Abstract
The importance of alternative splicing in regulating apoptosis has been suggested by findings of functionally antagonistic proteins generated by alternative splicing of several genes involved in apoptosis. Among these, Ich-1 (also named as caspase-2) encodes a member of the caspase family of proteases. Two forms of Ich-1 are produced as a result of alternative splicing: Ich-1L, which causes apoptosis, and Ich-1S, which prevents apoptosis. The precise nature of Ich-1 alternative splicing and its regulation remain unknown. Here, we show that the production of Ich-1L and Ich-1S transcripts results from alternative exclusion or inclusion of a 61-bp exon. Several splicing factors can regulate Ich-1 splicing. Serine-arginine-rich proteins SC35 and ASF/SF2 promote exon skipping, decreasing the ratio of Ich-1S to Ich-1L transcripts; whereas heterogeneous nuclear ribonucleoprotein A1 facilitates exon inclusion, increasing this ratio. Furthermore, in cultured cells, SC35 overexpression increases apoptosis; whereas heterogeneous nuclear ribonucleoprotein A1 overexpression decreases apoptosis. These results provide the first direct evidence that splicing factors can regulate Ich-1 alternative splicing and suggest that alternative splicing may be an important regulatory mechanism for apoptosis.
Regulated alternative splicing of pre-mRNA is an important mechanism for generating functionally different proteins from the same gene. Recently, alternative splicing has been found to be involved in processing pre-mRNAs of a number of genes acting at different steps of apoptosis pathway, ranging from membrane-associated death signal receptors to members of caspase family (1–5). Most strikingly, alternative splicing of Bcl-x, Ced-4, and Ich-1 pre-mRNAs results in the formation of products that play opposite roles in apoptosis (1–3). In Bcl-x, usage of the proximal 5′ splice site gives rise to a transcript encoding a long form of product, Bcl-xL; while selection of the distal 5′ splice site leads to a short product, Bcl-xS. Similar to Bcl-2, Bcl-xL can inhibit cell death; whereas, Bcl-xS antagonizes the activity of Bcl-2 (1). In Ced-4 pre-mRNA splicing, alternative usage of two different 3′ splice sites results in the formation of two transcripts, Ced-4L and Ced-4S. Overexpression of Ced-4s promotes cell death, while Ced-4L inhibits cell death (3). In the case of Ich-1, alternative splicing also generates two products, Ich-1L and Ich-1S. Ich-1L induces cell death; whereas overexpression of Ich-1S prevents cell death (2). These observations suggest a potential role of alternative splicing in regulating apoptosis.
Splicing factors of the serine-arginine-rich (SR) and heterogeneous nuclear ribonucleoprotein (hnRNP) families are emerging as important alternative splicing regulators (6–12). The SR proteins are characterized by structural and functional similarities (8–11). They contain SR sequences and RNA recognition motifs of the ribonucleoprotein type. They play important roles in both constitutive splicing and regulated alternative splicing (reviewed in references 7–12). Several studies have suggested that SR proteins and an hnRNP protein, hnRNP A1, have antagonistic effects on splice site selection (13–15).
We have used Ich-1 as a model system to study the role of alternative splicing in regulation of apoptosis. We characterized the nature of Ich-1 alternative splicing and established a minigene system for studying Ich-1 alternative splicing. We have further examined effects of several splicing factors on Ich-1 alternative splicing and provided evidence for the role of splicing factors in regulation of apoptosis.
MATERIALS AND METHODS
Plasmid Purification, Cell Culture, and Transient Transfection.
cDNAs encoding individual splicing factors were inserted into pcDNA3 (Invitrogen) under control of the cytomegalovirus promoter. Murine Ich-1 genomic fragment was a gift from Junying Yuan (Harvard University). Ich-1 minigene construct was made by inserting the murine Ich-1 genomic fragment into pcDNA3 vector between HindIII and XhoI sites. For transfection experiments, all plasmid DNA samples were purified by double cesium chloride centrifugation. HeLa or 293T cells were grown in 6 cm dishes in DMEM supplemented with 10% fetal calf serum. At the time of transfection, HeLa cells were ≈60% confluent and 293T cells were ≈30% confluent. Transfection was performed by using 18 μg of lipofectin and 8 μg of plasmids expressing individual splicing factors. Cells were harvested 48 hr after transfection. Under these conditions, the transfection efficiency in these experiments was ≈2% for HeLa cells and 20% for 293T cells. For co-transfection experiments, 8 μg of Ich-1 mini-gene construct together with 2 μg of plasmids expressing individual splicing factors or vector control were used. DNA sequence analysis of Ich-1 genomic fragments and different expression plasmids was carried out on an ABI 373A automatic sequencer using PRISM Ready reaction DyeDeoxy Terminator cycle sequencing kit (Applied Biosystems).
Reverse Transcription (RT)–PCR Assay.
RNA was prepared from the transfected HeLa or 293T cells and RT-PCR was carried out in the presence of 32P-dCTP as previously described (16). Murine and human Ich-1 specific primers (2) were used to detect the transfected and endogenous Ich-1 transcripts, respectively. The PCR cycle number was kept to a minimum to maintain linearity. Eighteen cycles for the endogenous human Ich-1 and 23 cycles for the transfected murine Ich-1 were used in PCR reactions. The ratio of Ich-1S to Ich-1L was measured using a PhosphorImager (Molecular Dynamics).
Immunofluorescent Staining and Detection of Apoptosis.
HeLa cells were grown to 60% confluence on cover glass (Erie Scientific, Portsmouth, NH) in 24-well dishes. Cells were transfected by using 2 μg of lipofectin and 1 μg plasmids expressing SC35 or hnRNP A1 or SRp20 as myc-tagged proteins. Twenty four hours after transfection, serum was removed from the medium. After 48 hr of serum-deprivation, cells were fixed with 4% paraformaldehyde in PBS. Indirect immunofluorescence staining was carried out using 9E10 monoclonal anti-myc antibody (17) and cyanine-3 conjugated anti-mouse secondary antibody (Jackson ImmunoResearch). Cells were counterstained with Hoechst dye 33258 (Sigma) as described (2) to reveal the nuclear morphology. Cells with condensed and fragmented nuclei were scored as apoptotic. Cell counting was performed by two individuals in a double-blind manner.
RESULTS AND DISCUSSION
Characterization of Ich-1 Alternative Splicing.
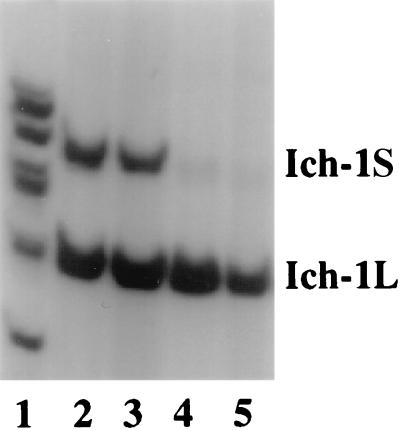
Using a sensitive RT-PCR assay, we found that both Ich-1L and Ich-1S can be detected in lymphoid and neural tissues. However, the ratio of Ich-1L and Ich-1S has distinct tissue specificity. Consistent with previous observations (2), in the lymphoid tissues such as thymus and spleen, Ich-1L is the predominant Ich-1 transcript; while in the adult brain, both Ich-1L and Ich-1S are expressed at similar levels (Fig. 1). The previous study suggested that alternative splicing of Ich-1 resulted from alternative usage of two competing 5′ splice sites (2). Our analyses of the genomic DNA and cDNA sequences indicate that production of Ich-1L and Ich-1S transcripts results from alternative exclusion or inclusion of a 61-bp exon, respectively (Fig. 2). Inclusion of the 61-bp exon results in translation termination using a more upstream stop codon in Ich-1S (Fig. 3A); therefore, the longer Ich-1S transcript encodes a shorter protein product. The sequence of the 61-bp exon that is present in Ich-1S but absent from Ich-1L is identical between human and mouse Ich-1 sequences. Eighty two of 93 nucleotides in the common upstream exon and 81 of 84 nucleotides in the intron upstream to the Ich-1S specific 61-bp exon are identical between human and mouse sequences. All the differences except one between the human and mouse sequences are purine to purine or pyrimidine to pyrimidine changes. The high degree of sequence conservation in the 61-bp exon and its upstream intron suggests that this region may contain cis-elements important for regulation of Ich-1 alternative splicing.
Figure 1.
Expression of Ich-1L and Ich-1S in neural and lymphoid tissues. RT-PCR was performed with murine Ich-1 specific primers and cDNA templates reverse transcribed from Poly(A)+ RNA prepared from different murine tissues: total brain (lane 2), cerebrum (lane 3) thymus (lane 4), and spleen (lane 5). Size markers are shown in lane 1. Ich-1S transcript is longer because inclusion of the alternatively spliced exon creates a stop codon (see Fig. 2 and Fig. 3A).
Figure 2.
Alignment of murine and human Ich-1 genomic and cDNA sequences. Sequences 1–3 are murine Ich-1L cDNA, Ich-1S cDNA and genomic DNA; sequences 4–6 are human genomic DNA, Ich-1S cDNA, and Ich-1L cDNA respectively. Identical nucleotides are indicated by “- - -” and gaps are marked by “⋅⋅⋅”. The positions of 5′ and 3′ splice sites are shown. The 61-bp exon included in the Ich-1S cDNA is underlined. A stop codon TGA in frame with Ich-1S is in bold form and the sequence of the 2.8 kb downstream exon is omitted (//).
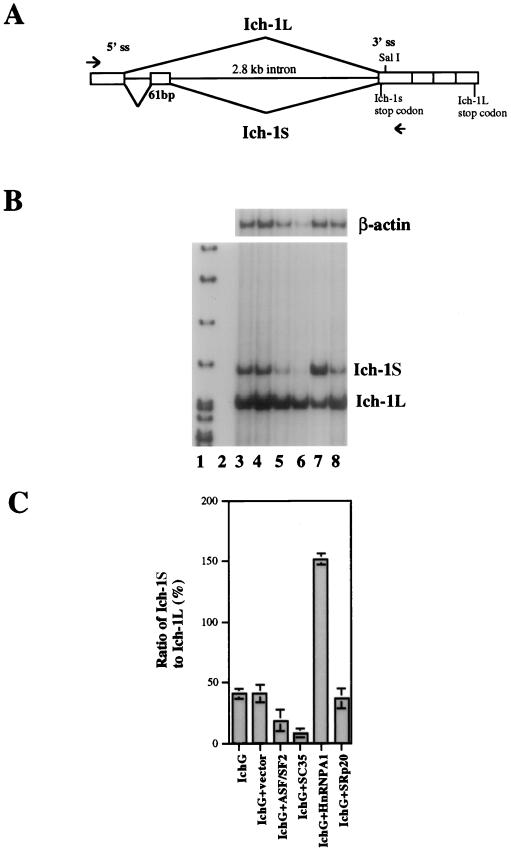
Figure 3.
Regulation of Ich-1 alternative splicing by SR proteins and hnRNP A1. (A) The Ich-1 minigene construct. The genomic DNA fragment containing the upstream exon, introns flanking the 61-bp exon, and the downstream exons was inserted under control of the cytomegalovirus promoter in a mammalian expression vector pcDNA3. The immediate downstream exon was fused at the SalI site to exons further downstream. The arrowheads indicate positions of specific primers used in the RT-PCR assay to detect the transfected Ich-1 minigene expression. (B and C) The Ich-1 minigene construct was co-transfected into HeLa cells with a plasmid encoding individual splicing regulators. Alternatively spliced Ich-1 products expressed from the transfected Ich-1 minigene were detected by RT-PCR with murine Ich-1 specific primers. The ratio of Ich-1S to Ich-1L was measured using a PhosphorImager. Lane 1 contains size markers. Lanes 2–8 contain RT-PCR products after transfection with pcDNA3 vector alone (lane 2), Ich-1 minigene construct (IchG) (lane 3), IchG+vector (lane 4), IchG+ASF/SF2 (lane 5), IchG+SC35 (lane 6), IchG+hnRNP A1 (lane 7), and IchG+SRp20 (lane 8). B shows a representative gel containing RT-PCR products. β-actin PCR products for the same samples were used as controls for amounts of input RNA. C shows the ratio of Ich-1S to Ich-1L as determined from three independent experiments.
Using the murine Ich-1 genomic fragment, we constructed a minigene plasmid containing the common upstream 5′ exon, the 61-bp exon with the flanking introns, and downstream exons of the murine Ich-1 gene (Fig. 3A). Because this construct does not include all of the upstream exons, it would not express functionally active proteins, thus allowing us to examine the regulation of Ich-1 alternative splicing without affecting cell death. Transfection of the Ich-1 minigene construct into HeLa cells led to the formation of two alternatively spliced Ich-1 RNA species, which were detected by RT-PCR (Fig. 3B, lane 3). The RT-PCR was specific because no transcripts were detected in HeLa cells transfected with the vector plasmid used for making the murine Ich-1 minigene construct (Fig. 3B, lane 2). DNA sequence analyses of both upper and lower bands (Fig. 3B) of the PCR products confirmed that the alternative splice sites used were indeed the same as those in Ich-1S and Ich-1L (2). These results indicated that the alternative splicing of Ich-1 pre-mRNA can be reproduced in cultured cells using the minigene construct.
Regulation of Ich-1 Alternative Splicing by Overexpression of SR Proteins and hnRNP A1.
Using the Ich-1 minigene construct, we tested whether known splicing factors can regulate Ich-1 alternative splicing. The Ich-1 minigene construct was cotransfected into HeLa cells together with a plasmid encoding one of splicing factors. The splicing products of the transfected murine Ich-1 minigene were then detected by RT-PCR using primers specific to murine Ich-1. The ratio of Ich-1S to Ich-1L was determined using a PhosphorImager. As shown in Fig. 3, transfection of SR family members, either SC35 or ASF/SF2, decreased the amount of Ich-1S relative to that of Ich-1L mRNA (Fig. 3B, lanes 6 and 5). By contrast, hnRNP A1 increased the production of Ich-1S relative to that of Ich-1L (Fig. 3 B and C, lane 7). Not all SR proteins affect Ich-1 splicing, because SRp20 did not alter the relative ratio of these transcripts (Fig. 3 B and C, lane 8). Furthermore, two other RS domain containing proteins, U2AF35 and U2AF65 did not affect Ich-1 splicing (W.-j.Z. and J.Y.W., unpublished results). These results demonstrate that in the Ich-1 alternative splicing, SR proteins SC35 and ASF/SF2 promote exon skipping, whereas hnRNP A1 promotes exon inclusion. Such effects are different from what has been observed on other known model substrate pre-mRNAs. SR protein ASF/SF2 has been shown to prevent exon skipping in β-tropomyosin and clathrin light chain B pre-mRNA alternative splicing (13–15). The hnRNP A1 protein, on the other hand, enhances exon skipping in these pre-mRNAs (14, 15).
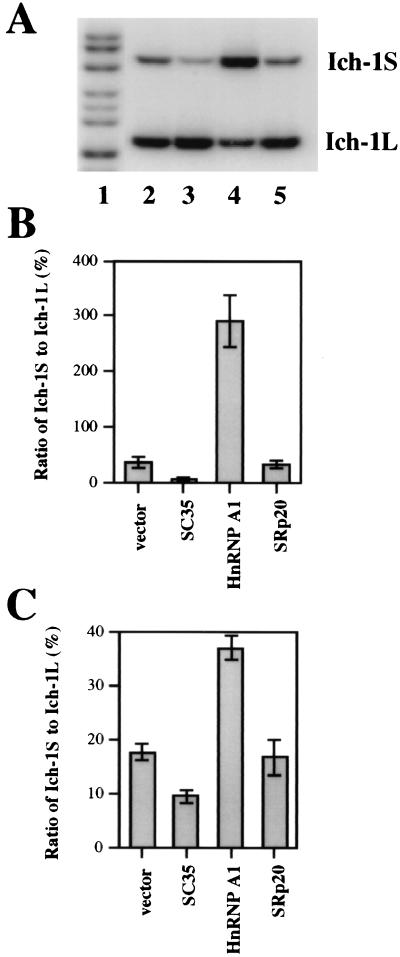
To determine whether this observation is specific to HeLa cells, we performed the same experiments using 293T cells and obtained similar results (Fig. 4). Higher transfection efficiency in 293T cells allowed us to examine the effects of these splicing regulators on endogenous Ich-1 alternative splicing using primers specific to endogenous human Ich-1 gene. After transfection with SC35 or hnRNP A1 expressing plasmids, small but reproducible changes in the ratio of endogenous Ich-1S to Ich-1L were observed. Similar effects on the alternative splicing of the transfected minigene and of the endogenous Ich-1 were noted. That is, SC35 decreased and hnRNP A1 increased the ratio of Ich-1S to Ich-1L (Fig. 4 B and C). These observations demonstrate that unique properties of Ich-1 alternative splicing regulation by SC35 and hnRNP A1 are intrinsic to the Ich-1 gene and are not due to an artifact of the transfected Ich-1 minigene construct. Changes in the ratio of Ich-1S to Ich-1L of the endogenous Ich-1 transcripts were not as dramatic as those in the ratio of Ich-1 transcripts expressed from the transfected minigene. This is likely the consequence of low transfection efficiency because only ≈20% of cells were transfected. To our knowledge, this is the first report of effects of SR proteins on splicing regulation of an endogenous gene in mammalian cells, although an hnRNP A1 related protein has been reported to regulate dopa decarboxylase gene alternative splicing in Drosophila (18).
Figure 4.
Regulation of Ich-1 alternative splicing by SC35 and hnRNP A1 in 293T cells. (A and B). Effects of SC35 and hnRNP A1 on alternative splicing of the Ich-1 minigene. Panel A shows a representative gel containing RT-PCR products detected from 293T cells transfected with the Ich-1 minigene construct together with either the vector alone or a plasmid expressing splicing factor: IchG+vector (lane 2), IchG+SC35 (lane 3), IchG+hnRNP A1 (lane 4), and IchG+SRp20 (lane 5). Lane 1 contains size markers. B shows the ratio of Ich-1S to Ich-1L as determined from three independent experiments. (C) Effects of SC35 and hnRNP A1 on endogenous Ich-1 alternative splicing. The plasmids expressing individual splicing regulators were transfected into 293T cells. The alternative splicing products of the endogenous Ich-1 were detected by RT-PCR with human Ich-1 primers. Shown in C is quantitation of the ratio of Ich-1S to Ich-1L as determined from three independent experiments.
Regulation of Apoptosis by Splicing Factors.
Potential roles for splicing factors in regulating apoptosis are suggested by our finding that SC35 increases the relative amount of Ich-1L transcript, which encodes a product promoting apoptosis, whereas hnRNP A1 increases the relative amount of Ich-1s, which encodes a product promoting cell survival. To directly test the function of these splicing factors in apoptosis, we transfected plasmids expressing the splicing factors into HeLa cells and then scored the number of apoptotic cells according to the nuclear morphology. We chose to use HeLa cells because Ich-1L overexpression is known to induce apoptosis in these cells (2), and adherence of HeLa cells to the culture dish surface is better than 293T cells, making the scoring of apoptotic cells more accurate. The assay for apoptosis was similar to that performed for Ich-1 (2). After transfection, cells were immunostained to detect transfected cells by using the mAb against the myc tag present on the expressed splicing regulators. Hoechst dye 33258 counterstaining revealed the nuclear morphology of cultured cells. Under normal conditions, transfection of plasmids encoding hnRNP A1, SC35 or SRp20 had no detectable effect on cell death (W.-j.Z. and J.Y.W., unpublished results). However, when cells were deprived of serum, SC35 overexpression increased cell death; whereas hnRNP A1 overexpression decreased the number of dead cells (Fig. 5 and Table 1). SRp20 overexpression, on the other hand, had no detectable effect. As shown in Table 1, ≈20% of cells overexpressing SC35 were apoptotic as assessed by fragmentation and condensation of nuclei, whereas, 4.3% of nontransfected cells in the same dish were apoptotic. However, in hnRNP A1 overexpressing cells only 1.9% were apoptotic as compared with 4.5% in the nontransfected cells. Under these conditions, expression level of different splicing factors was comparable as assessed by Western blot analysis of cell lysates of the transfected cells by using the mAb against the myc tag (data not shown). Furthermore, immunofluorescent staining using the anti-myc antibody revealed that the percentage of transfected cells was equivalent in cells expressing different splicing factors. The number of apoptotic cells was comparable in the nontransfected cells among different transfections, suggesting that the effects observed were not due to nonspecific toxicity of different DNA preparations. These results demonstrate that SC35 promotes apoptosis, whereas hnRNP A1 facilitates cell survival under certain conditions. Ich-1s overexpression prevents cell death induced by serum deprivation (2). This may offer an explanation for the protective effect of hnRNP A1 overexpression. It should be noted that only a fraction of SC35 overexpressing cells are apoptotic and not every cell overexpressing hnRNP A1 is resistant to death induced by serum removal. Because both SC35 and hnRNP A1 can regulate alternative splicing of a number of pre-mRNA substrates in vitro, it is possible that other genes involved in cell death are also affected, and the final outcome depends on the overall effects of overexpression of these splicing factors.
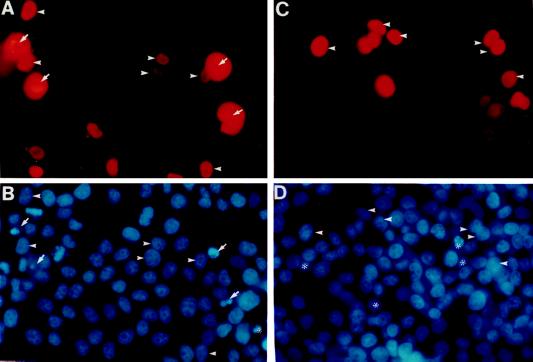
Figure 5.
Overexpression of SC35 or hnRNP A1 modulates apoptosis. HeLa cells were transiently transfected with plasmids expressing SC35 (A and B) or hnRNP A1 (C and D) as proteins containing a myc-tag. The transfected cells were shown by immunostaining using a mAb against the myc-tag (A and C). The cells were counterstained with Hoechst dye 33258 to show the nuclear morphology (B and D). Nuclei of a fraction of SC35 overexpressing cells are fragmented and condensed (B) (as marked by arrows). The nontransfected cells showing apoptotic condensed and fragmented nuclei are marked by *. Labeled with arrowheads are several cells that overexpress SC35 or hnRNP A1 and have normal nuclear morphology. The SC35 immunostaining in the transfected healthy cells was localized to the nucleus. However, in many dying cells overexpressing SC35, the immunostaining was no longer confined to the nucleus possibly due to defects in the nuclear envelope.
Table 1.
Effects of splicing factor overexpression on HeLa cells
| Transfected | Nontransfected | |
|---|---|---|
| SC35 | 19.9 ± 2.1 (2,931) | 4.3 ± 0.4 (4,190) |
| HnRNP A1 | 1.9 ± 0.2 (3,232) | 4.5 ± 0.5 (3,190) |
| SRp20 | 5.6 ± 0.6 (3,010) | 5.3 ± 0.3 (2,600) |
HeLa cells were transfected with myc-tagged SC35, hnRNPA, or SRp20 as described in Fig. 5. Cells were scored by immunostaining with anti-myc antibody for expression of the transfected splicing factors and by Hoechst dye 33258 staining for the nuclear morphology. The percentage of cells that had fragmented and condensed nuclei are shown with the total number of cells counted in parentheses. The data were collected from three independent experiments.
Our studies reveal that functionally important Ich-1 pre-mRNA splicing results from alternative exon inclusion. Our results demonstrate that several splicing factors can regulate Ich-1 alternative splicing of both the cotransfected minigene and the endogenous gene. Most interestingly, we have found that overexpression of these splicing factors can regulate apoptosis, in a manner consistent with their regulation of Ich-1 pre-mRNA splicing. While it remains possible that the observed effects on apoptosis may not be directly mediated by Ich-1 and that overexpression of these splicing factors could affect apoptosis in a indirect way, it is likely that these splicing factors may regulate activity of caspase family of apoptosis regulators because caspase inhibitor z-VAD significantly reduced the effects of SC35 and hnRNP A1 on apoptosis (W.-j.Z. and J.Y.W., unpublished result).
Our investigation of the effects of splicing factors on Ich-1 splicing has initiated the identification of molecular components that regulate alternative splicing of Ich-1. The observation that SC35 and ASF/SF2 promote Ich-1L production and that hnRNP A1 facilitates Ich-1S production raised the possibility that the relative concentrations of different splicing factors may contribute to tissue specific splicing of Ich-1. Thus, the thymus expresses predominantly Ich-1L whereas the brain expresses both Ich-1L and Ich-1S (Fig. 1; ref. 2). We have previously found that certain SR proteins, such as SC35, are expressed at a higher level in the thymus than in the brain (16). These results are consistent with the possibility that activities of SR proteins such as SC35 affect the relative ratio of Ich-1 transcripts. Although it is likely that relative concentration of SR proteins (such as SC35) vs. hnRNP A1 may regulate Ich-1 alternative splicing, it is possible that other splicing factors also participate in regulation of Ich-1 alternative splicing. It will be interesting to identify splicing factors responsible for tissue specific pattern of Ich-1 alternative splicing.
It is noteworthy that SC35 and ASF/SF2 promote exon skipping and that hnRNP A1 enhances exon inclusion in the Ich-1 system. These effects are opposite to the roles of these factors in β-tropomyosin and clathrin light chain B pre-mRNA alternative splicing, in which, ASF/SF2 prevents exon skipping whereas hnRNP A1 increases exon skipping (13–15). Our observations, when compared with results from the previous studies (13–15), suggest that Ich-1 system has properties distinct from the previously known alternative splicing model systems and that splicing factors regulate alternative exon skipping/inclusion in a substrate-dependent manner.
Our findings of the effects of splicing factors on apoptosis revealed a new class of proteins that could regulate apoptosis, in addition to those directly involved in the cell death machinery. The opposite effects of SC35 and hnRNP A1 on apoptosis suggest that the relative concentrations and activities of such splicing factors may provide a previously unsuspected regulatory mechanism for controlling cell death and survival.
Acknowledgments
We acknowledge Dr. Jun-Ying Yuan (Harvard Medical School) for the gift of Ich-1 genomic DNA and Dr. Raphael Kopan for providing 293T cells. We thank Drs. Ethan Bier, Stan Korsmeyer, Alan Schwartz and Arnold Strauss for critical reading of the manuscript. This work was supported by a grant from the National Institutes of Health (RO1 GM53945), a special fellowship from the Leukemia Society of America, and an institutional pilot research grant through Washington University from the Howard Hughes Medical Institute (76296–538202).
ABBREVIATIONS
- SR
serine-arginine-rich
- hnRNP
heterogeneous nuclear ribonucleoprotein
- RT
reverse transcription
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Boise L H, González-García M, Postema C E, Ding L, Lindstein T, Turka L A, Mao X, Nuñez G, Thompson C B. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Miura M, Bergeron L, Zhu H, Yuan J-Y. Cell. 1994;78:739–750. doi: 10.1016/s0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- 3.Shaham S, Horvitz H R. Cell. 1996;86:201–208. doi: 10.1016/s0092-8674(00)80092-6. [DOI] [PubMed] [Google Scholar]
- 4.Alnemri E S, Fernandes-Alnemri T, Litwack G. J Biol Chem. 1995;270:4312–4317. doi: 10.1074/jbc.270.9.4312. [DOI] [PubMed] [Google Scholar]
- 5.Papoff G, Cascino I, Eramo A, Starace G, Lynch D H, Ruberti G. J Immunol. 1996;156:4622–4630. [PubMed] [Google Scholar]
- 6.Rio D C. Curr Opin Genet Dev. 1993;3:574–584. doi: 10.1016/0959-437x(93)90093-5. [DOI] [PubMed] [Google Scholar]
- 7.Berget S M. J Biol Chem. 1995;2:2411–2414. doi: 10.1074/jbc.270.6.2411. [DOI] [PubMed] [Google Scholar]
- 8.Fu X-D. RNA. 1995;1:663–680. [PMC free article] [PubMed] [Google Scholar]
- 9.Krämer A. Annu Rev Biochem. 1996;65:367–409. doi: 10.1146/annurev.bi.65.070196.002055. [DOI] [PubMed] [Google Scholar]
- 10.Reed R. Curr Opin Genet Dev. 1996;6:215–220. doi: 10.1016/s0959-437x(96)80053-0. [DOI] [PubMed] [Google Scholar]
- 11.Manley J L, Tacke R. Genes Dev. 1996;10:1569–1579. doi: 10.1101/gad.10.13.1569. [DOI] [PubMed] [Google Scholar]
- 12.Valcarcel J, Green M R. Trends Biochem Sci. 1996;21:296–301. [PubMed] [Google Scholar]
- 13.Cáceres J F, Stamm S, Helfman D M, Krainer A R. Science. 1994;265:1706–1709. doi: 10.1126/science.8085156. [DOI] [PubMed] [Google Scholar]
- 14.Mayeda A, Krainer A R. Cell. 1992;68:365–375. doi: 10.1016/0092-8674(92)90477-t. [DOI] [PubMed] [Google Scholar]
- 15.Mayeda A, Helfman D M, Krainer A R. Mol Cell Biol. 1993;13:2993–3001. doi: 10.1128/mcb.13.5.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W-J, Wu J Y. Mol Cell Biol. 1997;16:5400–5408. doi: 10.1128/mcb.16.10.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evan G I, Lewis G K, Ramsey G, Bishop M. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen J, Zu K, Cass C L, Beyer A L, Hirsh J. Proc Natl Acad Sci USA. 1995;92:1822–1825. doi: 10.1073/pnas.92.6.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]