Abstract
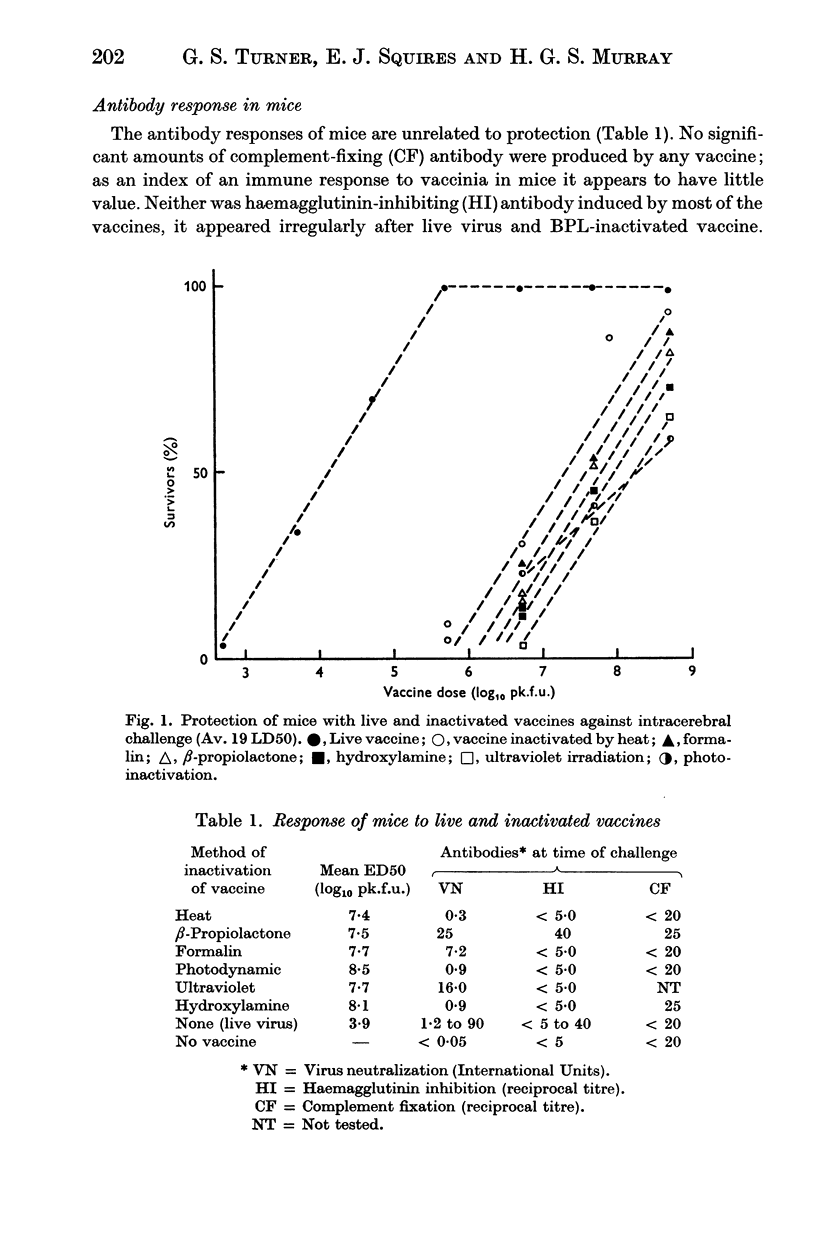
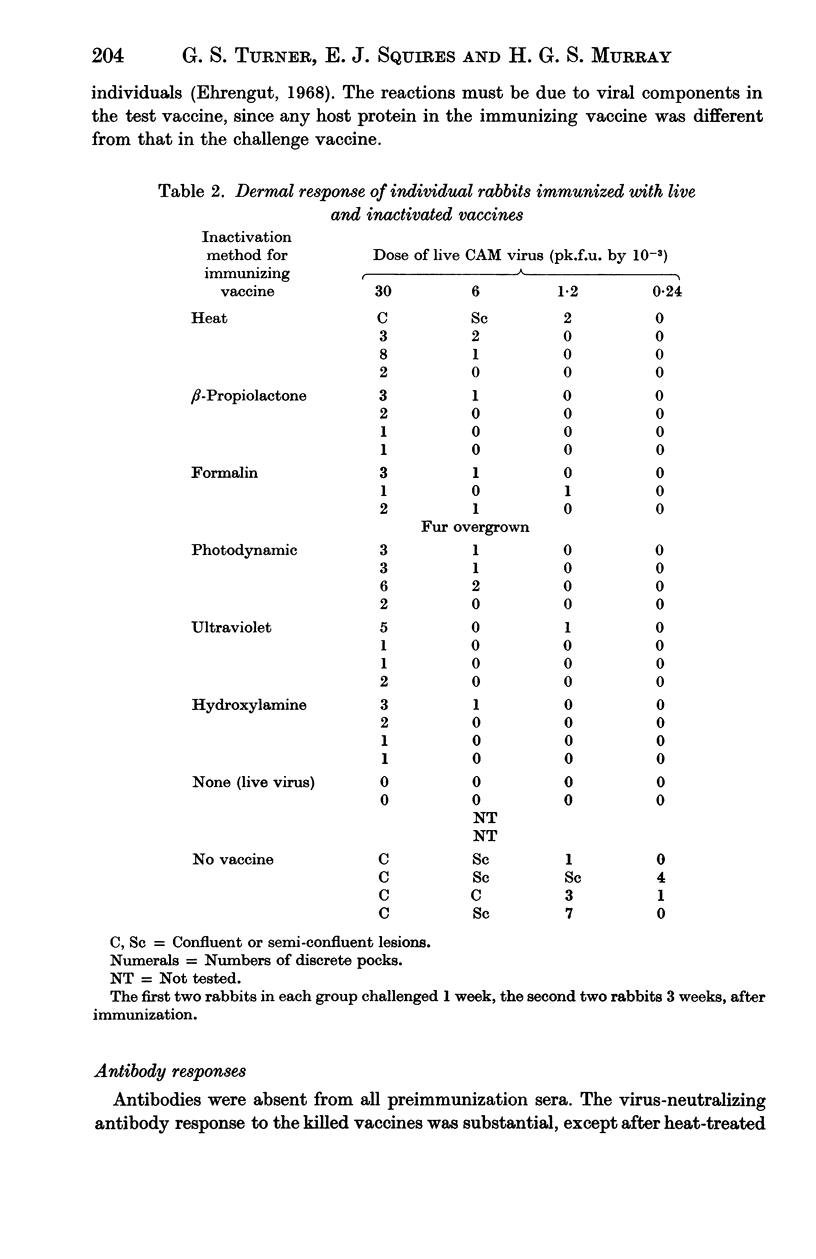
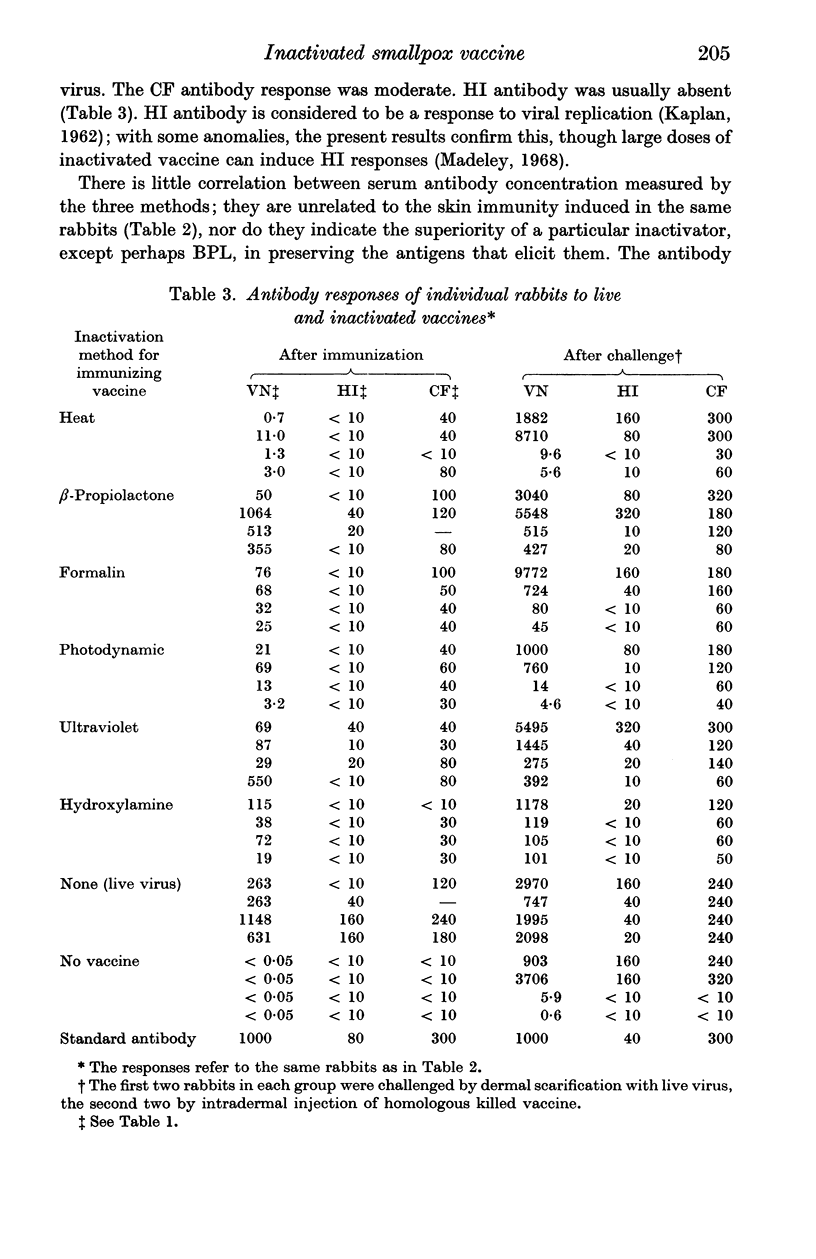
Vaccines were prepared from a single pool of high-titred vaccinia virus and inactivated by six methods, namely heat, formalin, hydroxylamine, β-propiolactone, ultraviolet irradiation, and visible light and methylene blue. Large doses of the vaccines were required to protect mice against intracerebral challenge. Differences in protection were not attributable to the method of their inactivation. The vaccines also induced similar degrees of skin immunity in rabbits which showed no severe dermal reactions when challenged with either homologous killed vaccine or live virus. The virus-neutralizing, haemagglutinin-inhibiting and complement fixing antibody responses to the vaccines differed; heat-inactivation preserved these antigens least well and β-propiolactone apparently the best. In both rabbits and mice there was little association between the different antibody responses to each vaccine or between the degrees of antibody response and the protection they induced. The relation of these findings to pox-virus immunity and the use of inactivated smallpox vaccine in man is discussed.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABEL P. REACTIVATION OF HEATED VACCINIA VIRUS IN VITRO. Z Vererbungsl. 1963 Nov 21;94:249–252. doi: 10.1007/BF00894768. [DOI] [PubMed] [Google Scholar]
- AMIES C. R. Loss of immunogenic properties of vaccinia virus inactivated by formaldehyde. Can J Microbiol. 1961 Apr;7:141–152. doi: 10.1139/m61-019. [DOI] [PubMed] [Google Scholar]
- Baron S., Buckler C. E., Friedman R. M., McCloskey R. V. Role of interferon during viremia. II. Protective action of circulating interferon. J Immunol. 1966 Jan;96(1):17–24. [PubMed] [Google Scholar]
- Boulter E. A. Protection against poxviruses. Proc R Soc Med. 1969 Mar 3;62(3):295–297. [PMC free article] [PubMed] [Google Scholar]
- COLLIER L. H., MCCLEAN D., VALLET L. The antigenicity of ultra-violet irradiated vaccinia virus. J Hyg (Lond) 1955 Dec;53(4):513–534. doi: 10.1017/s0022172400001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrengut W. Impfreaktionen und Pockenimpfschutz. Dtsch Med Wochenschr. 1968 May 10;93(19):948–954. doi: 10.1055/s-0028-1105170. [DOI] [PubMed] [Google Scholar]
- FENNER F. The reactivation of animal viruses. Br Med J. 1962 Jul 21;2(5298):135–142. doi: 10.1136/bmj.2.5298.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREESE E., BAUTZ-FREESE E., BAUTZ E. Hydroxylamine as a mutagenic and inactivating agent. J Mol Biol. 1961 Apr;3:133–143. doi: 10.1016/s0022-2836(61)80040-5. [DOI] [PubMed] [Google Scholar]
- Fellowes O. N. Hydroxylamine as an inactivating agent for foot-and-mouth disease virus. J Immunol. 1966 May;96(5):772–776. [PubMed] [Google Scholar]
- GARD S. Theoretical considerations in the inactivation of viruses by chemical means. Ann N Y Acad Sci. 1960 Jan 13;83:638–648. doi: 10.1111/j.1749-6632.1960.tb40934.x. [DOI] [PubMed] [Google Scholar]
- GIFFORD G. E. STUDIES ON THE SPECIFICITY OF INTERFERON. J Gen Microbiol. 1963 Dec;33:437–443. doi: 10.1099/00221287-33-3-437. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Nahmias A. J., Murphy F. A., Kramer J. H. Cellular immunity in vaccinia infection of mice. Anti-thymocyte serum effects on primary and secondary responsiveness. J Exp Med. 1968 Jul 1;128(1):121–132. doi: 10.1084/jem.128.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN C., BENSON P. F., BUTLER N. R. IMMUNOGENICITY OF ULTRAVIOLET-IRRADIATED, NON-INFECTIOUS, VACCINIA-VIRUS VACCINE IN INFANTS AND YOUNG CHILDREN. Lancet. 1965 Mar 13;1(7385):573–574. doi: 10.1016/s0140-6736(65)91146-3. [DOI] [PubMed] [Google Scholar]
- KAPLAN C., McCLEAN D., VALLET L. A note on the immunogenicity of ultra-violet irradiated vaccinia virus in man. J Hyg (Lond) 1962 Mar;60:79–83. doi: 10.1017/s0022172400039322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Sharp D. G. Influence of the physical state of formalinized vaccinia virus particles on surviving plaque titer: evidence for multiplicity reactivation. J Immunol. 1967 Dec;99(6):1221–1225. [PubMed] [Google Scholar]
- Kimes R. C., Bussell R. H. The nucleic acid type and effect of pH and hydroxylamine on canine distemper virus. Arch Gesamte Virusforsch. 1968;24(4):387–395. doi: 10.1007/BF01243078. [DOI] [PubMed] [Google Scholar]
- LINDENMANN J., BUSER F. [The inactivated vaccinia antigen of Herrlich: an experiment in serological antigen testing in man]. Pathol Microbiol (Basel) 1962;25:478–488. [PubMed] [Google Scholar]
- Madeley C. R. The immunogenicity of heat-inactivated vaccinia virus in rabbits. J Hyg (Lond) 1968 Mar;66(1):89–107. doi: 10.1017/s0022172400040973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannweiler E., Geister R. Das Verhalten der Serumantikörper bei Wiederimpfung mit Vakzinevirus. Dtsch Med Wochenschr. 1967 Nov 24;92(47):2168–2171. doi: 10.1055/s-0028-1106109. [DOI] [PubMed] [Google Scholar]
- McNeill T. A. The antibody response of rabbits to inactivated vaccinia virus. J Hyg (Lond) 1965 Dec;63(4):525–535. doi: 10.1017/s0022172400045411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill T. A. The development of skin resistance and hypersensitivity following inactivated vaccinia virus vaccines in rabbits. J Hyg (Lond) 1966 Mar;64(1):23–31. doi: 10.1017/s0022172400040304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RONDLE C. J., DUMBELL K. R. Antigens of cowpox virus. J Hyg (Lond) 1962 Mar;60:41–49. doi: 10.1017/s0022172400039292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde W. Versuch einer Gegenüberstellung bisheriger Unterlagen zur Einschätzung der Enzephalitisprophylaxe mittels Vakzineantigen in der DDR. Z Arztl Fortbild (Jena) 1968 Feb 1;62(3):177–183. [PubMed] [Google Scholar]
- Subrahmanyan T. P., Mims C. A. Fate of intravenously administered interferon and the distribution of interferon during virus infections in mice. Br J Exp Pathol. 1966 Apr;47(2):168–176. [PMC free article] [PubMed] [Google Scholar]
- TAYLOR A. R. Effects of nonionizing radiations on animal viruses. Ann N Y Acad Sci. 1960 Jan 13;83:670–683. doi: 10.1111/j.1749-6632.1960.tb40938.x. [DOI] [PubMed] [Google Scholar]
- Turner G. S., Kaplan C. Observations on photodynamic inactivation of vaccinia virus and its effect on immunogenicity. J Hyg (Lond) 1965 Sep;63(3):395–410. doi: 10.1017/s0022172400045289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G. S. Respiratory infection of mice with vaccinia virus. J Gen Virol. 1967 Jul;1(3):399–402. doi: 10.1099/0022-1317-1-3-399. [DOI] [PubMed] [Google Scholar]
- WOESE C. Thermal inactivation of animal viruses. Ann N Y Acad Sci. 1960 Jan 13;83:741–751. doi: 10.1111/j.1749-6632.1960.tb40943.x. [DOI] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Photodynamic inactivation of animal viruses: a review. Photochem Photobiol. 1966 Mar;4(2):159–170. doi: 10.1111/j.1751-1097.1965.tb05733.x. [DOI] [PubMed] [Google Scholar]


