Abstract
The action of trypsin and pronase on the haemagglutinins and neuraminidases of eight strains of influenza virus has been examined.
The haemagglutinins of all the strains were highly susceptible to digestion by pronase but there were great variations in resistance to trypsin.
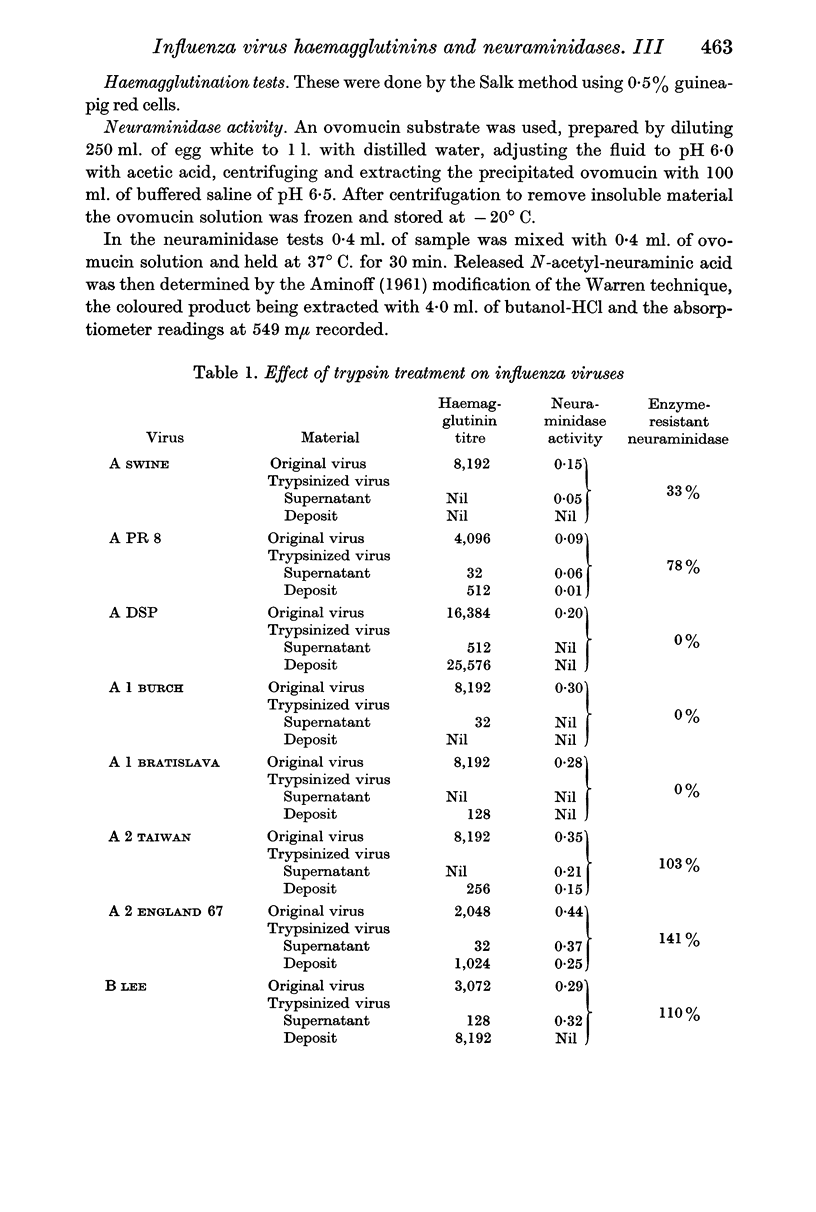
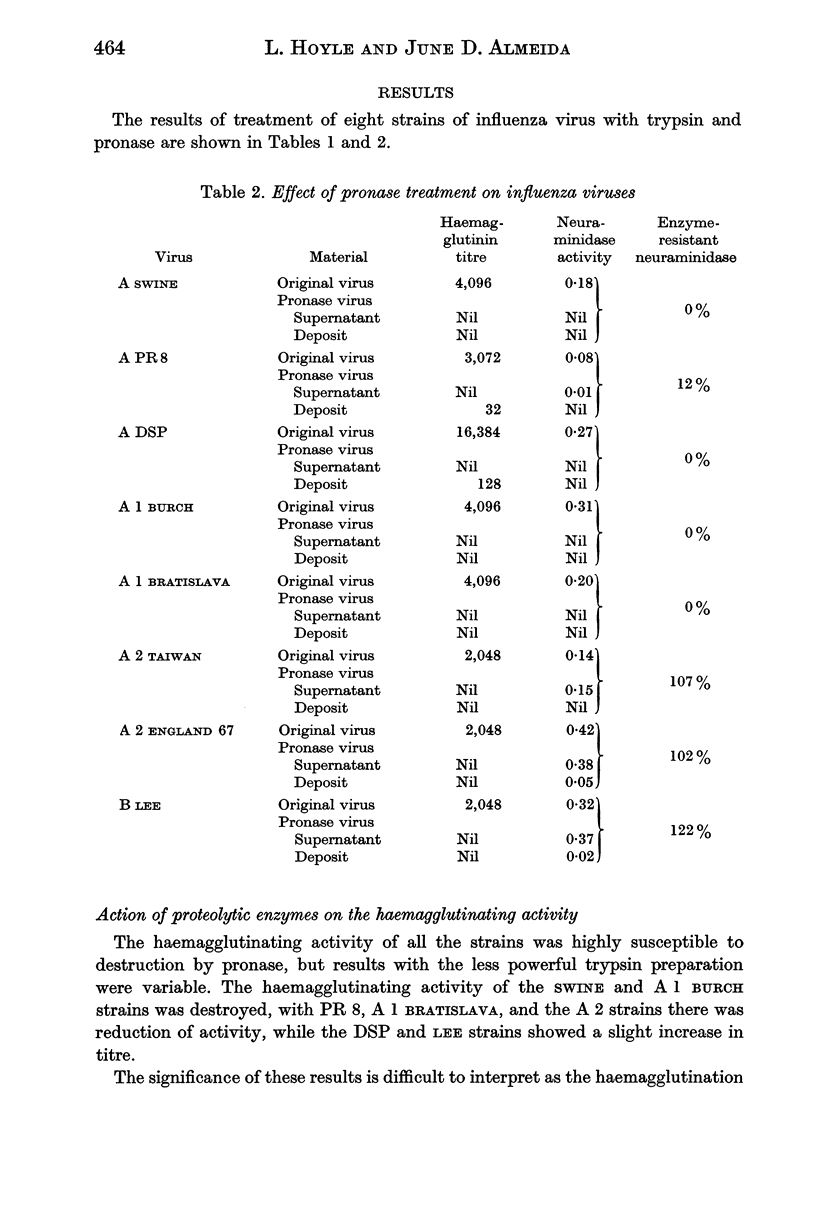
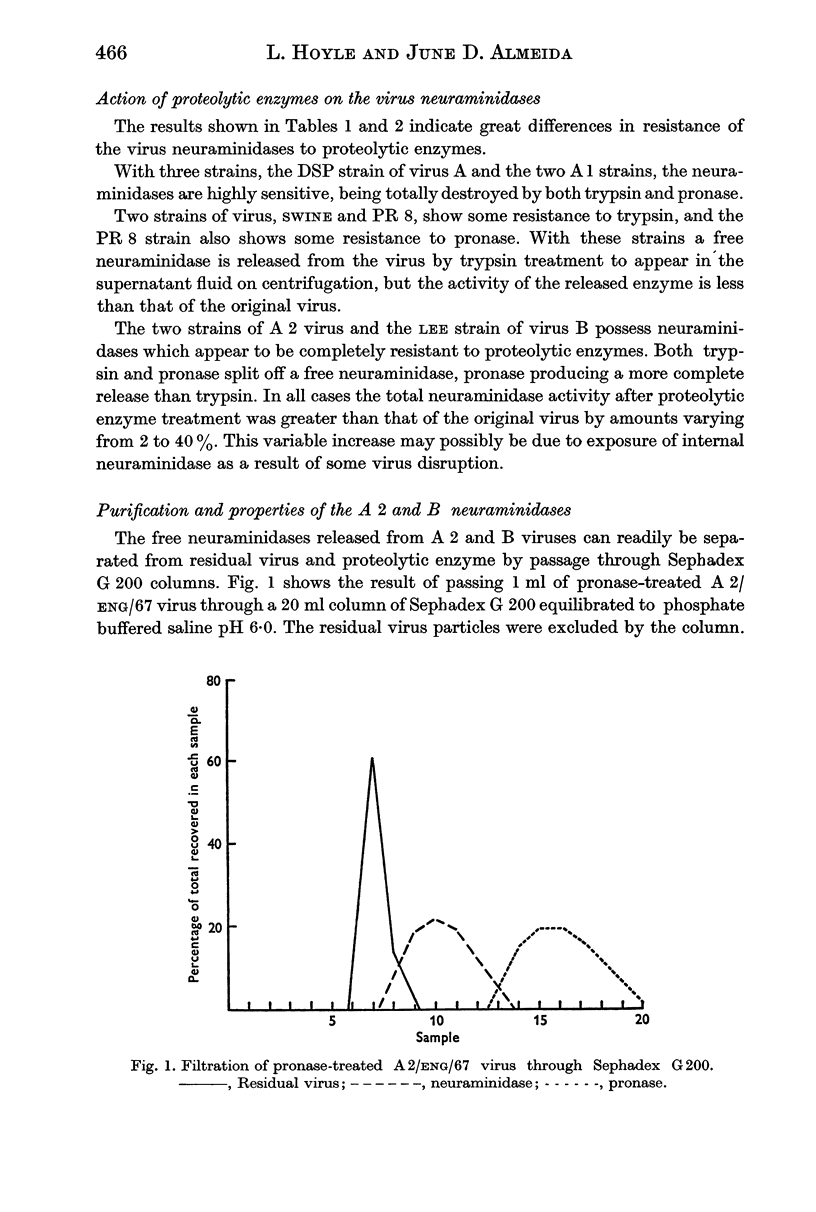
The neuraminidases of the eight strains were of three types. The neuraminidases of the A 1 strains and the DSP strain of virus A were highly susceptible to destruction by both enzymes. The neuraminidases of the PR 8 and Swine strains showed partial resistance especially to trypsin, while the A 2 strains and the LEE strains of virus B possessed neuraminidases that were completely resistant to both trypsin and pronase.
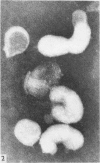
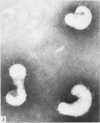
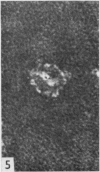
Proteolytic enzymes released free neuraminidases from the A 2 and LEE viruses the morphology of which was different from that of neuraminidases released by detergent treatment.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle L. The chemical reactions of the haemagglutinins and neuraminidases of different strains of influenza viruses. I. Effect of reagents reacting with amino acids in the active centres. J Hyg (Lond) 1969 Jun;67(2):289–299. doi: 10.1017/s0022172400041693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle L. The chemical reactions of the haemagglutinins and neuraminidases of different strains of influenza viruses. II. Effects of reagents modifying the higher order structure of the protein molecule. J Hyg (Lond) 1969 Jun;67(2):301–310. doi: 10.1017/s002217240004170x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAVER W. G. STRUCTURAL STUDIES ON THE PROTEIN SUBUNITS FROM THREE STRAINS OF INFLUENZA VIRUS. J Mol Biol. 1964 Jul;9:109–124. doi: 10.1016/s0022-2836(64)80094-2. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Valentine R. C. Morphology of the isolated hemagglutinin and neuraminidase subunits of influenza virus. Virology. 1969 May;38(1):105–119. doi: 10.1016/0042-6822(69)90132-9. [DOI] [PubMed] [Google Scholar]
- MAYRON L. W., ROBERT B., WINZLER R. J., RAFELSON M. E., Jr Studies on the neuraminidase of influenza virus. I. Separation and some properties of the enzyme from Asian and PR8 strains. Arch Biochem Biophys. 1961 Mar;92:475–483. doi: 10.1016/0003-9861(61)90387-3. [DOI] [PubMed] [Google Scholar]
- NOLL H., AOYAGI T., ORLANDO J. The structural relationship of sialidase to the influenza virus surface. Virology. 1962 Sep;18:154–157. doi: 10.1016/0042-6822(62)90193-9. [DOI] [PubMed] [Google Scholar]
- Reginster M. Release of influenza virus neuraminidase by caseinase C of Streptomyces albus G. J Gen Microbiol. 1966 Mar;42(3):323–331. doi: 10.1099/00221287-42-3-323. [DOI] [PubMed] [Google Scholar]
- SUGG J. Y., CLEELAND R. Differences in trypsin susceptibility among influenza viruses and relationship of the susceptibility to the antigenic type or subtype of the virus. J Immunol. 1962 Mar;88:369–376. [PubMed] [Google Scholar]
- Seto JT BRZENIEK R., Rott R. Isolation of a low molecular weight sialidase (neuraminidase) from influenza virus. Biochim Biophys Acta. 1966 Feb 14;113(2):402–404. doi: 10.1016/s0926-6593(66)80081-4. [DOI] [PubMed] [Google Scholar]