-KATP channel antibody (data not shown). Western blotting analysis using the anti-SUR2A antibody as well as the anti-Kir6.2 antibody (raised against the synthetic peptide comprising residues 33-47 in the Kir6.2 protein, see ref. 16) identified p38 kDa and p150 pkDa polypeptides as Kir6.2 and SUR2A (see Fig. 2C).
Figure 2. Anti-SUR2A antibody and immunoprecipitation.
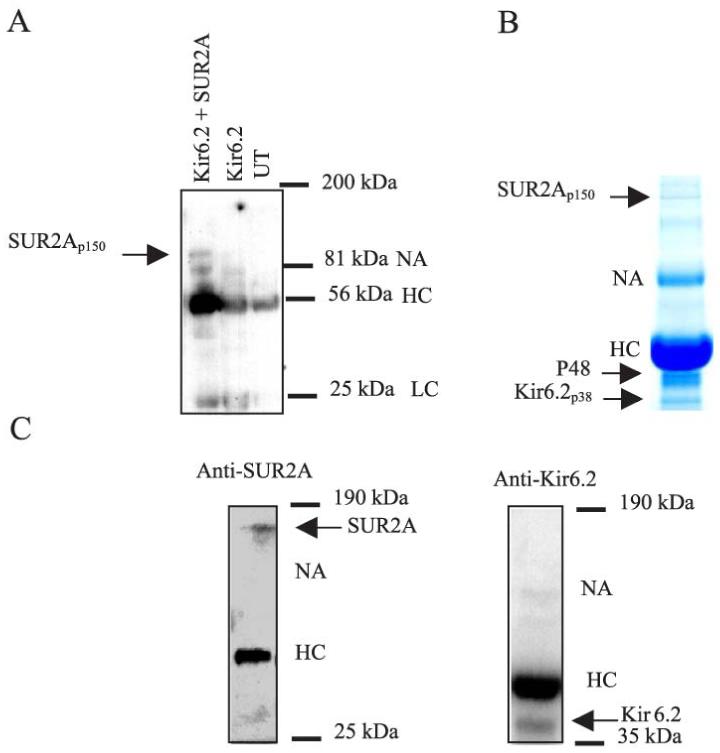
A) Western blotting analysis of imunoprecipitate pellets from untransfected (UT), transfected and cotransfected A549 cells. Note that apart from antibody fragments, SUR2A signal was detected only in cotransfected cells; that is, cells expressing SUR2A subunit. B) Coomasie blue stain of immunoprecipitate pellets obtained from cardiac membrane fraction precipitated with 40 μg anti-SUR2A antibody. Note the presence of proteins migrating at ∼38 kDa, ∼48 kDa, and ∼150 kDa. C) Western blot of anti-SUR2A immunoprecipitate pellets from guinea pig cardiac membrane fraction with the anti-SUR2A and anti-Kir6.2 (raised against the synthetic peptide consisting of residues 33 to 47, ARFVSKKGNCNVAHK, in the Kir6.2 protein) antibodies. Note that single signals in both cases and no cross-reactivity with any other proteins within 35-190 kDa range. HC-heavy chain.
CK presence in anti-SUR2A immunoprecipitate of cardiac membrane fraction
We examined the presence of CK in the anti-SUR2A immunoprecipitate by CK assay and MALDI-TOF analysis. The CK activity was present in anti-SUR2A immunoprecipitation pellets (Fig. 3AB), whereas no activity was observed when the experimental protocol was applied without the antibody. The level of the CK activity in the precipitate correlated well with the concentration of antibody (Fig. 3B). In addition, the obtained mass spectrum of tryptic mass fingerprints of p48 kDa (Fig. 3C) was identified clearly as muscle form creatine kinase (CK).
Figure 3. CK is present in anti-SUR2A immunoprecipitate.
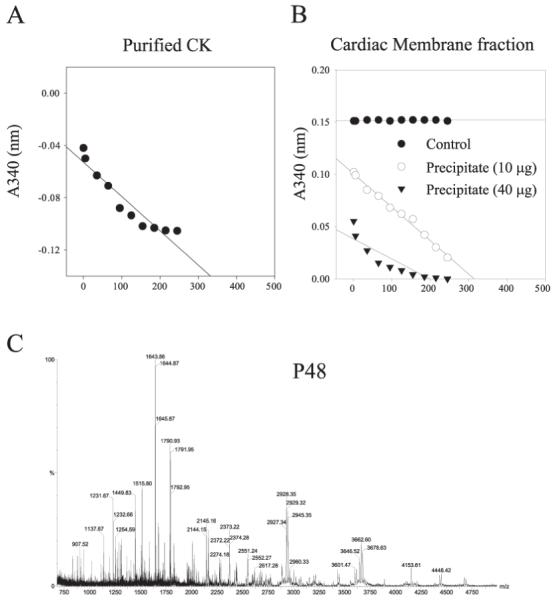
A) Creatine kinase assay with commercial CK (Sigma). B) Creatine kinase assay with anti-SUR2A immunoprecipitate of cardiac membrane fraction. Note that CK activity is recovered in pellets in an antibody concentration-dependent manner. C) Graph representing MALDI-TOF mass spectrum of tryptic mass fingerprint obtained from ∼48 kDa migrating protein. The protein was identified as muscle form of creatine kinase.
SUR2A presence in anti-CK immunoprecipitate of cardiac membrane fraction
We observed no cross-reactivity between the anti-SUR2A antibody and purified CK. (Fig. 4A). However, Western blotting of cardiac membrane anti-SUR2A immunoprecipitate with an anti-CK antibody revealed a single signal at the expected size of CK (48 kDa) (Fig. 4B). Conversely, the Western blotting of cardiac membrane anti-CK immunoprecipitate with an anti-SUR2A antibody revealed a single signal at the expected size of SUR2A (150 kDa, Fig. 4B).
Figure 4. CK is associated with cardiac KATP channel protein complex.
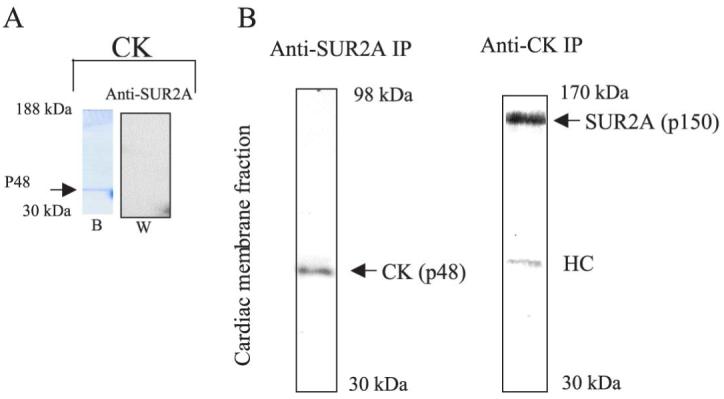
A) Coomasie blue stain of commercial muscular CK (Sigma) running at 48 kDA (b) and corresponding Western blot (w) with anti-SUR2A antibody. Note a lack of cross-reactivity between the anti-SUR2A antibody and CK. B) Western blotting of anti-SUR2A immunoprecipitate with anti-CK (anti-SUR2A IP) and anti-CK immunoprecipitate with anti-SUR2A (anti-CK IP) antibody of guinea pig cardiac membrane fraction. Note single “right” size signals (48 kDa for CK and 150 kDa for SUR2A) in both cases. HC = heavy chain.
CK and cardiac KATP channel subunits
A549 cells were cotransfected with genes encoding Kir6.2 and/or SUR2A plus the muscle form of CK cloned from mouse heart (see the Materials and Methods). Proteins from all cell groups were immunoprecipitated with the anti-SUR2A antibody except for cells cotransfected with Kir6.2/CK, where the anti-Kir6.2 antibody was used. CK assay revealed CK activity only in immunoprecipitate from cells transfected either with SUR2A plus CK or with SUR2A/Kir6.2 plus CK, but not in immunoprecipitate from untransfected cells or cells transfected with Kir6.2 plus CK (Fig. 5A). We obtained similar results by using Western blotting analysis of the immunoprecipitates with the CK antibody. A single band at 48-kDa size was obtained only in immunoprecipitate from cells cotransfected with SUR2A/CK and SUR2A/Kir6.2/CK, but not in immunoprecipitate from cells without gene encoding the SUR2A subunit (Fig. 5B).
Figure 5. CK directly associates with the SUR2A subunit.
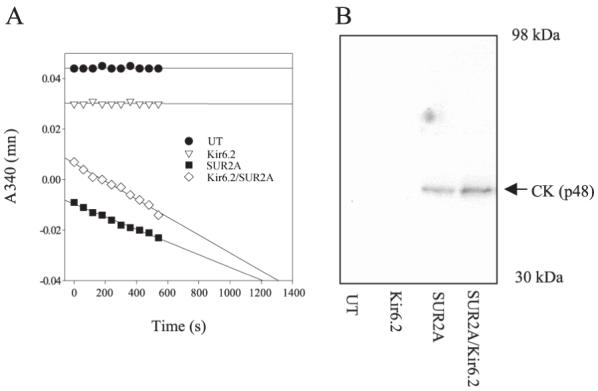
A) Creatine kinase assay with anti-SUR2A or anti-Kir6.2 immunoprecipitate of untransfected A549 cells (UT) or cells transfected with Kir6.2 plus CK (Kir6.2), SUR2A plus CK (SUR2A), and SUR2A/Kir6.2 plus CK (SUR2A/Kir6.2). Anti-Kir6.2 antibody was used for immunoprecipitation of Kir6.2 cells, whereas for all other groups anti-SUR2A antibody was applied. Note that CK activity is present only in SUR2A and SUR2A/Kir6.2 group of cells. B) Western blotting of anti-Kir6.2 and anti-SUR2A immunoprecipitate of A549 cells (same cell groups and methods as in (A). Note that single “right” size (48 kDa) band was observed only in SUR2A and SUR2A/Kir6.2 group of cells.
DISCUSSION
We report here that CK is physically associated with the SUR2A subunit of the cardiac KATP channels where it regulates the channel activity.
In previous studies, regulation of cardiac KATP channel activity by CK was established by using open cell-attach configuration of patch clamp technique, which preserves the structural and metabolic integrity of a cell (11, 19). Here, we tested the possibility that CK activity may change the probability of KATP channel opening by using inside-out configuration of the patch clamp technique, as well as whole cell electrophysiology. Inside-out configuration allowed direct application of CK substrates on the KATP channel protein complex and excluded the possibility that the response of the channel may be due to the action of a cytosolic/unbound fraction of CK (for this strategy see also 20). CK catalyses Phosphocreatine + ADP ↔ Creatine + ATP reaction; thereby, this enzyme has the potential both to close (by favoring ATP production) and to open (by favoring ADP production and ATP removal) KATP channels. The obtained results, at both single-channel and whole-cell levels, demonstrate that CK substrates activate or inhibit KATP channels opening, which is in agreement with the hypothesis that CK is within close proximity of the channels or is bound to the sarcolemma (11). In this respect, results from this as well as from previous studies (11, 19) are, at least, compatible with the possibility that CK is an integrative part of the cardiac KATP channel complex.
To examine whether CK is a part of the cardiac KATP channel complex in cardiac myocytes, we have applied a coimmunoprecipitation strategy—the strategy that may give a proof of a physical association between two proteins (16). The key step in developing a successful immunoprecipitation protocol is the design of specific antibodies. In this regard, we designed several antipeptide antibodies, based on hydrophilicity and antigenic index, against specific epitopes from each KATP channel subunit, Kir6.2 and SUR2A. Our preliminary studies demonstrated that an antibody raised against a SUR2A epitope CIVQRVNETQNGTNN (residues 321-334) was the most suitable for these experiments. Indeed, we have provided evidence that this anti-SUR2A antibody is specific for SUR2A (see also ref. 17). The level of specificity of the SUR2A antibody allowed us to investigate the nature of proteins forming the functional channel complex in sarcolemma. Thus, if CK is tightly associated with the SUR2A subunit, then it is expected to coprecipitate with the antibody. Coomassie blue staining of the anti-SUR2A immunoprecipitate revealed polypeptides with sizes corresponding to Kir6.2, SUR2A and CK (2, 16, 21). The appearance of these polypeptides was specific for the anti-SUR2A antibody and it was blocked with the antigenic peptide. These results suggest that the presence of polypeptides in immunoprecipitate was probably due to interaction of anti-SUR2A antibody with the SUR2A subunit. Thus, the obtained results support previous studies that showed physical association between the channel subunits (16, 21), and provide further evidence that the SUR2A and Kir6.2 subunits are physically associated to form the cardiac KATP channels in vivo. At present, Coomassie blue staining did not visualize the presence of AK in immunoprecipitate, but this can be explained by the fact that the size of AK is very close to the size of the primary antibody heavy chain, which overlays AK (11).
In the present study, we have provided several types of evidence to suggest that the 48 kDa protein was CK. First, we have demonstrated the presence of CK activity in anti-SUR2A immunoprecipitation pellets, whereas no activity was observed when the experimental protocol was applied without the antibody and the level of the CK activity in the precipitate correlated well with the concentration of antibody. Second, Western blotting of cardiac membrane anti-SUR2A immunoprecipitate with an anti-CK antibody revealed a single polypeptide migrating at the CK size. Third, the obtained mass spectrum of tryptic mass fingerprints of p48 kDa was identified as muscle form creatine kinase (CK). Therefore, taken together, the obtained results strongly indicate that 48 kDa protein is actually CK.
It should be said, however, that in principle, immunoprecipitations could have nonspecific contaminants (22), and therefore we used extreme care to classify CK as an associated protein to cardiac KATP channel complex. First, we have shown that there is no cross-reactivity between the anti-SUR2A antibody and purified CK itself, thus excluding the possibility that CK was precipitated directly with our anti-SUR2A antibody. Second, and most important, not only was CK found in anti-SUR2A immunoprecipitate but, conversely, SUR2A was identified in anti-CK immunoprecipitate of cardiac membrane fraction. Because coprecipitation with antibodies specific for both members of a complex is the most reliable evidence of physical association (22), these findings provide a proof that CK is physically associated with the cardiac KATP channel protein complex.
To define the exact subunit of the KATP channel that directly interacts with CK, we have applied an immunoprecipitation strategy at the level of recombinant proteins. Both CK assay and Western blotting with the anti-CK antibody revealed the presence of CK only in cells (co)transfected with SUR2A subunit, which suggests that CK directly and physically associates specifically with the SUR2A subunit. It is the predominant view that ATP inhibition of KATP channels occurs through interaction with the Kir6.2 subunit (23, 24), whereas the SUR subunit confers channel activation by ADP (25, 26). The fact that CK is associated specifically with the SUR2A subunit implies that this enzyme is sitting close to the ADP binding-site, where it may “catch” ADP (produced from ATP by AK, 11) and, by removing ADP and producing ATP, prevents ADP-induced channel opening. Thus, observations of simultaneous decrease in CK velocity and increase in opening of the KATP channels (27, 28) could be explained by tight regulation of KATP channel activity with CK, which is an integral part of the channel complex.
Recently, a unique model of cardiac KATP channel regulation involving AK and CK as major components of the system was proposed. More specifically, it has been suggested that AK-mediated opening of the KATP channels (AMP plus ATP-induced opening of KATP channels, 11, 20) is reversed by CK activity (ADP plus phosphocreatine-induced closing of KATP channels; refs. 11, 19, and also this study). The present result that CK is a part of the KATP channel protein complex supports this model. Physical association between AK (11) and CK (present study) with KATP channels actually means that the main intracellular producers of ATP (CK) and ADP (AK) form a complex with the KATP channel, which transduces ATP and ADP levels into the changes in membrane excitability. Under physiological condition, CK catalyses the production of ATP from phosphocreatine and ADP, maintaining high ATP/ADP ratio around the channel microenvironment and thereby keeping the channel in a closed state despite the presence of AK (11). During severe metabolic stress in the heart, CK velocity dramatically decreases together with a decrease in the phosphocreatine/ATP ratio (28). Both of these processes promote a drop in production of ATP (decrease in CK velocity) with concomitant increase in ADP levels within channel vicinity, catalyzed by AK or even by CK itself (decrease in the phosphocreatine/ATP ratio and increase in creatine), leading to opening of cardiac KATP channels (11). Thus, the complex of both AK and CK with cardiac KATP channels would allow highly regulated transduction of metabolic status into the membrane excitability.
In conclusion, we have demonstrated that CK is an integral part of the cardiac sarcolemmal KATP channel protein complex in vivo where may regulate the activity of KATP channels. These data provide a new avenue for investigating relationships between cardiac metabolism and cardiac membrane excitability.
ACKNOWLEDGMENTS
The authors acknowledge the kind gifts of Kir6.2 and SUR2A cDNAs received from S. Seino (Chiba University, Chiba, Japan) and Y. Kurachi (Osaka University, Osaka, Japan), respectively. We thank S. Jovanovic for critical reading of the manuscript. This research was supported by grants from the Biotechnology and Biological Sciences Research Council, British Heart Foundation, National Heart Research Fund, TENOVUS-Scotland and the Wellcome Trust to A.J.
REFERENCES
- 1.Dzeja PP, Terzic A. Phosphotransfer reactions in the regulation of ATP-sensitive K+ channels. FASEB J. 1998;12:523–529. doi: 10.1096/fasebj.12.7.523. [DOI] [PubMed] [Google Scholar]
- 2.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol. Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 3.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature (London) 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 4.Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ. Res. 1999;84:973–979. doi: 10.1161/01.res.84.9.973. [DOI] [PubMed] [Google Scholar]
- 5.Jovanovic A, Jovanovic S, Lorenz E, Terzic A. Recombinant cardiac ATP-sensitive K+ channel subunits confer resistance towards chemical hypoxia-reoxygenation injury. Circulation. 1998;98:1548–1555. doi: 10.1161/01.cir.98.15.1548. [DOI] [PubMed] [Google Scholar]
- 6.Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzales G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 7.Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 8.Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J. Biol. Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- 9.Terzic A, Jahangir A, Kurachi Y. Cardiac ATP-sensitive K+ channels regulation by intracellular nucleotides and K+ channel opening drugs. Am. J. Physiol. 1995;269:C525–C545. doi: 10.1152/ajpcell.1995.269.3.C525. [DOI] [PubMed] [Google Scholar]
- 10.Terzic A, Findlay I, Hosoya Y, Kurachi Y. Dualistic behavior of ATP-sensitive K+ channels toward intracellular nucleoside diphosphates. Neuron. 1994;12:1049–1058. doi: 10.1016/0896-6273(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 11.Carrasco AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman LV, Abraham MR, Hodgson D, Bienengraeber M, Puceat M, Janssen E, Wieringa B, Terzic A. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc. Acad. Natl. Sci. USA. 2001;98:7623–7628. doi: 10.1073/pnas.121038198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jovanovic S, Jovanovic A, Shen WK, Terzic A. Low concentrations of 17β-estradiol protect single cardiac cells against hypoxia-reoxygenation induced Ca2+ loading. J. Am. Coll. Cardiol. 2000;36:948–952. doi: 10.1016/s0735-1097(00)00798-1. [DOI] [PubMed] [Google Scholar]
- 13.Jovanovic S, Jovanovic A. Diadenosine tetraphosphate-gating of cardiac KATP channels requires intact actin cytoskeleton. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2001;364:276–280. doi: 10.1007/s002100100401. [DOI] [PubMed] [Google Scholar]
- 14.Jovanovic S, Jovanovic A. Delivery of genes encoding KATP channel subunits in conjuction with pinacidil prevents membrane depolarisation in cells exposed to chemical hypoxia-reoxygenation. Biochem. Biophys. Res. Commun. 2001;282:1098–1102. doi: 10.1006/bbrc.2001.4691. [DOI] [PubMed] [Google Scholar]
- 15.Jovanovic N, Jovanovic S, Jovanovic A, Terzic A. Gene delivery of Kir6.2/SUR2A in conjunction with pinacidil handles intracellular Ca2+ homeostasis under metabolic stress. FASEB J. 1999;13:923–929. doi: 10.1096/fasebj.13.8.923. [DOI] [PubMed] [Google Scholar]
- 16.Lorenz E, Alekseev AE, Krapivinsky GB, Carrasco AJ, Clapham DE, Terzic A. Evidence for direct physical association between a K+ channel (Kir6.2) and in ABC protein (SUR1) which affects cellular distribution and kinetic behavior of an ATP-sensitive K+ channel. Mol. Cell. Biol. 1998;18:1652–1659. doi: 10.1128/mcb.18.3.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranki HJ, Budas GR, Crawford RM, Jovanovic A. Gender-specific difference in cardiac ATP-sensitive K+ channels. J. Am. Coll. Cardiol. 2001;38:906–915. doi: 10.1016/s0735-1097(01)01428-0. [DOI] [PubMed] [Google Scholar]
- 18.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 19.Bienengraeber M, Alekseev AE, Abraham MR, Carrasco AJ, Moreau C, Vivaudou M, Dzeja PP, Terzic A. ATPase activity of the sulfonylurea receptor: a catalytic function for the KATP channel complex. FASEB J. 2000;14:1943–1952. doi: 10.1096/fj.00-0027com. [DOI] [PubMed] [Google Scholar]
- 20.Elvir-Mairena JR, Jovanovic A, Gomez LA, Alekseev AE, Terzic A. Reversal of the ATP-liganded state of ATP-sensitive K+ channels by adenylate kinase activity. J. Biol. Chem. 1996;271:31903–31908. doi: 10.1074/jbc.271.50.31903. [DOI] [PubMed] [Google Scholar]
- 21.Lorenz E, Terzic A. Physical association between recombinant cardiac ATP-sensitive K+ channel subunits Kir6.2 and SUR2A. J. Mol. Cell. Cardiol. 1999;31:425–434. doi: 10.1006/jmcc.1998.0876. [DOI] [PubMed] [Google Scholar]
- 22.Harlow E, Lane D. Immunoprecipitation. In: Harlow E, Lane D, editors. Using Antibodies: A Laboratory Manual. Woodbury, NY: Cold Spring Harbor Laboratory Press; 1999. [Google Scholar]
- 23.Tucker SJ, Gribble SM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature (London) 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- 24.Tanabe K, Tucker SJ, Matsuo M, Proks P, Ashcroft FM, Seino S, Amachi T, Ueda K. Direct photoaffinity labeling of the Kir6.2 subunit of the ATP-sensitive K+ channel by 8-azido-ATP. J. Biol. Chem. 1999;274:3931–3933. doi: 10.1074/jbc.274.7.3931. [DOI] [PubMed] [Google Scholar]
- 25.Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, Boyd AE, Gonzales G, Hererra-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 26.Nichols CG, Shying SL, Nestorowicz A, Glaser B, Clement JP, Gonzales G, Aguilar-Bryan L, Permutt MA, Bryan J. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science. 1996;272:1785–1787. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- 27.Knopp A, Thierfelder S, Koopmann R, Biskup C, Bohle T, Benndorf K. Anoxia generates rapid and massive opening of KATP channels in ventricular cardiac myocytes. Cardiovasc. Res. 1999;41:629–640. doi: 10.1016/s0008-6363(98)00238-7. [DOI] [PubMed] [Google Scholar]
- 28.Cave AC, Ingwall JS, Friedrich J, Liao R, Saupe KW, Apstein CS, Eberli FR. ATP synthesis during low-flow ischemia: influence of increased glycolytic substrate. Circulation. 2000;101:2090–2096. doi: 10.1161/01.cir.101.17.2090. [DOI] [PubMed] [Google Scholar]


