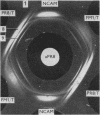
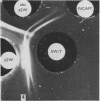
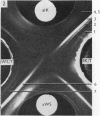
Abstract
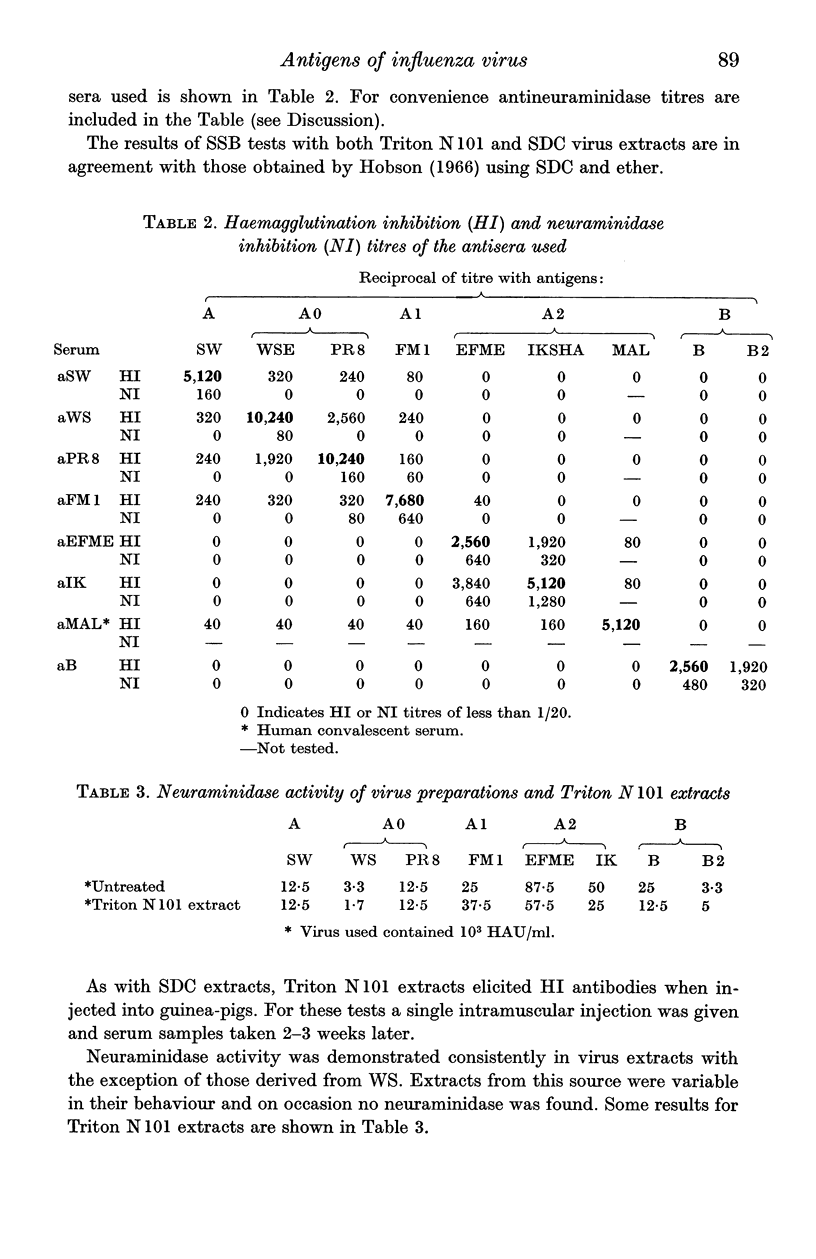
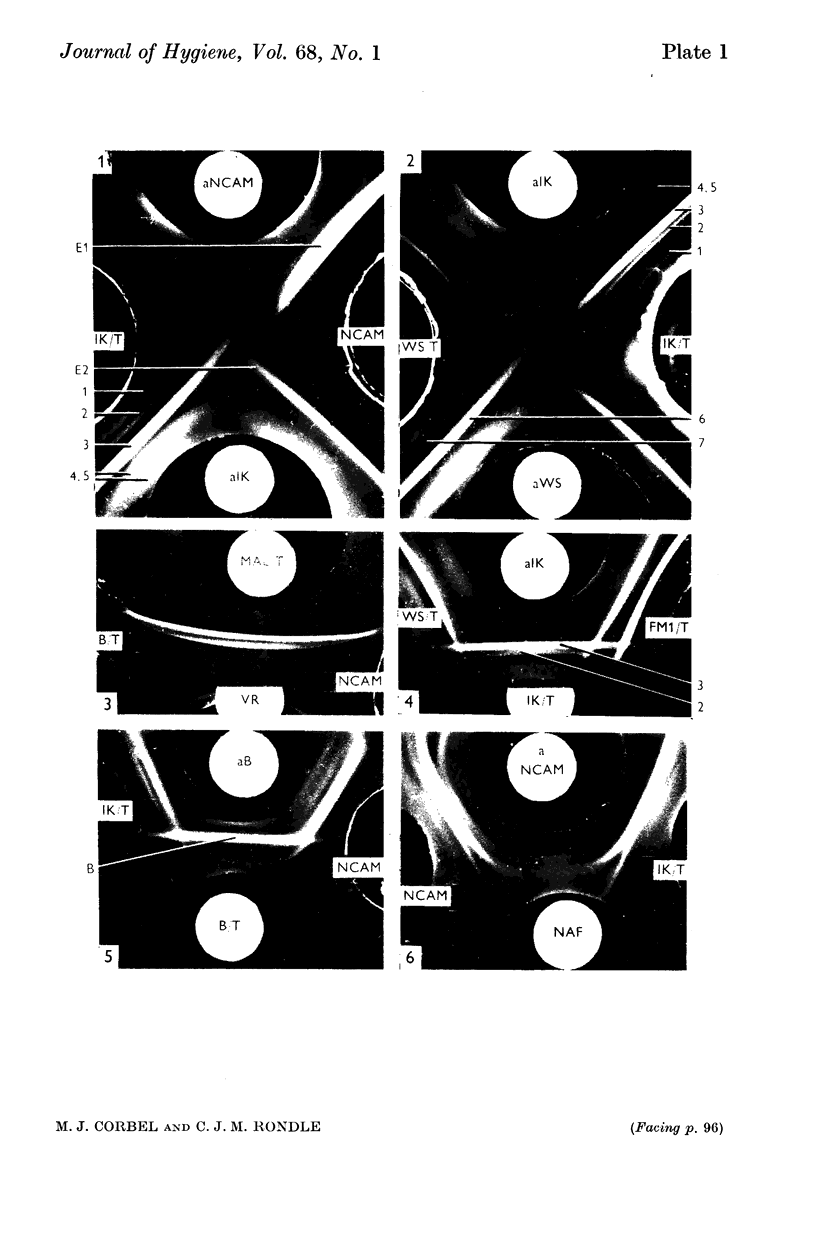
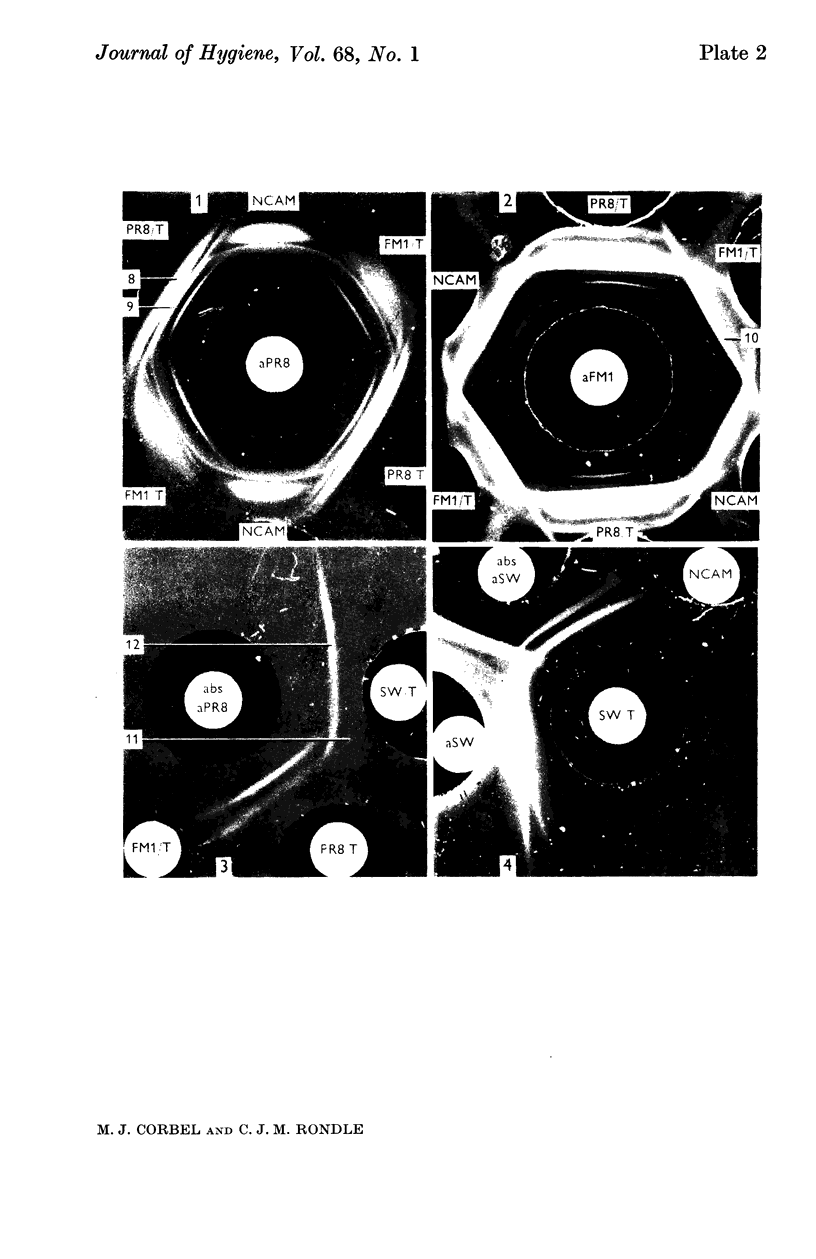
Highly purified influenza virus was degraded using anionic and non-ionic detergents. Best results were obtained using the non-ionic detergent Triton N 101. Tests showed that virus extracts contained neuraminidase and a substance that reacted specifically with rabbit antibody to virus haemagglutinin (specific serum blocking substance). Haemagglutination-inhibiting antibody was produced when virus extracts were inoculated into guinea-pigs. Immunodiffusion tests showed that extracts were complex. Host-specific material was regularly found. Under appropriate conditions S-antigen was detected as a single line pattern component. Two or more virus-specific materials were also present. One of these was probably neuraminidase and the other the specific serum blocking substance.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANANTHANARAYAN R. The fabric of virus elementary bodies. Br J Exp Pathol. 1954 Aug;35(4):381–388. [PMC free article] [PubMed] [Google Scholar]
- BARTALOS M. A possible chemical term for the operatorgene. Nature. 1963 Apr 6;198:109–109. doi: 10.1038/198109a0. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOPPIN P. W., STOECKENIUS W. INTERACTIONS OF ETHER-DISRUPTED INFLUENZA A2 VIRUS WITH ERYTHROCYTES, INHIBITORS, AND ANTIBODIES. Virology. 1964 Apr;22:482–492. doi: 10.1016/0042-6822(64)90069-8. [DOI] [PubMed] [Google Scholar]
- Corbel M. J., Rondle C. J., Bird R. G. Degradation of influenza virus by non-ionic detergent. J Hyg (Lond) 1970 Mar;68(1):77–80. doi: 10.1017/s0022172400028527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVENPORT F. M., HENNESSY A. V., BRANDON F. M., WEBSTER R. G., BARRETT C. D., Jr, LEASE G. O. COMPARISONS OF SEROLOGIC AND FEBRILE RESPONSES IN HUMANS TO VACCINATION WITH INFLUENZA A VIRUSES OR THEIR HEMAGGLUTININS. J Lab Clin Med. 1964 Jan;63:5–13. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- FABIYI A., LIEF F. S., HENLE W. Antigenic analysis of influenza viruses by complement fixation. II. The production of antisera to strain-specific V antigens in guinea pigs. J Immunol. 1958 Dec;81(6):467–477. [PubMed] [Google Scholar]
- HARBOE A., BORTHNE B., BERG K. Antibody against normal egg material resulting from influenza vaccination. Acta Pathol Microbiol Scand. 1961;53:95–103. doi: 10.1111/j.1699-0463.1961.tb00388.x. [DOI] [PubMed] [Google Scholar]
- HENNISCH M. P. Virus antigen antibody reactions by gel diffusion. Int Arch Allergy Appl Immunol. 1960;16:153–156. doi: 10.1159/000229083. [DOI] [PubMed] [Google Scholar]
- HOWE C., LEE L. T., ROSE H. M. Collocalia mucoid: a substrate for myxovirus neuraminidase. Arch Biochem Biophys. 1961 Dec;95:512–520. doi: 10.1016/0003-9861(61)90184-9. [DOI] [PubMed] [Google Scholar]
- HOYLE L. Structure of the influenza virus; the relation between biological activity and chemical structure of virus fractions. J Hyg (Lond) 1952 Jun;50(2):229–245. doi: 10.1017/s0022172400019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukenes G., Harboe A., Mortensson-Egnund K. A uronic and sialic acid free chick allantoic mucopolysaccharide sulphate which combines with influenza virus hi-antibody to host material. 1. Purification of the substance. Acta Pathol Microbiol Scand. 1965;64(4):534–542. doi: 10.1111/apm.1965.64.4.534. [DOI] [PubMed] [Google Scholar]
- Howe C., Lee L. T., Harboe A., Haukenes G. Immunochemical study of influenza virus and associated host tissue components. J Immunol. 1967 Mar;98(3):543–557. [PubMed] [Google Scholar]
- Ivanicová S. Immunodiffusion reaction with influenza virus haemagglutinin. Acta Virol. 1968 Mar;12(2):171–172. [PubMed] [Google Scholar]
- JENSEN K. E., FRANCIS T., Jr Antigen-antibody precipitates in solid medium with influenza virus. J Immunol. 1953 Mar;70(3):321–325. [PubMed] [Google Scholar]
- KROEGER A. V. Evidence for the absence of anaphylactogenic host protein in highly purified PR8 influenza virus. J Immunol. 1962 Jul;89:136–144. [PubMed] [Google Scholar]
- LIEF F. S., FABIYI A., HENLE W. Antigenic analyses of influenza viruses by complement fixation. I. The production of antibodies to the soluble antigen in guinea pigs. J Immunol. 1958 Jan;80(1):53–65. [PubMed] [Google Scholar]
- NEURATH A. R., SOKOL F. ASSOCIATION OF MYXOVIRUSES WITH AN ADENOSINE DIPHOSPHATASE/ADENOSINE TRIPHOSPHATASE/AS REVEALED BY CHROMATOGRAPHY ON DEAE-CELLULOSE AND BY DENSITY GRADIENT CENTRIFUGATION. Z Naturforsch B. 1963 Dec;18:1050–1052. doi: 10.1515/znb-1963-1208. [DOI] [PubMed] [Google Scholar]
- NORRBY E. Hemagglutination by measles virus. 4. A simple procedure for production of high potency antigen for hemagglutination-inhibition (HI) tests. Proc Soc Exp Biol Med. 1962 Dec;111:814–818. doi: 10.3181/00379727-111-27930. [DOI] [PubMed] [Google Scholar]
- Paniker C. K. Serological relationships between the neuraminidases in influenza viruses. J Gen Virol. 1968 May;2(3):385–394. doi: 10.1099/0022-1317-2-3-385. [DOI] [PubMed] [Google Scholar]
- Reginster M. Inactivation of influenza virus by caseinase C from Streptomyces albus G culture-filtrate. J Gen Microbiol. 1965 Aug;40(2):157–169. doi: 10.1099/00221287-40-2-157. [DOI] [PubMed] [Google Scholar]
- Reginster M. Release of influenza virus neuraminidase by caseinase C of Streptomyces albus G. J Gen Microbiol. 1966 Mar;42(3):323–331. doi: 10.1099/00221287-42-3-323. [DOI] [PubMed] [Google Scholar]
- SMITH W., BELYAVIN G., SHEFFIELD F. W. The host-tissue component of influenza viruses. Proc R Soc Lond B Biol Sci. 1955 May 17;143(913):504–522. doi: 10.1098/rspb.1955.0026. [DOI] [PubMed] [Google Scholar]
- SMITH W. The structural and functional plasticity of influenza virus. Lancet. 1952 May 3;1(6714):885–891. doi: 10.1016/s0140-6736(52)90584-9. [DOI] [PubMed] [Google Scholar]
- Schild G. C., Pereira H. G. Characterization of the ribonucleoprotein and neuraminidase of influenza A viruses by immunodiffusion. J Gen Virol. 1969 Apr;4(3):355–363. doi: 10.1099/0022-1317-4-3-355. [DOI] [PubMed] [Google Scholar]
- Styk B., Hána L. Immunodiffusion studies on the reaction of influenza virus with specific antibody and nonspecific serum beta-inhibitor. Acta Virol. 1966 Jul;10(4):281–290. [PubMed] [Google Scholar]
- Styk B., Hána L., Sedíleková M. Antibody response to natural influenza A2 infection of man as studied by gel double diffusion. Acta Virol. 1968 May;12(3):208–213. [PubMed] [Google Scholar]
- TYRRELL D. A., HORSFALL F. L., Jr Disruption of influenza virus; properties of degradation products of the virus particle. J Exp Med. 1954 Apr 1;99(4):321–342. doi: 10.1084/jem.99.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADSWORTH C. A slide microtechnique for the analysis of immune precipitates in gel. Int Arch Allergy Appl Immunol. 1957;10(6):355–360. doi: 10.1159/000228394. [DOI] [PubMed] [Google Scholar]